Abstract
Alzheimer’s disease (AD), the most prevalent form of dementia, is characterized by the accumulation of amyloid-beta (Aβ) plaques and hyperphosphorylated tau tangles. Currently, Alzheimer’s disease (AD) impacts 50 million individuals, with projections anticipating an increase to 152 million by the year 2050. Despite the increasing global prevalence of AD, its underlying pathology remains poorly understood, posing challenges for early diagnosis and treatment. Recent research suggests a link between gut dysbiosis and the aggregation of Aβ, the development of tau proteins, and the occurrence of neuroinflammation and oxidative stress are associated with AD. However, investigations into the gut–brain axis (GBA) in the context of AD progression and pathology have yielded inconsistent findings. This review aims to enhance our understanding of microbial diversity at the species level and the role of these species in AD pathology. Additionally, this review addresses the influence of confounding elements, including diet, probiotics, and prebiotics, on AD throughout different stages (preclinical, mild cognitive impairment (MCI), and AD) of its progression.
1. Introduction
Unlike the typical aging process, Alzheimer’s disease (AD) is a progressive neurodegenerative condition characterized by a range of cognitive impairments affecting various aspects of daily life. These impairments impact memory, thinking, decision making, communication, problem solving, personality, and mobility [1,2]. In AD, the formation of amyloid-beta (Aβ) plaques and hyperphosphorylated tau neurofibrillary tangles (NFTs) leads to inflammation and a gradual decline in cognitive function [3]. Despite various hypotheses about the development of AD, its onset and progression remain unclear.
Recent evidence suggests that the gut microbiota–brain axis could offer insights into the early diagnosis and treatment of neurodegenerative disorders, including depression and AD [4,5,6]. Gut health is significantly influenced by microbiota, which is largely composed of diverse microorganisms and resides primarily in the gastrointestinal tract (GIT). The gut microbiota’s role in AD pathogenesis has been extensively explored, revealing that individuals with AD and mild cognitive impairment (MCI) exhibit a lower gut microbiota diversity index than healthy controls [7,8].
Additionally, studies indicate similarities in the gut microbiota of individuals with MCI and AD, offering potential insights into pre-dementia pathogenesis and the identification of at-risk individuals [9,10]. Moreover, numerous studies are pursuing the goal of understanding and mitigating changeable risk factors for AD pathology, such as lifestyle, different types of dietary patterns, and obesity. These external factors play a critical role in AD development [11]. Conversely, research has shown that a healthy diet may offer a non-pharmacotherapeutic approach to modulating AD neuropathological markers [12]. Therefore, researchers are studying several lifestyle and dietary patterns in order to determine which patterns are most effective in preventing AD, focusing primarily on the Mediterranean diet, DASH diet, MIND diet, and ketogenic diet [13].
Gut microbiota can be affected by several factors, including genetics, age, antibiotics, and diet. Hence, this review aims to enhance our understanding of gut microbiota function, the role of diet, and the connections of these factors to AD.
2. Alzheimer’s Disease
AD is a neurodegenerative disease that affects 50 million people worldwide. It is estimated that this number will reach 152 million by 2050 [14,15]. AD is the 6th leading cause of death among adults due to a decline in memory and cognitive functions [16]. Currently, in Australia, one in ten people over 65 have AD, and three in ten people over 85 have the disease [17]. In Australia, dementia is the second leading cause of death for all residents, and according to provisional data, it is expected to become the leading cause of death within the next few years. According to Austrian statistics, there are estimated to be almost 29,000 people suffering from young-onset dementia in 2024, and the number is expected to rise to over 41,000 individuals by 2054. It can include individuals in their 30s, 40s, and 50s [18]. A global estimate of the annual cost of AD and other forms of dementia is USD $605 billion, equivalent to 1% of the global gross domestic product [19]. It is predicted that by 2030, the costs associated with AD and dementia will more than double from US$1.3 trillion per year to $2.8 trillion dollars per year, according to the World Alzheimer Report 2023 [15,20]. There is no effective treatment for AD, which results in symptoms worsening as the condition progresses. AD has been recognized as a global public health priority by the World Health Organization (WHO) [21].
2.1. Pathology of Alzheimer’s Disease
The neuropathological hallmarks of AD are extracellular Aβ plaques and the formation of NFTs. The development of Aβ and NFTs results in the loss of synapses and neurons [22]. Aβ plaques develop initially in the basal, temporal, and orbitofrontal neocortex regions of the brain and eventually spread into the neocortex, hippocampus, amygdala, diencephalon, and basal ganglia [23]. There are several hypotheses that have been proposed to explain the mechanism of action of Aβ peptides and NFTs in neurodegeneration of AD [22]. These include the amyloid cascade hypothesis, the tau hyperphosphorylation hypothesis, and the oxidative stress hypothesis. In addition, oxidative stress, mitochondrial dysfunction, and neuroinflammation play crucial roles in neuropathological changes in the brain [24].
2.2. Amyloid-Beta Peptides
Aβ is a transmembrane protein that is produced by hydrolysing the amyloid precursor protein (APP) via the amyloidogenic pathway [25]. This process is initiated by beta-site APP cleaving enzyme 1 (Beta-Secretase 1 (BACE1)), which forms a large soluble protein and a 99-amino-acid C-terminal fragment (C99). The C99 fragment is further processed by a γ-secretase to produce Aβ in either its 40- or 42-amino acid form [26]. Aβ42 levels have been identified as being important in early events in AD pathogenesis, especially the ratio of Aβ42/Aβ40 [26]. Further, Aβ monomers aggregate into oligomers, protofibrils, and amyloid fibrils. Fibrils of amyloid are larger and insoluble, and they can form plaques, whereas oligomers of amyloid can travel throughout the brain [26,27].
2.3. Tau Proteins
An NFT consists of paired helical filaments and straight filaments, which contain an abnormally phosphorylated form of the microtubule-associated protein tau. Tau is mainly found in neuronal axons of the brain [28]. When tau is acetylated or truncated, it is unable to bind to microtubules, which promotes tau aggregation, mitochondrial dysfunction, and synaptic deficiencies [29].
2.4. Oxidative Stress
Oxidative stress plays a significant role in the development and progression of AD pathology. Oxidative stress occurs when there is an increase in free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species [30]. Under stressful conditions, ROS formation increases within mitochondria and increases the risk of developing AD. In AD, oxidative stress promotes Aβ deposition and tau hyperphosphorylation, as well as subsequent loss of synapses and neurons [31].
3. The Gut–Brain Axis
The bidirectional communication between the enteric nervous system (ENS) and the central nervous system (CNS), known as the gut–brain axis (GBA), establishes a connection between the emotional and cognitive functions of the brain and peripheral intestinal functions [32,33]. Recent studies highlight the significant role of gut microbiota in GBA function, influencing the nervous, immune, and endocrine systems [34]. The microbiota in the gut produces various of neuroactive substances, such as neurotransmitters, short-chain fatty acids (SCFAs), and other bacterial metabolites, which influence neural activity and brain function [34,35,36,37,38]. These substances are generated through the fermentation of dietary fibre and other components (such as nitric oxide, ammonia, and ethanol) by gut bacteria [35,39].
However, gut dysbiosis has been linked to conditions such as anxiety, depression, autism spectrum disorders, and AD [40,41]. Consequently, the GBA has emerged as a potential target for therapeutic interventions aimed at enhancing brain health and treating neurological and psychiatric disorders. Enhancing brain health and treating brain-related disorders can be achieved by modifying the gut microbiota through various interventions, including dietary adjustments, prebiotics, probiotics, antibiotics, and faecal microbiota transplantation [42,43,44]. Further research is required to gain a more profound understanding of the complex interactions between the gut microbiota and the brain and to develop effective therapeutic strategies based on this knowledge.
4. Gut Microbiota
The human gut microbiota comprises bacteria, fungi, archaea, viruses, and protozoans existing in symbiotic relationships within the gastrointestinal tract [45]. In the human intestine, there are approximately 1000 species and 7000 strains of bacteria, with Firmicutes (such as Lactobacillus, Clostridium, and Eubacterium) and Bacteroidetes (including Bacteroides, and Prevotella) being the predominant phyla [33,45,46]. Recent studies on the human microbial flora emphasize the importance of maintaining a healthy intestinal microbiome, as there is a continual fluctuation in the structure, quantity, distribution, and biological characteristics of the endogenous intestinal flora.
An imbalance in intestinal flora is implicated in various diseases, including AD. Gut microbiota imbalance is closely associated with deficiencies in gut barrier function and intestinal permeability. A compromised gut barrier can lead to the release of microbial metabolites into the bloodstream. If the blood–brain barrier (BBB) experiences leakage, several proinflammatory cytokines can enter the central nervous system, triggering neuroinflammation by activating microglia and astrocytes [34,47].
5. Relationship between Gut Microbiota and AD
The microbiome’s involvement in AD pathogenesis has been observed in both animal and human studies. Specifically, research has identified associations between certain microbial organisms and the levels of cerebrospinal fluid (CSF) biomarkers related to AD. As an example, associations were noted between lower levels of cerebrospinal fluid (CSF) biomarkers, including the Aβ42/Aβ40 ratio, phosphorylated tau (p-tau), and the p-tau/Aβ42 ratio, and the presence of Clostridiaceae (SMB53) and Erysipelotrichaceae (cc115). Conversely, Blautia and Bacteroides spp. were associated with higher CSF biomarker levels [48]. Verhaar et al. (2022) introduced a machine learning model that identified Lachnospiraceae spp., Lachnoclostridium edouard, and Blautia faecis (Firmicutes) as the leading microbes in predicting the presence of tau, based on the area under the curve (AUC). This study also highlighted an association between gut microbiota composition and amyloid levels in the brain [49]. In five cross-sectional studies, associations were detected between the phylum Bacillota (Lachnospiraceae, Ruminococcus torques, Roseburia hominis, Lachnoclostridium, Marvinbryantia spp.) and the levels of Aβ and tau in both plasma and cerebrospinal fluid (CSF) [48,50,51,52,53]. Conversely, research identified higher levels of Alistipes spp. and Odoribacter splanchicus associated with increased amyloid in the CSF and decreased p-tau in the CSF [50]. Li et al. (2019) identified a negative correlation between amyloid burden and Lactobacillus abundance, as well as a positive correlation between Akkermansia muciniphila (phylum: Verrucomicrobiota) and medial temporal lobe atrophy [9]. Evidence from clinical and preclinical studies indicated that A. muciniphila plays a significant role in the development of depression, anxiety, Alzheimer’s disease, Parkinson’s disease, and other neuropsychiatric disorders [54]. However, some researchers discovered that A. muciniphila significantly reduced cognitive impairment in AD mouse models. It also improved the abundance of gut microbes that produce SCFAs and neurotransmitters. Additionally, they found that A. muciniphila reduced Aβ1–42 deposition in AD mice [55,56,57]. However, the underlying mechanism of A. muciniphila’s effect remains controversial.
Furthermore, Li et al.’s study detected associations between higher abundances of Fusicatenibacter, Blautia, and Dorea (family: Lachnospiraceae) and lower scores on the Mini-Mental State Examination (MMSE) among AD patients (18.1), individuals with mild cognitive impairment (MCI) (27.2), and normal controls (29.1). However, the presence of Hungatella, Faecalibacterium, and Butyricicoccus (family: Clostridiaceae) was associated with higher MMSE scores [9].
Both animal and human clinical research have reported alterations at the phylum level in the gut microbiota of AD patients and AD transgenic animal models, specifically in Firmicutes, Proteobacteria, and Bacteroidetes [58]. Although changes in Actinobacteria and Verrucomicrobia have been observed, these phyla are less prevalent in the gut of individuals with AD [58,59]. Cattaneo et al. (2017) demonstrated that individuals with amyloidosis exhibit distinct gut microbiota compositions compared to those without brain amyloidosis [48]. This study also revealed increased levels of proinflammatory cytokines, such as interleukin (IL)-6, CXCL2, NLRP3, and IL1β, in amyloid-positive patients compared to the anti-inflammatory cytokine IL-10. Proinflammatory cytokines were positively correlated with Escherichia/Shigella and negatively correlated with Eubacterium [48]. Additionally, Vogt et al. (2017) observed a reduction in gut microbiome bacterial diversity in AD patients compared to healthy age- and sex-matched control subjects. This was determined by sequencing 16S rRNA amplicons from faeces isolated from AD patients with dementia. Furthermore, they noted a decrease in Firmicutes and Bifidobacterium along with an increase in Bacteroidetes in AD patients compared to healthy controls (HCs) [50]. Microbiome studies conducted in China have also revealed differences in gut microbiota composition between AD patients and HCs [9,51,53,60]. Studies of human cohorts have also revealed links between specific gut bacteria and AD based on differentially abundant taxa (Table 1).
Li et al. (2019) study revealed higher prevalence of Bacillota (Blautia, Dorea), Firmicutes (Lactobacillus, Streptococcus), Verrucomicrobiota (Akkermansia), Actinobacteria (Bifidobacterium), and Pseudomonadota (Acinetobacter) in individuals with AD compared to healthy controls (HCs) [9]. Additionally, five cross-sectional studies identified Odoribacter splanchnicus, Bacteroides, Prevotella, and Alistipes spp. as highly abundant in AD, while Faecalibacterium prausnitzii, Eubacterium, Anaerostipes, Ruminococcus, and Roseburia spp. showed lower abundances in AD patients than in HCs [50,51,52,53,61]. Other reports also observed increased relative abundance of Actinobacteria and Bacilli, along with decreased relative abundance of Negativicutes and Bacteroidia in the AD group [48,50,51,52].
Although these findings collectively suggest alterations in gut microbiota composition in AD patients, it is essential to note that these studies primarily establish correlations, and there is a lack of uniformity in the outcomes regarding the bacterial phyla altered in AD patients.

Table 1.
Alterations in microbial diversity associated with AD from human studies.
Table 1.
Alterations in microbial diversity associated with AD from human studies.
| Sequencing Methods | Sample Size | Year | Results | Reference | |
|---|---|---|---|---|---|
| Decreased Microbiota Diversity in AD | Increased Microbiota Diversity in AD | ||||
| 16S rRNA amplicon sequencing for faecal samples | 25 AD 25 HCs | 2017 | Phyla: Firmicutes, Actinobacteria Genera: Bifidobacterium, SMB53, Dialister, Clostridium, Turicibacter, Adlercreutzia, cc115 | Phylum: Bacteroidetes Genera: Blautia, Bacteroides, Alistipes, Phascolarctobacterium, Bilophila, Gemella | [50] |
| qPCR for faecal samples | 40 amyloid-positive 33 amyloid-negative 10 HCs | 2017 | Amyloid-positive group showed lower abundance of E. rectale than other groups | Amyloid-positive group showed higher abundance of Escherichia/Shigella than other groups | [48] |
| 16S rRNA amplicon sequencing for faecal samples | 43 AD 43 HCs | 2018 | Phylum: Actinobacteria Classes: Negativicutes, Bacteroidia Orders: Bacteroidales, Selenomonadales Families: Lanchnospiraceae, Bacteroidaceae, Veillonellaceae Genera: Lachnoclostridium | Phylum: Bacteroidetes Classes: Actinobacteria, Bacilli Order: Lactobacillales Families: Ruminococcaceae, Enterococcaceae, Lactobacillaceae Genera: Bacteroides, Ruminococcus, Subdoligranulum | [51] |
| NextSeq500/Metagenomic analysis for faecal samples | 24 AD 33 with other dementia types 51 HCs | 2019 | Genus: Lachnoclostridium | Genera: Bacteroides, Alistipes, Odoribacter, Barnesiella | [52] |
| 16S rRNA amplicon sequencing for faecal and blood samples | 30 AD 30 MCI 30 HCs | 2019 | Genera: Alistipes, Bacteroides, Parabacteroides, Sutterella, Paraprevotella | Genera: Dorea, Lactobacillus, Streptococcus, Bifidobacterium, Blautia, Escherichia | [9] |
| 16S rRNA amplicon sequencing for faecal samples | 33 AD 32 amnestic MCI 32 HCs | 2019 | Phylum: Firmicutes | Phylum: Proteobacteria Orders: Gammaproteobacteria, Enterobacteriales Family: Enterobacteriaceae | [53] |
| 16S rRNA amplicon sequencing for faecal samples | 100 AD 71 HCs | 2021 | Genus: butyrate-producing Faecalibacterium | Genus: lactate-producing Bifidobacterium | [62] |
| 16S rRNA amplicon sequencing | 20 MCI 22 HCs | 2021 | Genus: Bacteroides Families: Veillonellaceae, Ruminococcaceae | Genera: Blautia, Bacteroide Family: Lachnospiraceae | [63] |
| 16S rRNA amplicon sequencing for faecal samples | 11 MCI 11 AD 34 HCs | 2022 | Phylum: Firmicutes Genera: Bilophila, Faecalibacterium Classes: Clostridia, Deltaproteobacteria Orders: Clostridiales, Desulfovibrionales Families: Lachnospiraceae, Desulfovibrionaceae, Ruminococcaceae | Phylum: Bacteroidetes Class: Bacteroidia Order: Bacteroidal | [64] |
| 16S rRNA amplicon sequencing for faecal samples | 27 MCI 47 AD 51 HC | 2022 | Genera: Roseburia, Lactobacillus, Fusicatenibacter | Genera: Prevotella, Bacteroides | [65] |
AD, Alzheimer’s disease; MCI, mild cognitive impairment; HC, healthy control.
6. Diet, Alzheimer’s Disease, and Microorganisms
The impact of diet on health can be either beneficial or detrimental. There is a possibility that diet plays a role in influencing microbial communities [66,67]. Various factors such as dietary patterns, microbiome-specific interventions, and the consumption of natural supplements have the potential to significantly modify the composition of the microbiota. This alteration, in turn, affects the gut–brain axis (GBA), potentially leading to the alleviation of AD-related pathology [68,69] (Figure 1).
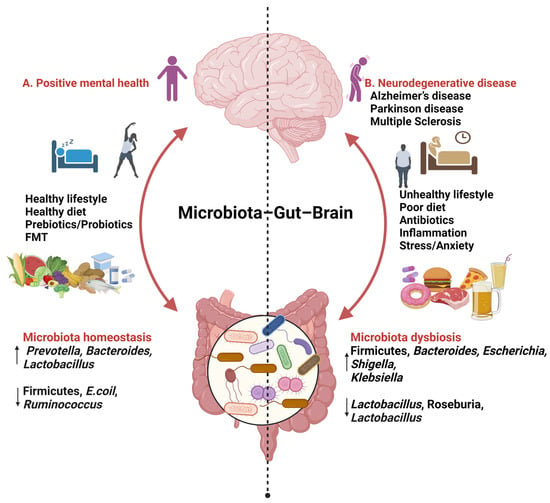
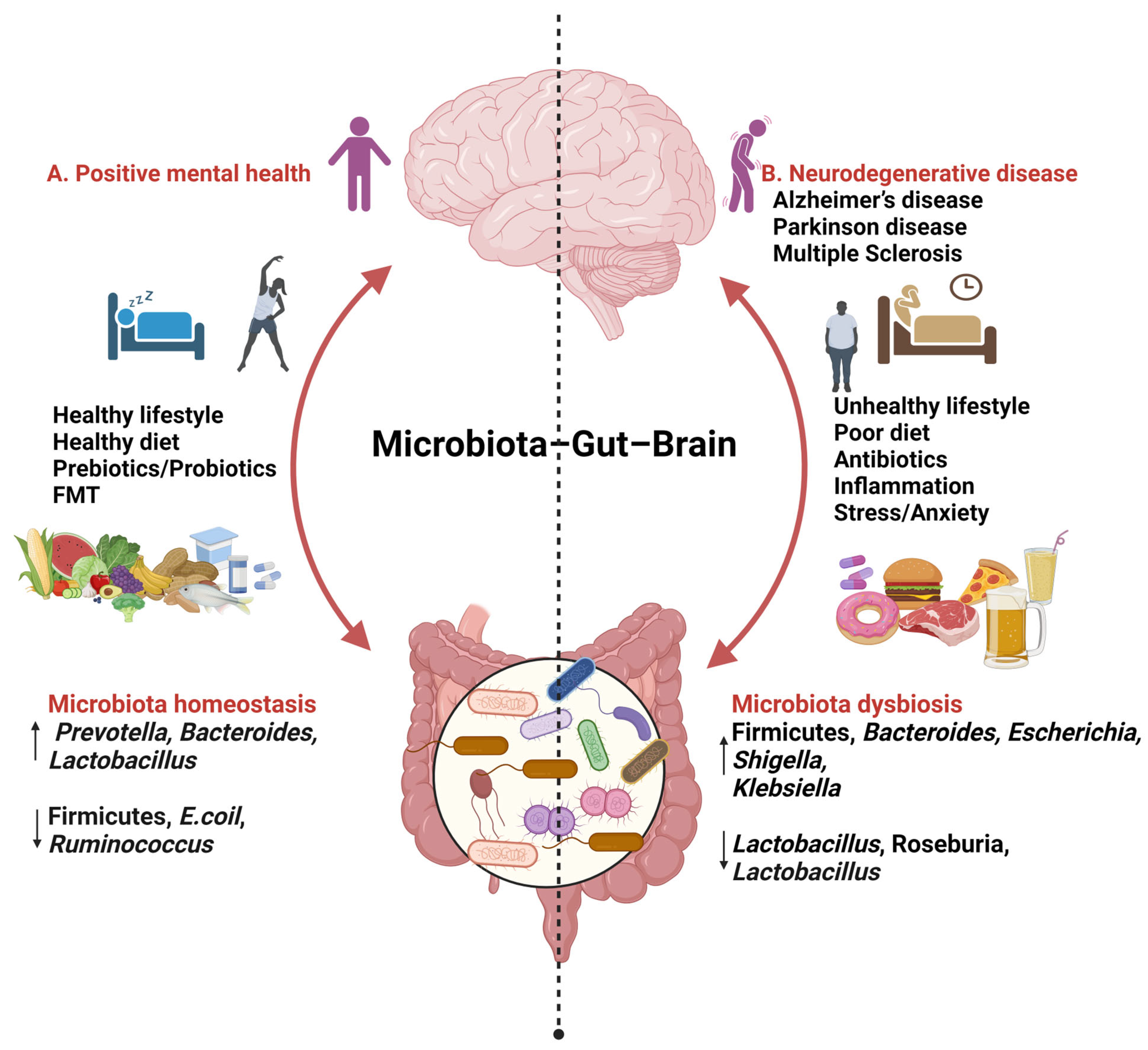
Figure 1.
Findings relating to gut–brain–microbiome interactions (created with BioRender.com, 24 January 2024). ↑ increase ↓ decrease.
A. Consuming a diet rich in fats (MUFAs, PUFAs), carbohydrates (fibre), and proteins, along with incorporating probiotics, prebiotics, and engaging in daily activities such as good sleep and exercise, has been associated with improved mental health. This dietary pattern is linked to an increase in beneficial microbial species such as Prevotella, Bacteroidetes, and Lactobacillus, while concurrently reducing levels of Firmicutes, Escherichia coli, and Ruminococcus.
B. An unhealthy lifestyle marked by stress; anxiety; and the consumption of high-fat, high-sugar, and processed foods has been associated with an increase in Firmicutes, Bacteroides, Escherichia, Shigella, and Klebsiella, while simultaneously decreasing levels of Lactobacillus, Roseburia, and Bacteroides.
6.1. Dietary Protein and Gut Microbiota
Protein stands as an essential macronutrient required by the human body. The quantity and source of protein intake, whether animal- or plant-based, can impact overall health and brain function [70]. The prolonged consumption of protein may influence the risk of cognitive decline, with higher protein intake being associated with a lower level of subjective cognitive impairment [71]. According to Fernando et al. (2018), a diet rich in protein may have a protective effect against brain amyloid-beta (Aβ) burden, especially before the onset of objective memory decline in older adults [72]. During the digestive process, unabsorbed dietary protein undergoes fermentation by proteolytic bacteria, resulting in beneficial end products that influence both host function and the composition of the microbiota [73]. Protein-rich diets have been linked to a reduction in anti-inflammatory bacteria, such as Bifidobacterium adolescentis, in the intestine, along with an increase in proinflammatory bacteria such as Bacteroides and Clostridium spp. [74]. Furthermore, studies have indicated a correlation between the intake of animal-derived proteins and a higher prevalence of AD due to the production of neurotoxic end products [75]. Conversely, substituting plant-derived proteins has been associated with reduced dementia-related mortality and improved brain health. Additionally, animal proteins may contribute to inflammation by promoting the growth of anaerobic bacteria such as Bacteroides, Alistipes, and Bilophila [76,77,78]. In contrast, plant-based proteins stimulate the growth of probiotic microorganisms such as Bifidobacterium and Lactobacillus while reducing the growth of pathogenic taxa such as Bacteroides fragilis and Clostridium perfringens [76,79,80,81]. Studies have shown a reduction in Roseburia and Eubacterium rectale in the intestinal microflora and a decrease in butyrate in the faecal matter of individuals following a high-protein or low-carbohydrate diet.
6.2. Dietary Fibre and Gut Microbiota
In general, carbohydrates can be categorized into simple sugars (monosaccharides, disaccharides) and complex sugars (starch, fibre). Dietary fibre serves as a beneficial reservoir of “microbiota-accessible carbohydrates” (MACs), enabling microbes to provide the host with both energy and carbon. Additionally, fibre has the ability to alter the flora of the intestinal tract. Fibres are therefore recognized as prebiotics, and increased intake of simple sugars is associated with an elevated risk of AD, whereas higher fibre intake is linked to a reduced risk of AD [79,82]. The consumption of soluble fibre promotes the production of short-chain fatty acids (SCFAs) by gut bacteria [83,84]. Evidence suggests that soluble fibre intake reduces propionate formation, enhances butyrate production, diminishes the activation of astrocytes, and improves cognitive function in the APP/PS1 mouse model of AD; these effects are attributed to gut microbiota dysbiosis [85].
A study indicated that individuals with a high fibre intake exhibited an increased prevalence of probiotic bacteria, including Lactobacillus, Bifidobacterium, and Roseburia, consequently reducing the Firmicutes:Bacteroidetes ratio [78,80].
6.3. Dietary Fat and Gut Microbiota
Dietary fats come in two main types, namely, saturated and unsaturated; the consumption of fats such as free fatty acids (FFAs), monounsaturated fats (MUFAs), and polyunsaturated fats (PUFAs) can influence the functions of numerous beneficial microorganisms such as Prevotella, Bifidobacterium [78]. MUFAs and PUFAs are associated with positive effects, including enhanced brain function and the prevention of neurodegenerative diseases [86,87,88]. A high intake of saturated fats and trans fats is associated with an increase in proinflammatory bacteria, while a high intake of MUFAs and PUFAs enhances the production of short-chain fatty acid (SCFA)-producing bacteria.
Studies have shown that saturated fats reduce anti-inflammatory bacteria (Lactobacillus intestinalis) and increase proinflammatory bacteria (Clostridial, Bacteroides, Bilophila, and Enterobacteriaceae) in mouse models [89]. Studies related to humans showed that saturated fat consumption led to an increase in the phylum Actinobacteria, while the phylum Firmicutes decreased in human studies. Additionally, the consumption of fish oil, rich in omega-3 PUFAs, increased the abundance of beneficial microbes, including Bifidobacterium, Adlercreutzia, Lactobacillus, Streptococcus, and Akkermansia muciniphila in transgenic mouse models [90]. In human studies, omega-3 PUFAs were shown to decrease the Firmicutes:Bacteroidetes ratio and increase the abundance of SCFA-producing bacterial genera, such as Bifidobacterium, Lachnospiraceae, and Roseburia [91,92,93]. High MUFA intake leads to increased levels of bacteria such as Parabacteroides, Roseburia, and Oscillospira, while decreasing proinflammatory bacteria such as Prevotella [94].
6.4. Polyphenols
Polyphenols are micronutrients with antioxidant properties that naturally occur in plants and plant-based foods [95]. The majority of polyphenols can be found in fruits and vegetables such as grapes, blackcurrants, cocoa, black and green olives, oranges, apples, almonds, flax seeds, pomegranates, red onions, and tomatoes. It is also possible to find polyphenols in coffee, green tea, and wine [96,97]. Polyphenols have been shown to affect the composition and diversity of intestinal microbiota. A diet rich in polyphenols promotes the growth of beneficial microbes, such as Bifidobacterium, and Lactobacillus, and lowers levels of pathogens, including Staphylococcus aureus, Salmonella typhimurium, and Clostridium spp. [98,99]. In addition to their anti-inflammatory properties, polyphenols and their metabolites have been shown to prevent cognitive decline. It has also been found that dietary polyphenols may prevent neurodegenerative conditions through the AGEs-RAGE axis, as well as by regulating the microbiota–gut–brain axis [100].
6.5. Dietary Patterns and Gut Microbiota
A growing body of evidence indicates that alteration of gut microbiota due to diets rich in vegetables, legumes, grains, nuts, and fish, with a preference for plant-based foods over animal products, holds the potential to prevent the intestinal inflammatory processes that underlie many chronic diseases including AD [101,102]. The most studied dietary patterns are the Mediterranean diet (MeD), Dietary Approaches to Stop Hypertension (DASH), the Mediterranean–DASH Intervention for Neurodegenerative Delay (MIND), and the ketogenic diet (KD) in related to AD in the elderly population [68,103,104] (Figure 2).
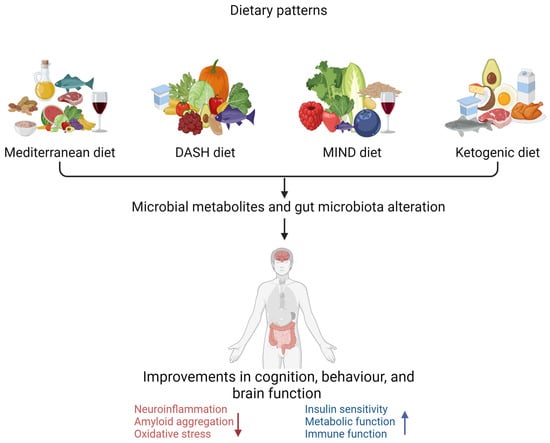
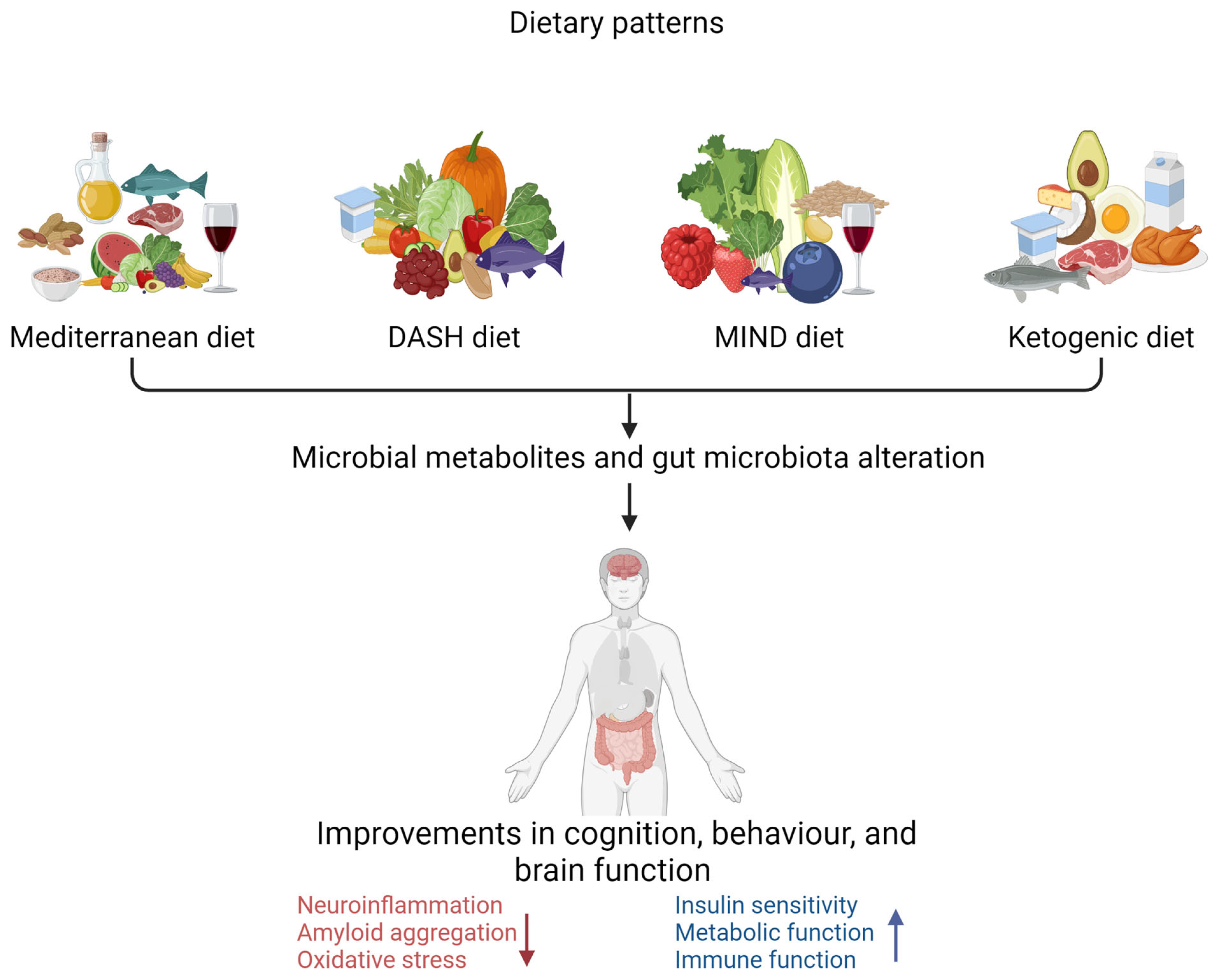
Figure 2.
Dietary pattern interventions to delay the progression of Alzheimer’s disease (created with BioRender.com). Dietary patterns such as the Mediterranean diet, DASH diet (Dietary Approaches to Stop Hypertension), MIND diet (Mediterranean–DASH Intervention for Neurodegenerative Delay), and ketogenic diet are associated with improved cognitive, behavioural, and brain function. ↑ increase ↓ decrease.
There are key differences among the Mediterranean, MIND, DASH, and ketogenic diets, as illustrated in Table 2, regarding the types of foods consumed in each dietary pattern [105]. All these dietary patterns have been associated with neuroprotective properties. The Mediterranean diet is associated with a reduced risk of cognitive decline in populations that consume it [105,106]. Consumption of the DASH diet has also been associated with improved cognitive function and a lowered risk of AD [107,108]. Evidence indicates that the MIND diet reduces cognitive impairment risk [109]. There is evidence that a ketogenic diet reduces or delays cognitive impairment in older individuals through different pathophysiological mechanisms [110,111].

Table 2.
Components of the Mediterranean, MIND, DASH, and ketogenic diets.
Several studies have been conducted to examine the relationship among the MeD, gut bacteria, and AD. The MeD consists of a diet rich in vegetables, fruits, nuts, whole grains, and olive oil, with moderate consumption of fish, poultry, and red wine, as well as polyphenols, fibre, and carbohydrates with a low glycaemic index. Therefore, the MeD promotes the growth of saccharolytic microbial species (Bacteroidetes, Firmicutes, and Actinobacteria) as well as the release of beneficial metabolites [115,116,117]. Consumption of the MeD has been shown to result in elevated levels of gut bacteria producing SCFAs, such as Bifidobacterium, Roseburia, and Lactobacillus, and reduced levels of proinflammatory bacteria such as Prevotella and Clostridium [97,118,119,120]. Additionally, the MeD has been linked to reduced human systemic inflammation through the promotion of beneficial fibre-degrading bacteria such as Cellulosilyticus, Faecalibacterium prausnitzii, and Eubacterium eligens [121]. Based on a meta-analysis, MeD diet compliance is also associated with reduced MCI and AD risk. This study included 34,168 participants, demonstrating a reduction of 17% in the risk of MCI and a reduction of 40% in the risk of AD. In addition, 612 non-frail or pre-frail individuals across five European countries were examined over a 12-month period; the findings showed that inflammation was reduced and that cognitive function was improved [122,123]. Mediterranean-diet followers had a 20% lower risk of dementia, according to a study conducted in 16,160 elderly participants in the EPIC-Spain Dementia Cohort [124]. Based on a systematic review, the Mediterranean diet has been shown to have beneficial effects on the cognitive function of the aging population after 10 weeks of adherence [125,126].
The ketogenic diet (KD), characterized by high fat (75%) and protein (20%) intake, with minimal carbohydrates (5%), aims to induce a state of ketosis [127]. Studies on individuals with MCI or AD show significant improvements in cognitive function with KD consumption, along with alterations in the microbiota, affecting species such as Akkermansia and Parabacteroides [128,129]. However, research on humans suggests that KD consumption reduces beneficial microbes, including Bifidobacteria, Dialister, E. rectale, Bacteroides, and Roseburia, while increasing proinflammatory bacteria such as E. Coli and Desulfovibrio spp. [130,131,132,133].
A study in MCI individuals on a high-fat modified Mediterranean KD (MMKD) observed changes in GABA-producing bacteria and GABA levels, emphasizing the need for caution in prolonged fasting due to potential risks of toxic levels and ketoacidosis in older individuals [128]. Moreover, high adherence to the DASH and MIND diets has been associated with reduced AD risk. Moreover, these dietary patterns contain nutrients with antioxidant and anti-inflammatory properties, contributing to the suppression of Aβ deposition [103,122].
7. Prebiotics, Probiotics, and Alzheimer’s Disease
Probiotics are living bacteria that promote the health of the host, while prebiotics are fibre substances degraded by gut microbiota [43,134]. The use of probiotics, such as lactic acid bacteria and Bifidobacterium, to reduce neuroinflammation has attracted attention [135,136]. However, there is still limited research on the therapeutic effects of probiotics and prebiotics in AD.
7.1. Prebiotics and Alzheimer’s Disease
Prebiotics are substrates selectively metabolized by host microorganisms to generate health benefits [137]. Recent studies in both animals and humans have investigated the impact of prebiotics on mental health. As an illustration, Liu et al. (2021) treated 5XFAD mice with the prebiotic mannan oligosaccharide, noting decreases in cognitive deficits, amyloid plaques, oxidative stress, and microglial activation, alongside modifications in the gut microbiome [138]. Additionally, Chen et al. (2017) noted that the prebiotic R13 tropomyosin receptor kinase B (TrkB) inhibited the proinflammatory pathway in the gut, leading to reduced amyloidogenesis and oxidative stress [28]. The prebiotic sodium oligomannate (GV-971) is utilized to enhance cognitive function and treat mild to moderate AD. Evidence suggests that GV-971 can reverse cognitive impairment; rectify gut dysbiosis; suppress neuroinflammation; and permeate the blood–brain barrier to directly bind to Aβ, inhibiting Aβ fibril formation [139].
7.2. Probiotics and Alzheimer’s Disease
The microbiota associated with probiotics can enhance cognitive function and play a positive role in preventing memory loss in Alzheimer’s disease (AD) [140,141]. Lactobacillus and Bifidobacterium are among the most commonly utilized probiotic genera [43]. Eight weeks of consumption of Bifidobacterium breve, Bifidobacterium longum, and Bifidobacterium infantis resulted in altered intestinal microbiome composition and increased levels of SCFAs in serum (acetate), brain (lactate and acetate), and serum (acetate and lactate). Notably, the proliferative marker Aβ and glial fibrillary acid protein did not exhibit significant changes [142,143].
An additional study demonstrated that combining probiotics with vitamin formulations resulted in reduced Aβ levels and improved cognitive performance in transgenic mouse models [144]. The same research group observed that when Bifidobacterium lactis Probio-M8 was administered for 45 days, it resulted in a reduced number of Aβ plaques, alterations in gut microbiota composition, and improved cognitive performance [145]. As per Akbari et al. (2016), probiotic milk containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum is associated with significantly improved Mini-Mental State Examination (MMSE) scores, reduced plasma malondialdehyde (MDA), and decreased plasma C-reactive protein (CRP) [146]. When MCI patients were treated with Bifidobacterium breve A1 for 16 weeks, the findings showed a significant improvement compared to the placebo group in neuropsychological assessments, including the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and the Japanese version of the MCI Screen (JMCIS) [147].
These studies concluded that probiotics, prebiotics, synbiotics (combinations of probiotics and prebiotics), and postbiotics (functional bioactive compounds such as SCFAs), could modify AD-related neuropathology and disease progression effectively, presenting a novel therapeutic approach [12,147].
8. Conclusions
Gut dysbiosis assumes a pivotal role in the pathology of AD, offering a non-invasive diagnostic and potential treatment avenue. The intricate interplay between gut microbiota and AD pathogenesis involves abnormalities in Aβ, tau phosphorylation, neuroinflammation, dysregulation of neurotransmitters, and oxidative stress. While various studies have identified functional bacteria linked to AD pathology and altered brain function, conclusive results remain elusive. Factors contributing to this ambiguity include a focus on genus-level associations without delving into species-level specifics and a lack of consideration for dietary changes. Ongoing research endeavours are dedicated to unravelling these mechanisms, promising valuable insights into the nuanced contributions of gut microbiota and dietary influences on cognition, dementia, and AD.
Author Contributions
Conceptualization, D.M.S.D. and W.M.A.D.B.F.; writing—original draft preparation, D.M.S.D. and W.M.A.D.B.F.; writing—review and editing, D.M.S.D., W.M.A.D.B.F., S.R.R.-S., R.N.M. and V.J.; supervision., W.M.A.D.B.F., S.R.R.-S., R.N.M. and V.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Delite Agro Polymers PVT–Industrial scholarship, Alzheimer’s Research Australia and Edith Cowan University (G1005890).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Helmes, E.; Østbye, T. Beyond memory impairment: Cognitive changes in Alzheimer’s disease. Arch. Clin. Neuropsychol. 2002, 17, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Wolk, D.A.; Dunfee, K.L.; Dickerson, B.C.; Aizenstein, H.J.; DeKosky, S.T. A medial temporal lobe division of labor: Insights from memory in aging and early Alzheimer disease. Hippocampus 2011, 21, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Mahalanobish, S.; Sil, P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yin, A.; Li, H.; Wang, R.; Wu, G.; Shen, J.; Zhang, M.; Wang, L.; Hou, Y.; Ouyang, H.; et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2015, 2, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Wang, X.; Huang, J.; Zhao, D.; Tan, Y.; Zhang, Z.; Zhang, Z.; Zhu, L.; Wu, B.; et al. Anti-Alzheimers molecular mechanism of icariin: Insights from gut microbiota, metabolomics, and network pharmacology. J. Transl. Med. 2023, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Ding, D.; Zhu, H.; Wang, R.; Su, F.; Wu, W.; Xiao, Z.; Liang, X.; Zhao, Q.; Hong, Z. Disturbed microbial ecology in Alzheimer’s disease: Evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021, 21, 226. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, X.; Wu, J.; Xiao, Z.; Wu, W.; Ding, S.; Zheng, L.; Liang, X.; Luo, J.; Ding, D.; et al. Altered Gut Microbiota and Its Clinical Relevance in Mild Cognitive Impairment and Alzheimer’s Disease: Shanghai Aging Study and Shanghai Memory Study. Nutrients 2022, 14, 3959. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, D.; Zhou, G.; Li, C. Dietary Pattern, Gut Microbiota, and Alzheimer’s Disease. J. Agric. Food Chem. 2020, 68, 12800–12809. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Ellouze, I.; Sheffler, J.; Nagpal, R.; Arjmandi, B. Dietary Patterns and Alzheimer’s Disease: An Updated Review Linking Nutrition to Neuroscience. Nutrients 2023, 15, 3204. [Google Scholar] [CrossRef]
- Romanenko, M.; Kholin, V.; Koliada, A.; Vaiserman, A. Nutrition, gut microbiota, and Alzheimer’s disease. Front. Psychiatry 2021, 12, 712673. [Google Scholar] [CrossRef]
- Wei, R.; Wei, P.; Yuan, H.; Yi, X.; Aschner, M.; Jiang, Y.-M.; Li, S.-J. Inflammation in Metal-Induced Neurological Disorders and Neurodegenerative Diseases. Biol. Trace Elem. Res. 2024, 202, 1–23. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W.; Svercauski, J. Alzheimer Disease (Nursing); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dementia Statistics: Dementia Australia. 2022. Available online: https://www.dementia.org.au/statistics (accessed on 1 January 2022).
- Dementia in Australia, Web Report. 2023. Available online: https://www.aihw.gov.au/reports/dementia/dementia-in-aus/contents/summary (accessed on 21 September 2023).
- Alzheimer’s Disease Statistics. Available online: https://alzheimersnewstoday.com/alzheimers-disease-statistics/ (accessed on 1 January 2022).
- World Alzheimer Report 2023. Available online: https://www.alzint.org/resource/world-alzheimer-report-2023/ (accessed on 21 September 2023).
- Flyer, B.A. Dementia: A Public Health Priority; World Health Organization (WHO): Geneva, Switzerland, 24 August 2012; Available online: https://www.who.int/publications/i/item/dementia-a-public-health-priority (accessed on 21 September 2023).
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J. New insights into the pathogenesis of Alzheimer’s disease. Front. Neurol. 2020, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Crews, L.; Masliah, E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010, 19, R12–R20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Yan, S.S. Role of mitochondrial amyloid-β in Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, S569–S578. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in Alzheimer’s disease: Molecular defects and therapeutic approaches. Mol. Psychiatry 2023, 28, 202–216. [Google Scholar] [CrossRef]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Soeda, Y.; Takashima, A. New insights into drug discovery targeting tau protein. Front. Mol. Neurosci. 2020, 13, 590896. [Google Scholar] [CrossRef]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Szablewski, L. Human gut microbiota in health and Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 62, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium butyricum against microglia-mediated neuroinflammation in Alzheimer’s disease via regulating gut microbiota and metabolites butyrate. Mol. Nutr. Food Res. 2020, 64, 1900636. [Google Scholar] [CrossRef]
- Saresella, M.; Calabrese, E.; Marventano, I.; Piancone, F.; Gatti, A.; Calvo, M.G.; Nemni, R.; Clerici, M. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 21, 927–938. [Google Scholar] [CrossRef]
- Clarke, G.; Sandhu, K.V.; Griffin, B.T.; Dinan, T.G.; Cryan, J.F.; Hyland, N.P. Gut reactions: Breaking down xenobiotic–microbiome interactions. Pharmacol. Rev. 2019, 71, 198–224. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Santos, S.F.; de Oliveira, H.L.; Yamada, E.S.; Neves, B.C.; Pereira, A., Jr. The Gut and Parkinson’s Disease-A Bidirectional Pathway. Front. Neurol. 2019, 10, 574. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.; Ling, Z.; Wang, F.; Ling, Y.; Gong, T.; Fang, N.; Ye, S.; Si, J.; Liu, J. Fructooligosaccharides ameliorating cognitive deficits and neurodegeneration in APP/PS1 transgenic mice through modulating gut microbiota. J. Agric. Food Chem. 2019, 67, 3006–3017. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Khanna, S.; Vazquez-Baeza, Y.; González, A.; Weiss, S.; Schmidt, B.; Muñiz-Pedrogo, D.A.; Rainey, J.F.; Kammer, P.; Nelson, H.; Sadowsky, M. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 2017, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Faruqui, N.A.; Prium, D.H.; Mowna, S.A.; Ullah, M.A.; Araf, Y.; Sarkar, B.; Zohora, U.S.; Rahman, M.S. Gut microorganisms and neurological disease perspectives. Future Neurol. 2021, 16, FNL53. [Google Scholar] [CrossRef]
- Askarova, S.; Umbayev, B.; Masoud, A.-R.; Kaiyrlykyzy, A.; Safarova, Y.; Tsoy, A.; Olzhayev, F.; Kushugulova, A. The links between the gut microbiome, aging, modern lifestyle and Alzheimer’s disease. Front. Cell. Infect. Microbiol. 2020, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, B.J.; Hendriksen, H.M.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M. Gut microbiota composition is related to AD pathology. Front. Immunol. 2022, 12, 794519. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.-Q.; Shen, L.-L.; Li, W.-W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. MBio 2019, 10, e00632-19. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia muciniphila in neuropsychiatric disorders: Friend or foe? Front. Cell. Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D. Potential of Akkermansia muciniphila and its outer membrane proteins as therapeutic targets for neuropsychological diseases. Front. Microbiol. 2023, 14, 1191445. [Google Scholar] [CrossRef]
- He, X.; Yan, C.; Zhao, S.; Zhao, Y.; Huang, R.; Li, Y. The preventive effects of probiotic Akkermansia muciniphila on D-galactose/AlCl3 mediated Alzheimer’s disease-like rats. Exp. Gerontol. 2022, 170, 111959. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.; Frisoni, G.; Neher, J.; Fåk, F.; Jucker, M.; Lasser, T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Forster, S.C.; Kumar, N.; Anonye, B.O.; Almeida, A.; Viciani, E.; Stares, M.D.; Dunn, M.; Mkandawire, T.T.; Zhu, A.; Shao, Y. A human gut bacterial genome and culture collection for improved metagenomic analyses. Nat. Biotechnol. 2019, 37, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Peng, J.; Huang, X.; Xiao, L.; Huang, F.; Zuo, Z. Gut Microbiome Features of Chinese Patients Newly Diagnosed with Alzheimer’s Disease or Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 80, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Shinkai, S.; Shiroma, H.; Taniguchi, Y.; Tsuchida, S.; Kariya, T.; Kawahara, T.; Kobayashi, Y.; Kohda, N.; Ushida, K. Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s-type dementia. Cell Rep. Med. 2021, 2, 100398. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef]
- Liu, P.; Jia, X.Z.; Chen, Y.; Yu, Y.; Zhang, K.; Lin, Y.J.; Wang, B.H.; Peng, G.P. Gut microbiota interacts with intrinsic brain activity of patients with amnestic mild cognitive impairment. CNS Neurosci. Ther. 2021, 27, 163–173. [Google Scholar] [CrossRef]
- Sheng, C.; Yang, K.; He, B.; Du, W.; Cai, Y.; Han, Y. Combination of gut microbiota and plasma amyloid-β as a potential index for identifying preclinical Alzheimer’s disease: A cross-sectional analysis from the SILCODE study. Alzheimer’s Res. Ther. 2022, 14, 1–15. [Google Scholar] [CrossRef]
- Yıldırım, S.; Nalbantoğlu, Ö.U.; Bayraktar, A.; Ercan, F.B.; Gündoğdu, A.; Velioğlu, H.A.; Göl, M.F.; Soylu, A.E.; Koç, F.; Gülpınar, E.A. Stratification of the gut microbiota composition landscape across the alzheimer’s disease continuum in a Turkish cohort. Msystems 2022, 7, e00004-22. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J. Alzheimer’s Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M. Relationships of dietary patterns, foods, and micro-and macronutrients with Alzheimer’s disease and late-life cognitive disorders: A systematic review. J. Alzheimer’s Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Hughes, C.F.; Ward, M.; Hoey, L.; McNulty, H. Diet, nutrition and the ageing brain: Current evidence and new directions. Proc. Nutr. Soc. 2018, 77, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Wallace, R.B.; Shadyab, A.H.; Kroenke, C.H.; Haring, B.; Howard, B.V.; Shikany, J.M.; Valdiviezo, C. Association of Major Dietary Protein Sources With All-Cause and Cause-Specific Mortality: Prospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e015553. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-S.; Yuan, C.; Ascherio, A.; Rosner, B.A.; Blacker, D.; Willett, W.C. Long-term dietary protein intake and subjective cognitive decline in US men and women. Am. J. Clin. Nutr. 2022, 115, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.; Rainey-Smith, S.R.; Gardener, S.L.; Villemagne, V.L.; Burnham, S.C.; Macaulay, S.L.; Brown, B.M.; Gupta, V.B.; Sohrabi, H.R.; Weinborn, M. Associations of dietary protein and fiber intake with brain and blood amyloid-β. J. Alzheimer’s Dis. 2018, 61, 1589–1598. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hentges, D.J.; Maier, B.R.; Burton, G.C.; Flynn, M.A.; Tsutakawa, R.K. Effect of a high-beef diet on the fecal bacterial flora of humans. Cancer Res. 1977, 37, 568–571. [Google Scholar] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Weisburger, J.H.; Wynder, E.L. Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J. Nutr. 1975, 105, 878–884. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Dominika, Ś.; Arjan, N.; Karyn, R.P.; Henryk, K. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef]
- Romond, M.-B.; Ais, A.; Guillemot, F.; Bounouader, R.; Cortot, A.; Romond, C. Cell-free whey from milk fermented with Bifidobacterium breve C50 used to modify the colonic microflora of healthy subjects. J. Dairy. Sci. 1998, 81, 1229–1235. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed]
- Satokari, R. High intake of sugar and the balance between pro-and anti-inflammatory gut bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Cuervo-Zanatta, D.; Syeda, T.; Sánchez-Valle, V.; Irene-Fierro, M.; Torres-Aguilar, P.; Torres-Ramos, M.A.; Shibayama-Salas, M.; Silva-Olivares, A.; Noriega, L.G.; Torres, N. Dietary fiber modulates the release of gut bacterial products preventing cognitive decline in an Alzheimer’s mouse model. Cell. Mol. Neurobiol. 2023, 43, 1595–1618. [Google Scholar] [CrossRef]
- Syeda, T.; Sanchez-Tapia, M.; Pinedo-Vargas, L.; Granados, O.; Cuervo-Zanatta, D.; Rojas-Santiago, E.; Díaz-Cintra, S.; Torres, N.; Perez-Cruz, C. Bioactive food abates metabolic and synaptic alterations by modulation of gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 66, 1657–1682. [Google Scholar] [CrossRef]
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G.A. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001, 161, 1581–1586. [Google Scholar] [CrossRef]
- Gardener, S.; Gu, Y.; Rainey-Smith, S.R.; Keogh, J.B.; Clifton, P.M.; Mathieson, S.; Taddei, K.; Mondal, A.; Ward, V.K.; Scarmeas, N. Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Transl. Psychiatry 2012, 2, e164. [Google Scholar] [CrossRef] [PubMed]
- Matura, S.; Prvulovic, D.; Mohadjer, N.; Fusser, F.; Oertel, V.; Reif, A.; Pantel, J.; Karakaya, T. Association of dietary fat composition with cognitive performance and brain morphology in cognitively healthy individuals. Acta Neuropsychiatr. 2021, 33, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat-diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515. [Google Scholar] [CrossRef] [PubMed]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Balfegó, M.; Canivell, S.; Hanzu, F.A.; Sala-Vila, A.; Martínez-Medina, M.; Murillo, S.; Mur, T.; Ruano, E.G.; Linares, F.; Porras, N. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: A pilot randomized trial. Lipids Health Dis. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Andersen, A.D.; Mølbak, L.; Michaelsen, K.F.; Lauritzen, L. Molecular fingerprints of the human fecal microbiota from 9 to 18 months old and the effect of fish oil supplementation. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 303–309. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Haro, C.; Montes-Borrego, M.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Tinahones, F.J.; Landa, B.B. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J. Clin. Endocrinol. 2016, 101, 233–242. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Iskandar, M.M.; Sleno, L.; Sabally, K.; Khairallah, J.; Prakash, S.; Kubow, S. Absorption and metabolism of phenolics from digests of polyphenol-rich potato extracts using the Caco-2/HepG2 co-culture system. Foods 2018, 7, 8. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 9816–9842. [Google Scholar] [CrossRef] [PubMed]
- Staubo, S.C.; Aakre, J.A.; Vemuri, P.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C. Mediterranean diet, micronutrients and macronutrients, and MRI measures of cortical thickness. Alzheimer’s Dement. 2017, 13, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease—A review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Judd, S.; Letter, A.J.; Alexandrov, A.V.; Howard, G.; Nahab, F.; Unverzagt, F.W.; Moy, C.; Howard, V.J.; Kissela, B. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013, 80, 1684–1692. [Google Scholar] [CrossRef]
- Duplantier, S.C.; Gardner, C.D. A critical review of the study of neuroprotective diets to reduce cognitive decline. Nutrients 2021, 13, 2264. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Agarwal, P.; Leurgans, S.E.; Agrawal, S.; Aggarwal, N.T.; Cherian, L.J.; James, B.D.; Dhana, K.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Association of Mediterranean-DASH Intervention for Neurodegenerative Delay and Mediterranean diets with Alzheimer disease pathology. Neurology 2023, 100, e2259–e2268. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Businaro, R.; Vauzour, D. The role of diet in preventing and reducing cognitive decline. Curr. Opin. Psychiatry 2020, 33, 432–438. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Beck, T.; Bennett, D.A.; Schneider, J.A.; Hayden, K.M.; Shadyab, A.H.; Rajan, K.B.; Morris, M.C.; Cornelis, M.C. Adherence to MIND Diet, Genetic Susceptibility, and Incident Dementia in Three US Cohorts. Nutrients 2022, 14, 2759. [Google Scholar] [CrossRef] [PubMed]
- Morrill, S.J.; Gibas, K.J. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes Metab. Syndr. 2019, 13, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Sai, K.S.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: A pilot study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittitta, L.; Tumminia, A. Influence of the mediterranean and ketogenic diets on cognitive status and decline: A narrative review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef]
- Seo, Y.; Gang, G.; Kim, H.K.; Kim, Y.; Kang, S.; Kim, H.; Lee, S.G.; Go, G.-W. Effect of MIND diet on cognitive function in elderly: A narrative review with emphasis on bioactive food ingredients. Food Sci. Biotechnol. 2024, 33, 297–306. [Google Scholar] [CrossRef]
- Tangney, C.C.; Li, H.; Wang, Y.; Barnes, L.; Schneider, J.A.; Bennett, D.A.; Morris, M.C. Relation of DASH-and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014, 83, 1410–1416. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.; Murphy, K.J. A Mediterranean diet with fresh, lean pork improves processing speed and mood: Cognitive findings from the MedPork randomised controlled trial. Nutrients 2019, 11, 1521. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean diet and health: Food effects on gut microbiota and disease control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R.; Matthews, D.C.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 2018, 90, e1789–e1798. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota–immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Reinón, M.E.; Chirlaque, M.D.; Gavrila, D.; Amiano, P.; Mar, J.; Tainta, M.; Ardanaz, E.; Larumbe, R.; Colorado-Yohar, S.M.; Navarro-Mateu, F.; et al. Mediterranean Diet and Risk of Dementia and Alzheimer’s Disease in the EPIC-Spain Dementia Cohort Study. Nutrients 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Devranis, P.; Vassilopoulou, Ε.; Tsironis, V.; Sotiriadis, P.M.; Chourdakis, M.; Aivaliotis, M.; Tsolaki, M. Mediterranean Diet, Ketogenic Diet or MIND Diet for Aging Populations with Cognitive Decline: A Systematic Review. Life 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Pullen, H.; Ritchie, C.W.; Shannon, O.M.; Stevenson, E.J.; Muniz-Terrera, G. Mediterranean diet and structural neuroimaging biomarkers of Alzheimer’s and cerebrovascular disease: A systematic review. Exp. Gerontol. 2023, 172, 112065. [Google Scholar] [CrossRef]
- Lilamand, M.; Porte, B.; Cognat, E.; Hugon, J.; Mouton-Liger, F.; Paquet, C. Are ketogenic diets promising for Alzheimer’s disease? A translational review. Alzheimer’s Res. Ther. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Dilmore, A.H.; Martino, C.; Neth, B.J.; West, K.A.; Zemlin, J.; Rahman, G.; Panitchpakdi, M.; Meehan, M.J.; Weldon, K.C.; Blach, C. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Q.; Yu, L.-L.; Qi, G.-Y.; Mi, Y.-S.; Wu, W.-Q.; Lee, Y.-K.; Zhai, Q.-X.; Tian, F.-W.; Chen, W. Can dietary patterns prevent cognitive impairment and reduce Alzheimer’s disease risk: Exploring the underlying mechanisms of effects. Neurosci. Biobehav. Rev. 2022, 135, 104556. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.-H.; Guschin, A.; Bock, M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, S.; Zhou, Y.; Yu, L.; Zhang, L.; Wang, Y. Altered gut microbiome composition in children with refractory epilepsy after ketogenic diet. Epilepsy Res. 2018, 145, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Arora, K.; Green, M.; Prakash, S. The microbiome and Alzheimer’s disease: Potential and limitations of prebiotic, synbiotic, and probiotic formulations. Front. Bioeng. Biotechnol. 2020, 8, 537847. [Google Scholar] [CrossRef]
- Pluta, R.; Ułamek-Kozioł, M.; Januszewski, S.; Czuczwar, S.J. Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging 2020, 12, 5539. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Krüger, J.F.; Hillesheim, E.; Pereira, A.C.; Camargo, C.Q.; Rabito, E.I. Probiotics for dementia: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2021, 79, 160–170. [Google Scholar] [CrossRef]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment—A meta-analysis of randomized controlled trials. Aging 2020, 12, 4010. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Golovko, S.; Golovko, M.Y.; Singh, S.; Darland, D.C.; Combs, C.K. Effects of probiotic supplementation on short chain fatty acids in the App NL-GF mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 76, 1083–1102. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.; Zhou, C.; Ohno, K.; Kuhara, T.; Taslima, F.; Abdullah, M.; Jung, C.-G.; Michikawa, M. Probiotic Bifidobacterium breve prevents memory impairment through the reduction of both amyloid-β production and microglia activation in APP knock-in mouse. J. Alzheimer’s Dis. 2022, 85, 1555–1571. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Amakye, W.K.; Qi, C.; Liu, X.; Ma, J.; Ren, J. Bifidobacterium Lactis Probio-M8 regulates gut microbiota to alleviate Alzheimer’s disease in the APP/PS1 mouse model. Eur. J. Nutr. 2021, 60, 3757–3769. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: A randomized, double-blind, placebo-controlled trial. J. Alzheimer’s Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).