Biochemical Profiling of Urine Metabolome in Premature Infants Based on LC−MS Considering Maternal Influence
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. LC−MS
3. Results
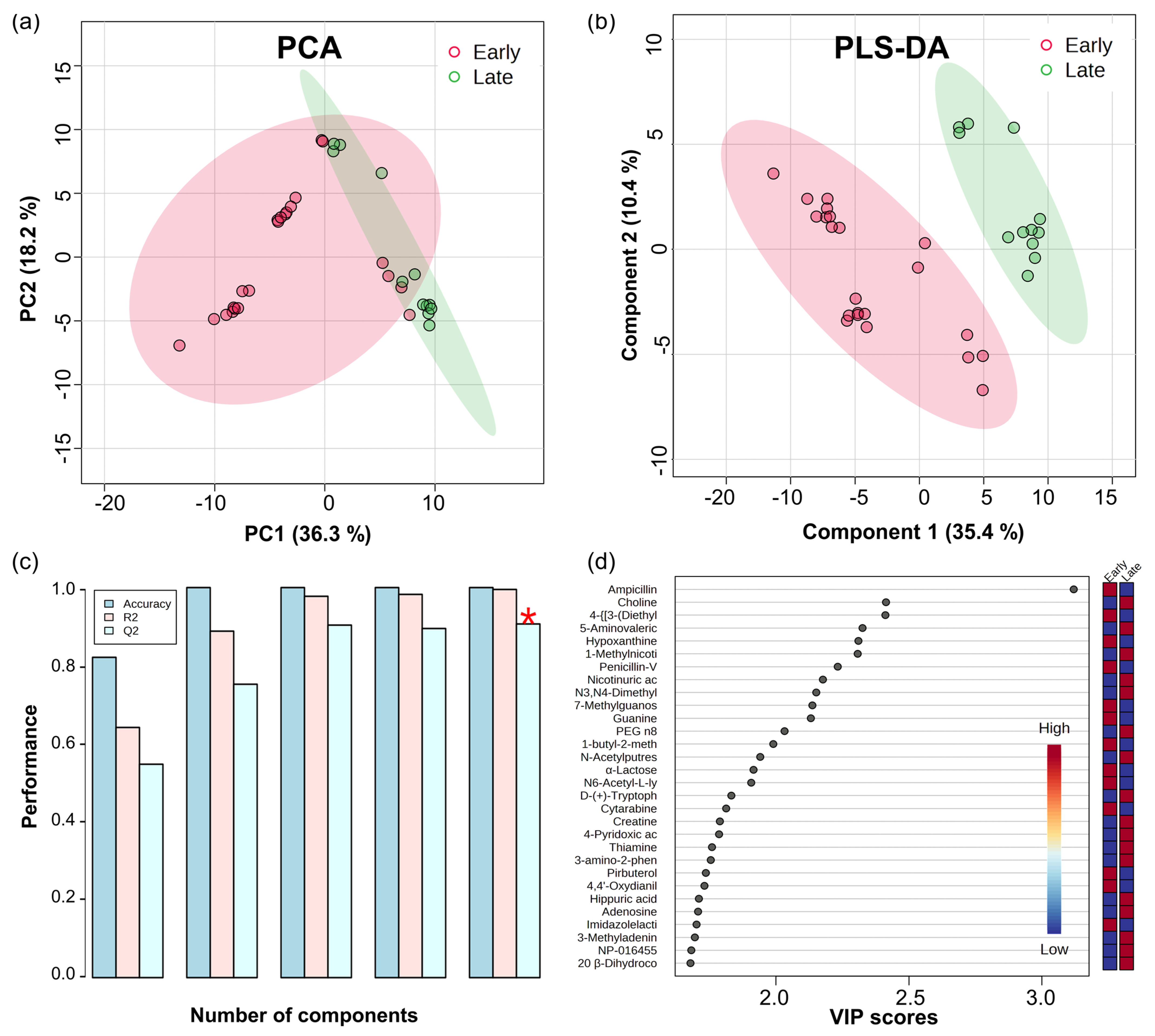
3.1. Multivariate Analysis
3.2. Differential Analysis
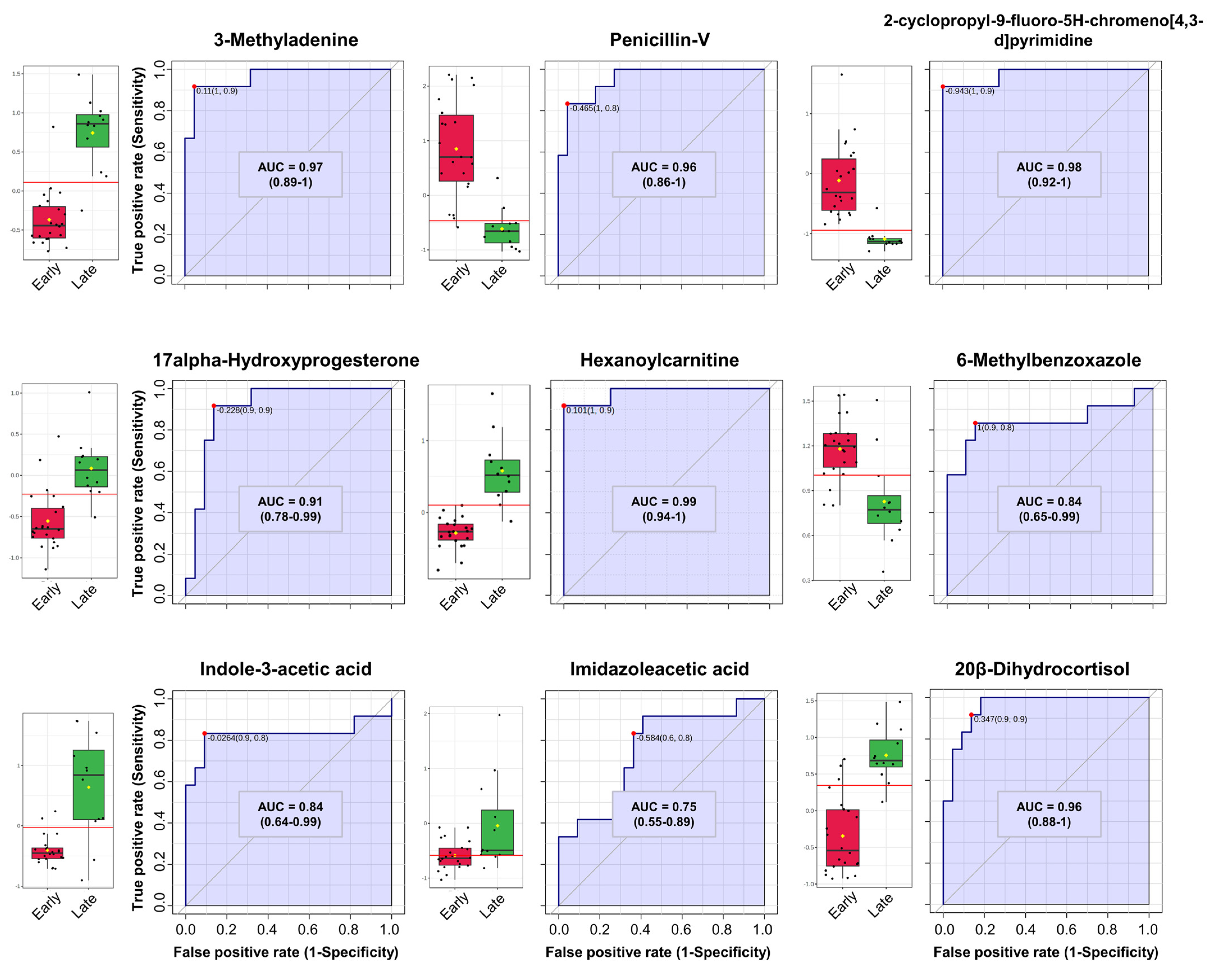
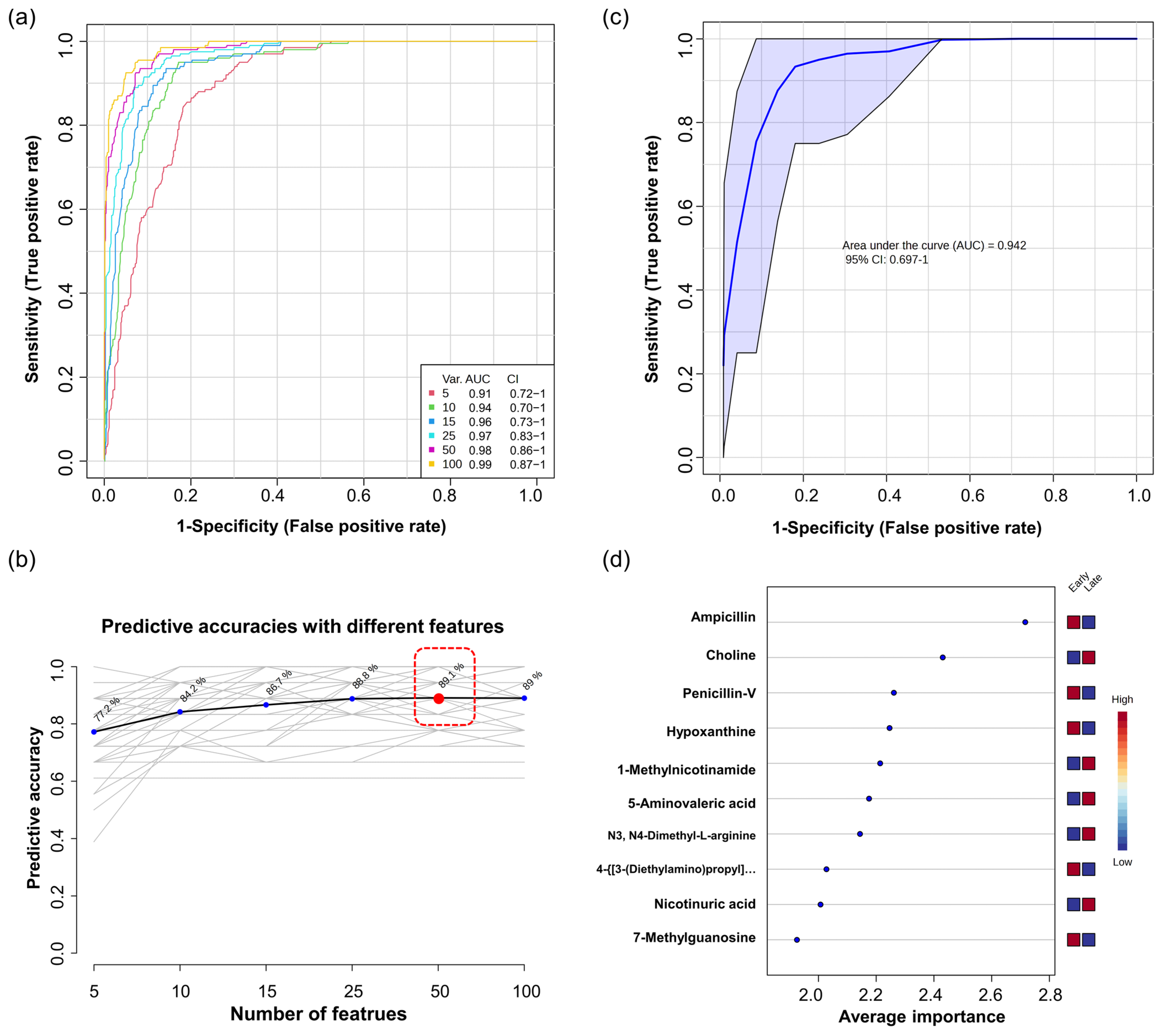
3.3. Univariate and Multivariate ROC Curve Analysis
4. Discussion
4.1. Investigating the Diverse Physiological Mechanisms and Drug Metabolism in Preterm Infant Urinary Metabolites Using Multivariate Analysis
4.2. Identification of Differential Metabolites in Preterm Infants’ Urine over Time via Differential Expression Analysis
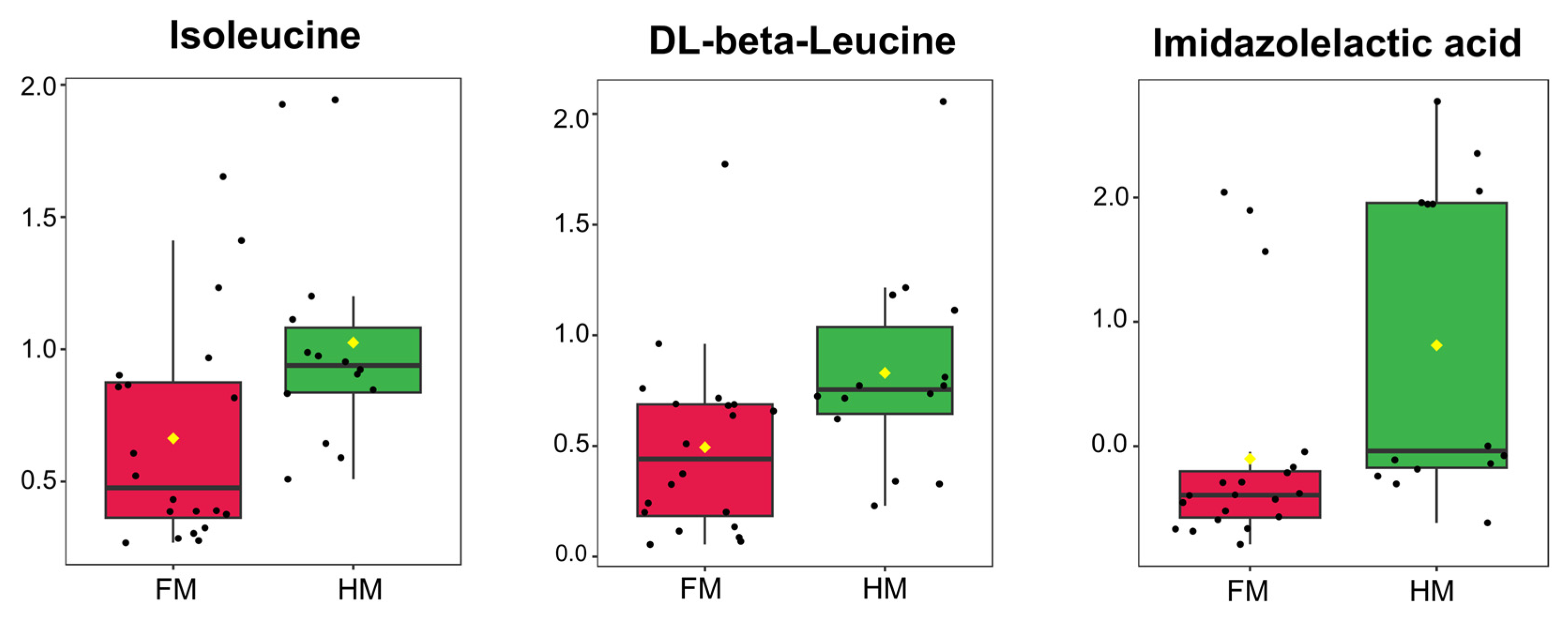
4.3. Efficacy of Human Milk in Premature Infants from a Substance Transfer Perspective and Essential Amino Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abu-Saad, K.; Fraser, D. Maternal Nutrition and Birth Outcomes. Epidemiol. Rev. 2010, 32, 5–25. [Google Scholar] [CrossRef]
- Dewey, K.G. Impact of Breastfeeding on Maternal Nutritional Status. In Protecting Infants through Human Milk; Springer: Boston, MA, USA, 2004; pp. 91–100. [Google Scholar]
- Alamri, S.H.; Abdeen, G.N. Maternal Nutritional Status and Pregnancy Outcomes Post-bariatric Surgery. Obes. Surg. 2022, 32, 1325–1340. [Google Scholar] [CrossRef]
- Khalili Doroodzani, A.; Dobaradaran, S.; Zarei, S.; Raeisi, A.; Mahmoodi, M.; Rahmani, E.; Nabipour, I.; Saeedi, R.; Mahmudpour, M.; Akbarzadeh, S.; et al. Maternal and fetal exposure to metal (loid)s, maternal nutrition status, and impact on prenatal growth in an energy rich zone and an urban area along the Persian Gulf. Environ. Pollut. 2022, 309, 119779. [Google Scholar] [CrossRef]
- Zaidi, A.Z.; Moore, S.E.; Okala, S.G. Impact of Maternal Nutritional Supplementation during Pregnancy and Lactation on the Infant Gut or Breastmilk Microbiota: A Systematic Review. Nutrients 2021, 13, 1137. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology; Abrams, S.A.; Fuchs, G.J., III; Kim, J.H.; Lindsey, C.W. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Datta, S.; Johnson, G.A.; Li, P.; Satterfield, M.C.; Spencer, T.E. Proline metabolism in the conceptus: Implications for fetal growth and development. Amino Acids 2008, 35, 691–702. [Google Scholar] [CrossRef]
- Haggarty, P. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 2010, 30, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Moltu, S.J.; Sachse, D.; Blakstad, E.W.; Strømmen, K.; Nakstad, B.; Almaas, A.N.; Westerberg, A.C.; Rønnestad, A.; Brække, K.; Veierød, M.B.; et al. Urinary Metabolite Profiles in Premature Infants Show Early Postnatal Metabolic Adaptation and Maturation. Nutrients 2014, 6, 1913–1930. [Google Scholar] [CrossRef]
- Emwas, A.H. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar] [CrossRef]
- West, C.R.; Harding, J.E.; Williams, C.E.; Gunning, M.I.; Battin, M.R. Quantitative electroencephalographic patterns in normal preterm infants over the first week after birth. Early Hum. Dev. 2006, 82, 43–51. [Google Scholar] [CrossRef]
- Knobel-Dail, R.B.; Tanaka, D.T.; Holditch-Davis, D.; White, J. Perfusion Index in Very Low Birth Weight Premature Infants During Their First 2 Weeks of Life. Biol. Res. Nurs. 2016, 19, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, C.; Alshaikh, B.; Harabor, A. Prolonged use of antibiotics after birth is associated with increased morbidity in preterm infants with negative cultures. J. Matern. Fetal Neonatal Med. 2019, 32, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Rigo, J. The nutrition of preterm infants. Early Hum. Dev. 2012, 88, S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Robertson, D.; Griffin, J.; van der Werf, M.; Nikolau, B.; Morrison, N.; Sumner, L.W.; Goodacre, R.; Hardy, N.W.; Taylor, C.; et al. The metabolomics standards initiative (MSI). Metabolomics 2007, 3, 175–178. [Google Scholar] [CrossRef]
- Smith, L.J.; McKay, K.O.; van Asperen, P.P.; Selvadurai, H.; Fitzgerald, D.A. Normal Development of the Lung and Premature Birth. Paediatr. Respir. Rev. 2010, 11, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, J.; Vakili, K. Neonatal liver physiology. Semin. Pediatr. Surg. 2013, 22, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.S.; Ferreira, T.S.; Lima, R.C.G.; Vieira, V.C.; Medeiros, D.S. Premature birth: Topics in physiology and pharmacological characteristics. Rev. Assoc. Med. Bras. 2021, 67, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Burns, J.L.; Cummings, P. Ampicillin Use in Infant Fever: A Systematic Review. Arch. Pediatr. Adolesc. Med. 2002, 156, 27–32. [Google Scholar] [CrossRef]
- Yoshioka, H.; Takimoto, M.; Riley, H.D., Jr. Pharmacokinetics of Ampicillin in the Newborn Infant. J. Infect. Dis. 1974, 129, 461–464. [Google Scholar] [CrossRef]
- Ginsburg, C.M.; McCracken, G.H., Jr.; Thomas, M.L.; Clahsen, J. Comparative Pharmacokinetics of Amoxicillin and Ampicillin in Infants and Children. Pediatrics 1979, 64, 627–631. [Google Scholar] [CrossRef]
- Axline, S.G.; Yaffe, S.J.; Simon, H.J. Clinical Pharmacology of Antimicrobials in Premature Infants: II. Ampicillin, Methicillin, Oxacillin, Neomycin, and Colistin. Pediatrics 1967, 39, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Greenberg, R.G.; Yoo, Y.; Clark, R.H.; Benjamin Jr, D.K.; Zimmerman, K.O.; Cohen-Wolkowiez, M.; Wade, K.C.; Best Pharmaceuticals for Children Act—Pediatric Trials Network Steering Committee. Ampicillin dosing in premature infants for early-onset sepsis: Exposure-driven efficacy, safety, and stewardship. J. Perinatol. 2022, 42, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.I.; Germovsek, E.; Sharland, M. What do I need to know about penicillin antibiotics? Arch. Dis. Child. Educ. Pract. Ed. 2017, 102, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kuppala, V.S.; Meinzen-Derr, J.; Morrow, A.L.; Schibler, K.R. Prolonged Initial Empirical Antibiotic Treatment is Associated with Adverse Outcomes in Premature Infants. J. Pediatr. 2011, 159, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Dashe, J.S.; Gilstrap, L.C. Antibiotic Use in Pregnancy. Obstet. Gynecol. Clin. N. Am. 1997, 24, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.; Bacci, C.; Volpato, A.; Bandiera, M.; Favero, L.; Zanette, G. Pregnancy and dentistry: A literature review on risk management during dental surgical procedures. Dent. J. 2021, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A. Liver-immaturity and treatment of kernicterus of the premature baby. Indian. J. Pediatr. 1958, 25, 184–200. [Google Scholar] [CrossRef]
- McEwen, B.S. Steroid Hormones: Effect on Brain Development and Function. Horm. Res. 2008, 37, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.F.; Pitkin, J.; Ho, C.S.; Baglin, J.; Hunt, R.W.; Zacharin, M.R. Hormone Modeling in Preterm Neonates: Establishment of Pituitary and Steroid Hormone Reference Intervals. J. Clin. Endocrinol. Metab. 2015, 100, 1097–1103. [Google Scholar] [CrossRef]
- Mitchell, F.L.; Shackleton, C.H.L. The Investigation of Steroid Metabolism in Early Infancy. In Advances in Clinical Chemistry; Bodansky, O., Stewart, C.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1969; Volume 12, pp. 141–215. [Google Scholar]
- Tomlinson, J.W.; Stewart, P.M. Cortisol metabolism and the role of 11β-hydroxysteroid dehydrogenase. Best. Pract. Res. Clin. Endocrinol. Metab. 2001, 15, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Honour, J.W. 17-Hydroxyprogesterone in children, adolescents and adults. Ann. Clin. Biochem. 2014, 51, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.M.; Ogundiya, A.; Didi, M.; Turner, M.A. Adrenal function of extremely premature infants in the first 5 days after birth. J. Pediatr. Endocrinol. Metab. 2019, 32, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Vegesna, S.R.; Welch, M.; Lieberman, S. Precursors of the neurosteroids. Proc. Natl. Acad. Sci. USA 1994, 91, 3220–3223. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yao, J.; Chen, S.; Mao, Z.; Brinton, R.D. Allopregnanolone Reverses Bioenergetic Deficits in Female Triple Transgenic Alzheimer’s Mouse Model. Neurotherapeutics 2020, 17, 178–188. [Google Scholar] [CrossRef]
- Kuhl, H. Pharmacology of estrogens and progestogens: Influence of different routes of administration. Climacteric 2005, 8, 3–63. [Google Scholar] [CrossRef]
- Bernhard, W.; Poets, C.F.; Franz, A.R. Choline and choline-related nutrients in regular and preterm infant growth. Eur. J. Nutr. 2019, 58, 931–945. [Google Scholar] [CrossRef]
- Bernhard, W.; Full, A.; Arand, J.; Maas, C.; Poets, C.F.; Franz, A.R. Choline supply of preterm infants: Assessment of dietary intake and pathophysiological considerations. Eur. J. Nutr. 2013, 52, 1269–1278. [Google Scholar] [CrossRef]
- McMahon, K.E.; Farrell, P.M. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin. Chim. Acta 1985, 149, 1–12. [Google Scholar] [CrossRef]
- Eder, L.; Hirt, L.; Dunant, Y. Possible involvement of thiamine in acetylcholine release. Nature 1976, 264, 186–188. [Google Scholar] [CrossRef]
- Speeg, K., Jr.; Chen, D.; McCandless, D.; Schenker, S. Cerebral acetylcholine in thiamine deficiency. Proc. Soc. Exp. Biol. Med. 1970, 134, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Cuisinier, C.; Ward, R.J.; Francaux, M.; Sturbois, X.; de Witte, P. Changes in plasma and urinary taurine and amino acids in runners immediately and 24 h after a marathon. Amino Acids 2001, 20, 13–23. [Google Scholar] [CrossRef]
- Silva, A.R.; Silva, C.G.; Ruschel, C.; Helegda, C.; Wyse, A.T.; Wannmacher, C.M.; Wajner, M.; Dutra-Filho, C.S. L-pyroglutamic acid inhibits energy production and lipid synthesis in cerebral cortex of young rats in vitro. Neurochem. Res. 2001, 26, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Kamatani, N.; Jinnah, H.A.; Hennekam, R.C.M.; van Kuilenburg, A.B.P. 6—Purine and Pyrimidine Metabolism. In Emery and Rimoin’s Principles and Practice of Medical Genetics and Genomics, 7th ed.; Pyeritz, R.E., Korf, B.R., Grody, W.W., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 183–234. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Vincent, M.F. Disorders of Purine and Pyrimidine Metabolism. In Inborn Metabolic Diseases: Diagnosis and Treatment; Fernandes, J., Saudubray, J.-M., Van den Berghe, G., Tada, K., Buist, N.R.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 289–302. [Google Scholar] [CrossRef]
- Perrone, S.; Laschi, E.; De Bernardo, G.; Giordano, M.; Vanacore, F.; Tassini, M.; Calderisi, M.; Toni, A.L.; Buonocore, G.; Longini, M. Newborn metabolomic profile mirrors that of mother in pregnancy. Med. Hypotheses 2020, 137, 109543. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.S.; Hopper, A.; Slater, L.; Asmerom, Y.; Esiaba, I.; Boskovic, D.S.; Angeles, D.M. Urinary Hypoxanthine as a Measure of Increased ATP Utilization in Late Preterm Infants. ICAN Infant Child Adolesc. Nutr. 2014, 6, 240–249. [Google Scholar] [CrossRef]
- Wingen, A.M.; Löffler, W.; Waldherr, R.; Schärer, K. Acute renal failure in an infant with partial deficiency of hypoxanthine-guanine phosphoribosyltransferase. Proc. Eur. Dial. Transplant. Assoc. Eur. Ren. Assoc. 1985, 21, 751–755. [Google Scholar]
- Holland, P.C.; Dillon, M.J.; Pincott, J.; Simmonds, H.A.; Barratt, T.M. Hypoxanthine guanine phosphoribosyl transferase deficiency presenting with gout and renal failure in infancy. Arch. Dis. Child. 1983, 58, 831–833. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, J.; Yang, M.; Len, J.; Yu, Y. Comprehensive analysis of 7-methylguanosine and immune microenvironment characteristics in clear cell renal cell carcinomas. Front. Genet. 2022, 13, 866819. [Google Scholar] [CrossRef]
- Raćkowska, E.; Bobrowska-Korczak, B.; Giebułtowicz, J. Development and validation of a rapid LC–MS/MS method for determination of methylated nucleosides and nucleobases in urine. J. Chromatogr. B 2019, 1128, 121775. [Google Scholar] [CrossRef]
- Embleton, N.D. Optimal protein and energy intakes in preterm infants. Early Human. Dev. 2007, 83, 831–837. [Google Scholar] [CrossRef]
- Dolezal, V.; Tucek, S. Utilization of citrate, acetylcarnitine, acetate, pyruvate and glucose for the synthesis of acetylcholine in rat brain slices. J. Neurochem. 1981, 36, 1323–1330. [Google Scholar] [CrossRef]
- Van Goudoever, J.B.; Sulkers, E.J.; Timmerman, M.; Huijmans, J.G.M.; Langer, K.; Carnielli, V.P.; Sauer, P.J.J. Amino Acid Solutions for Premature Neonates During the First Week of Life: The Role of N-Acetyl-L-Cysteine and N-Acetyl-L-Tyrosine. J. Parenter. Enter. Nutr. 1994, 18, 404–408. [Google Scholar] [CrossRef]
- du Toit, W.L.; Kruger, R.; Gafane-Matemane, L.F.; Schutte, A.E.; Louw, R.; Mels, C.M. Urinary metabolomics profiling by cardiovascular risk factors in young adults: The African Prospective study on Early Detection and Identification of Cardiovascular disease and Hypertension study. J. Hypertens. 2022, 40, 1545–1555. [Google Scholar] [CrossRef]
- Johansen, M.L.; Bak, L.K.; Schousboe, A.; Iversen, P.; Sørensen, M.; Keiding, S.; Vilstrup, H.; Gjedde, A.; Ott, P.; Waagepetersen, H.S. The metabolic role of isoleucine in detoxification of ammonia in cultured mouse neurons and astrocytes. Neurochem. Int. 2007, 50, 1042–1051. [Google Scholar] [CrossRef]
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine plays an important role for maintaining immune function. Curr. Protein Pept. Sci. 2019, 20, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Cain, A.; Holton, J. Histidinaemia: A child and his family. Arch. Dis. Child. 1968, 43, 62. [Google Scholar] [CrossRef] [PubMed]
- Kappelman, M.; Thomas, G.H.; Howell, R.R. Histidinemia in a Negro child. Am. J. Dis. Child. 1971, 122, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Pencharz, P.B.; Ball, R.O. Amino acid requirements of infants and children. Protein Energy Requir. Infancy Child. 2006, 58, 109–119. [Google Scholar]
- Heird, W.C. Amino acids in pediatric and neonatal nutrition. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.D.; Tonannavar Yenagi, J.; Kamat, V.; Tonannavar, J. XRD structure and vibrational analysis of DL-β-Leucine, as aided by DFT tetramer model and characterized by NBO, AIM and NCI calculations. J. Mol. Struct. 2020, 1218, 128495. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mok, J.-H.; Song, J.; Hahn, W.-H.; Cho, S.; Park, J.-M.; Koh, J.; Kim, H.; Kang, N.M. Biochemical Profiling of Urine Metabolome in Premature Infants Based on LC−MS Considering Maternal Influence. Nutrients 2024, 16, 411. https://doi.org/10.3390/nu16030411
Mok J-H, Song J, Hahn W-H, Cho S, Park J-M, Koh J, Kim H, Kang NM. Biochemical Profiling of Urine Metabolome in Premature Infants Based on LC−MS Considering Maternal Influence. Nutrients. 2024; 16(3):411. https://doi.org/10.3390/nu16030411
Chicago/Turabian StyleMok, Jeong-Hun, Junhwan Song, Won-Ho Hahn, Seonghyeon Cho, Jong-Moon Park, Jiwon Koh, Ho Kim, and Nam Mi Kang. 2024. "Biochemical Profiling of Urine Metabolome in Premature Infants Based on LC−MS Considering Maternal Influence" Nutrients 16, no. 3: 411. https://doi.org/10.3390/nu16030411
APA StyleMok, J.-H., Song, J., Hahn, W.-H., Cho, S., Park, J.-M., Koh, J., Kim, H., & Kang, N. M. (2024). Biochemical Profiling of Urine Metabolome in Premature Infants Based on LC−MS Considering Maternal Influence. Nutrients, 16(3), 411. https://doi.org/10.3390/nu16030411






