Exerkines, Nutrition, and Systemic Metabolism
Abstract
1. Introduction
2. Overview of Exerkines Associated with Macronutrient Metabolism
2.1. Classification of Exerkines Based on Their Source and Actions
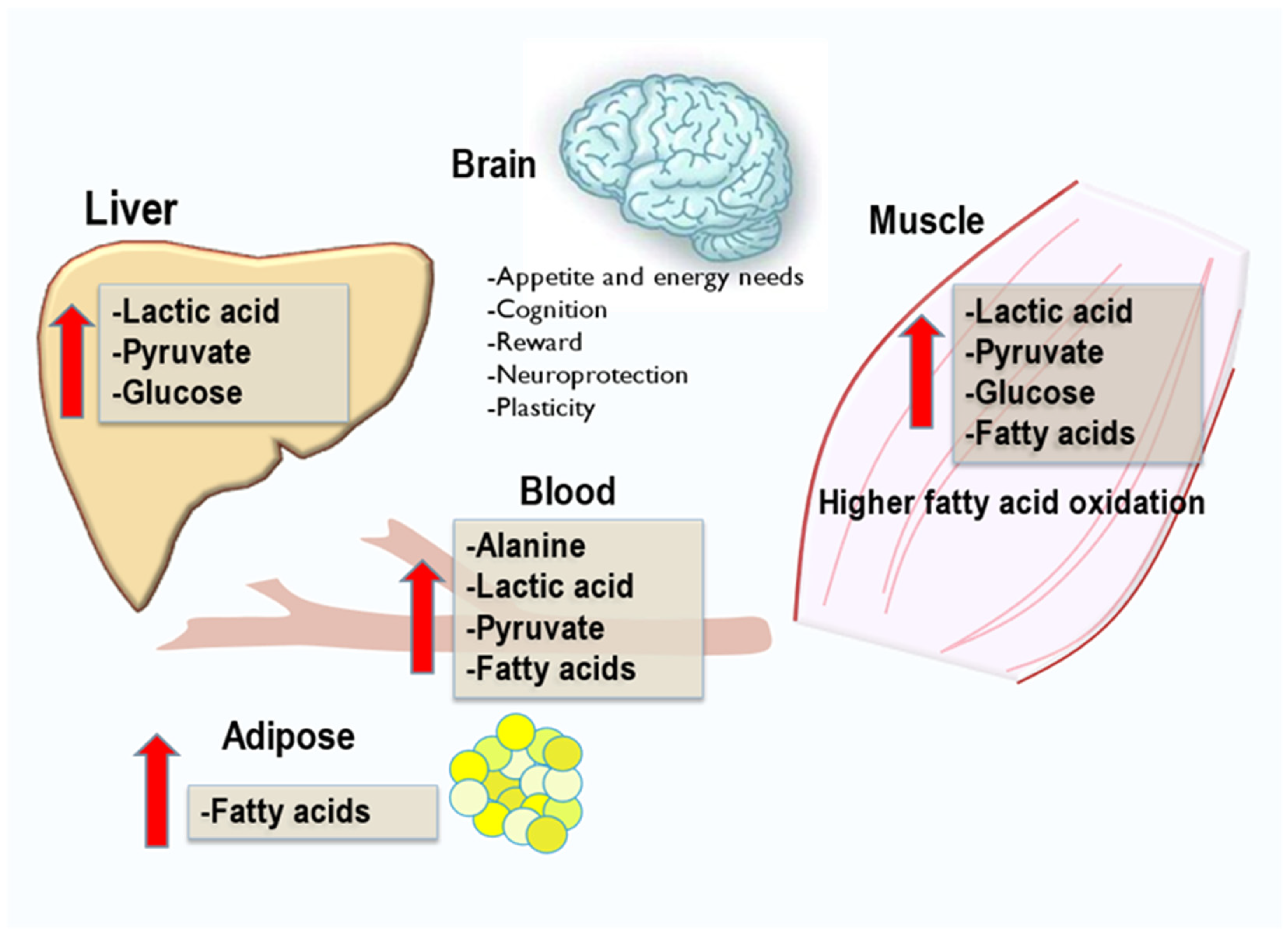
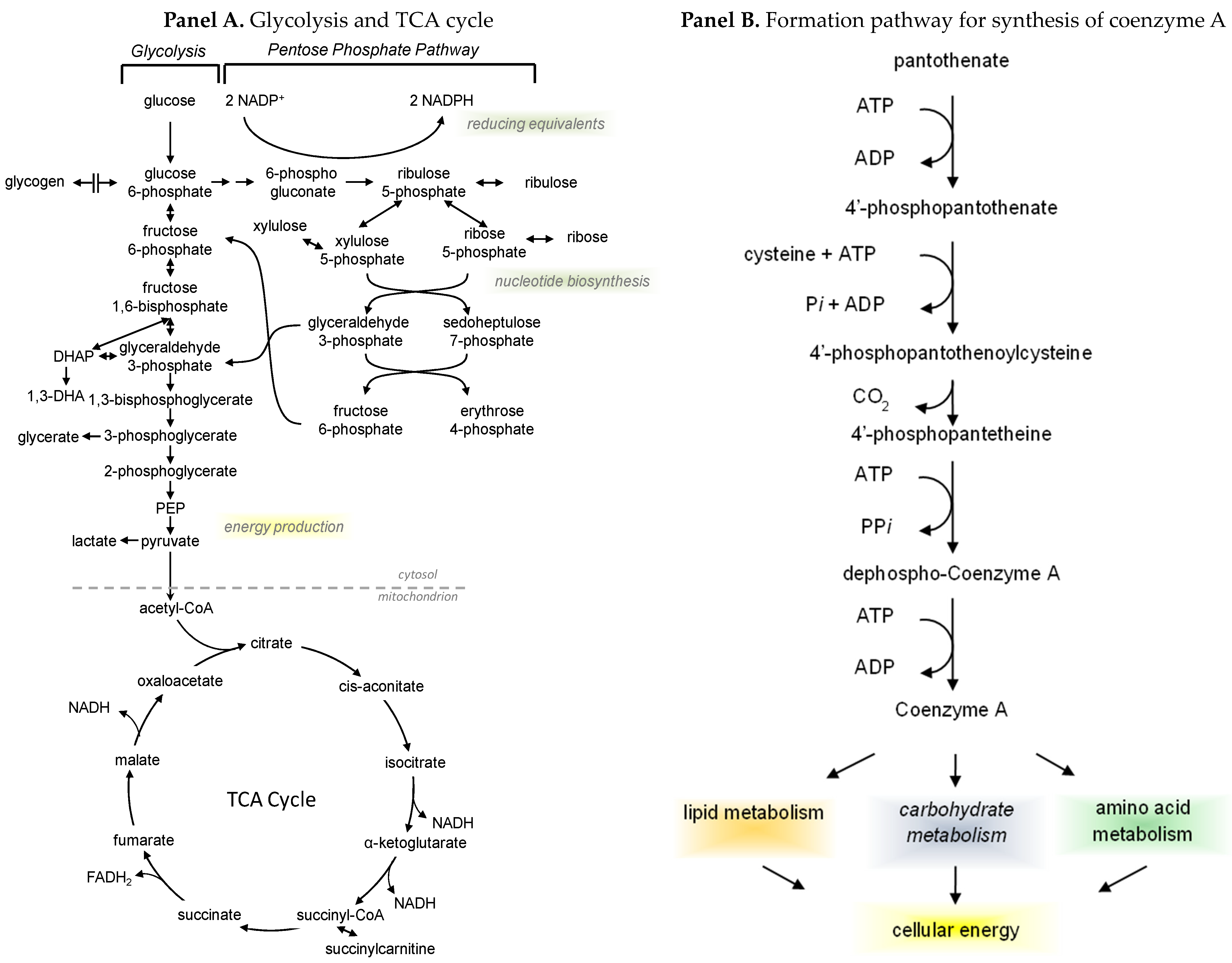
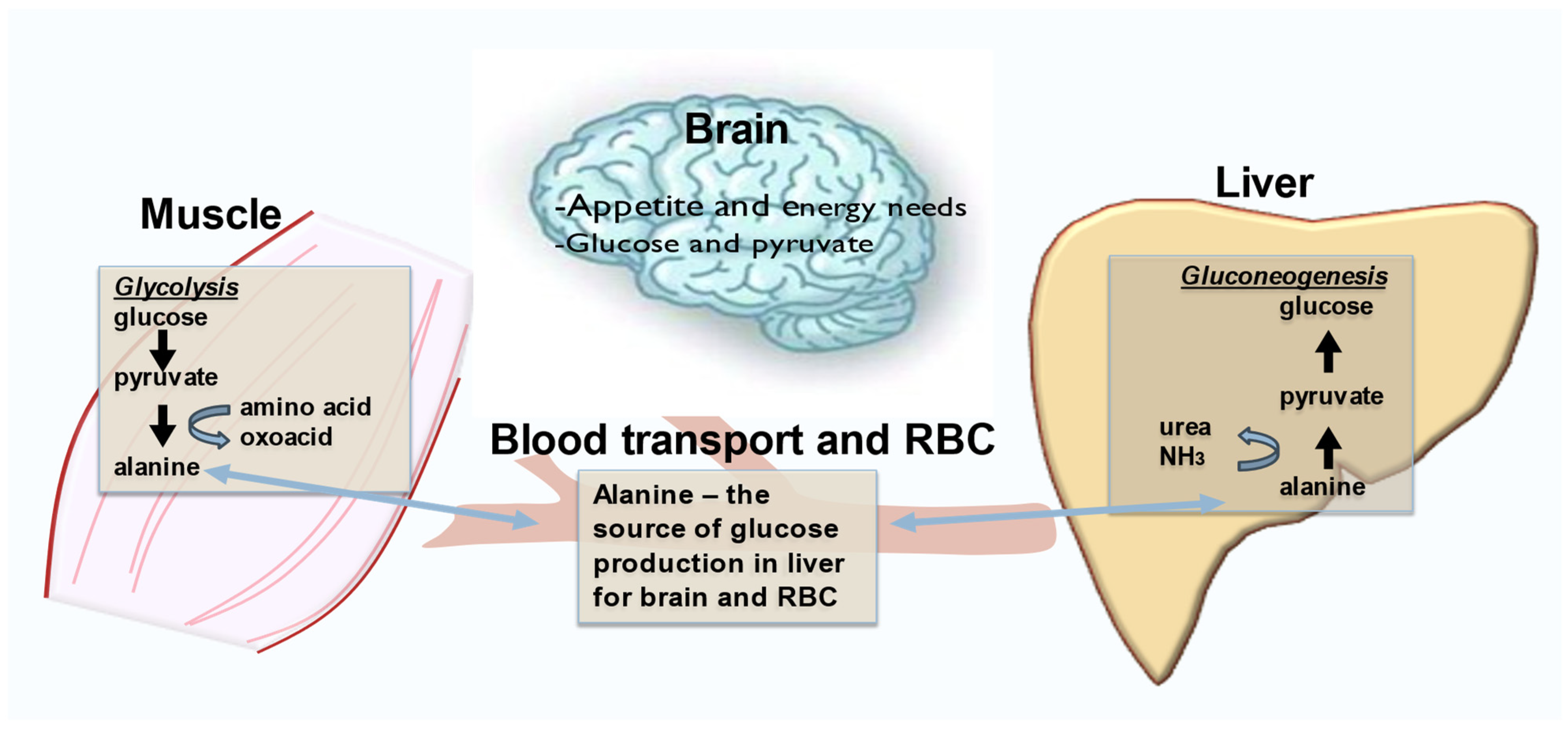
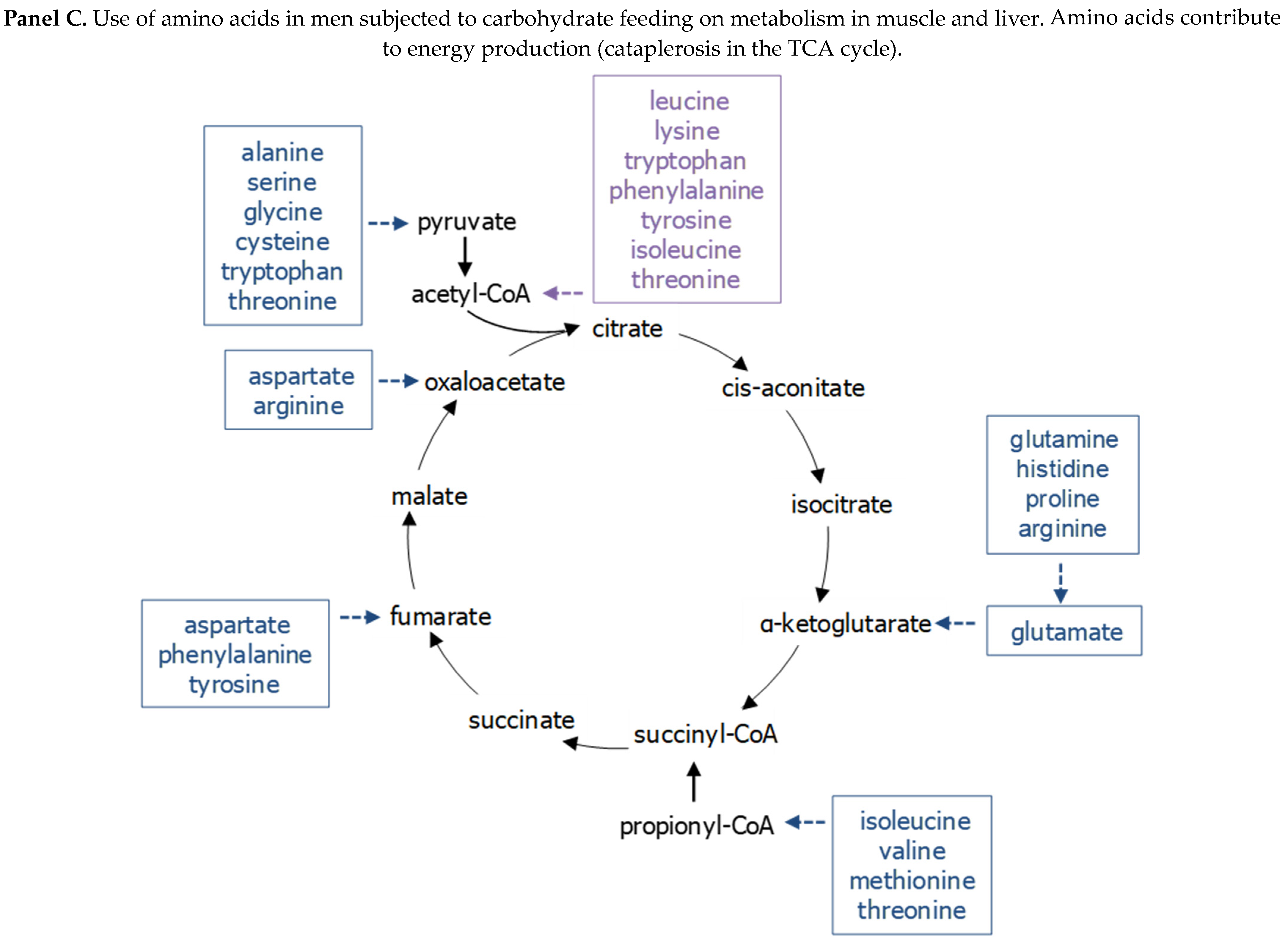
2.2. Exerkines of Metabolism and Energy Production
2.3. Hormones, Autocrine and Paracrine Exerkines, and Exercise Effects on Metabolism
3. Endocannabinoids, Oxylipins, and Polyunsaturated Fatty Acids in Exercise
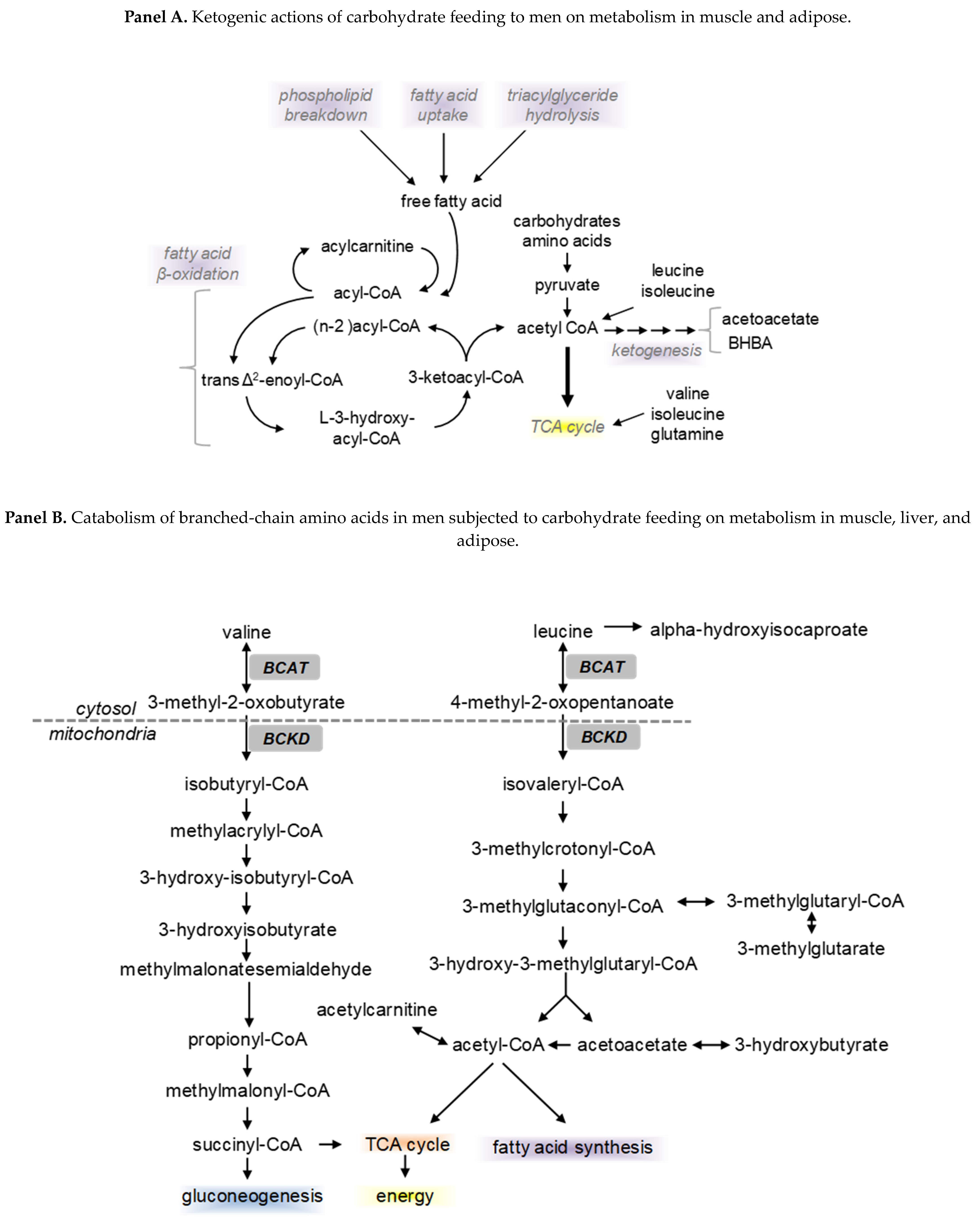
4. Diet, Nutrients, and Exerkine Effects on Metabolism
5. Exercise, Neurobiology, and Neuroinflammation
6. Exercise and the Brain
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AEA | Anandamide |
| 1-AG | 1-arachidonoylglycerol |
| 2-AG | 2-arachidonoylglycerol |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| DHA | Docosahexaenoic acid |
| DHEA | Docosahexaenoyl ethanolamide |
| EPA | Eicosapentaenoic acid |
| EPEA | Eicosapentaenoyl ethanolamide |
| eCB | Endocannabinoid |
| ECS | Endocannabinoid system |
| LEA | N-linoleylethanolamine |
| OxL | Oxylipin |
| OEA | Oleoylethanolamide |
| PUFA | Polyunsaturated fatty acid |
| (TGFβ2) | Transforming growth factor β2 |
References
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Castillo-Armengol, J.; Fajas, L.; Lopez-Mejia, I.C. Inter-organ communication: A gatekeeper for metabolic health. EMBO Rep. 2019, 20, e47903. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- So, K.-F.; Li, A.; Liang, Y.-Y.; Zhang, L.-D.; Luo, X.; Wu, L.-L.; Chen, Z.-W.; Wei, G.-H.; Zhang, K.-Q.; Du, Z.-A.; et al. All roads lead to Rome—A review of the potential mechanisms by which exerkines exhibit neuroprotective effects in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1210–1227. [Google Scholar] [CrossRef]
- Park, Y.; Watkins, B.A. Dietary PUFAs and Exercise Dynamic Actions on Endocannabinoids in Brain: Consequences for Neural Plasticity and Neuroinflammation. Adv. Nutr. 2022, 13, 1989–2001. [Google Scholar] [CrossRef]
- Li, A.; Yau, S.Y.; Machado, S.; Wang, P.; Yuan, T.F.; So, K.F. Enhancement of Hippocampal Plasticity by Physical Exercise as a Polypill for Stress and Depression: A Review. CNS Neurol. Disord. Drug Targets 2019, 18, 294–306. [Google Scholar] [CrossRef]
- Kornberg, M.D. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1486. [Google Scholar] [CrossRef]
- Choi, I.; Son, H.; Baek, J.H. Tricarboxylic Acid (TCA) Cycle Intermediates: Regulators of Immune Responses. Life 2021, 11, 69. [Google Scholar] [CrossRef]
- Gonzalez, S.V.; Nguyen, N.H.; Rise, F.; Hassel, B. Brain metabolism of exogenous pyruvate. J. Neurochem. 2005, 95, 284–293. [Google Scholar] [CrossRef]
- LeBlanc, P.J.; Peters, S.J.; Tunstall, R.J.; Cameron-Smith, D.; Heigenhauser, G.J. Effects of aerobic training on pyruvate dehydrogenase and pyruvate dehydrogenase kinase in human skeletal muscle. J. Physiol. 2004, 557 Pt 2, 559–570. [Google Scholar] [CrossRef]
- Heo, J.; Noble, E.E.; Call, J.A. The role of exerkines on brain mitochondria: A mini-review. J. Appl. Physiol. 2023, 134, 28–35. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Gallmeier, E.; Behrens, L.; Leal, V.V.; Misgeld, T.; Klinkert, W.E.; Kolbeck, R.; Hoppe, E.; Oropeza-Wekerle, R.-L.; Bartke, I.; et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: A neuroprotective role of inflammation? J. Exp. Med. 1999, 189, 865–870. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Watkins, B.A.; Kahathuduwa, C.; Chyu, M.-C.; Zabet-Moghaddam, M.; Elmassry, M.M.; Luk, H.-Y.; Brismée, J.-M.; Knox, A.; Lee, J.; et al. Tai Chi Improves Brain Functional Connectivity and Plasma Lysophosphatidylcholines in Postmenopausal Women With Knee Osteoarthritis: An Exploratory Pilot Study. Front. Med. 2021, 8, 775344. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Newman, J.W.; Elmassry, M.M.; Borkowski, K.; Chyu, M.-C.; Kahathuduwa, C.; Neugebauer, V.; Watkins, B.A. Tai Chi exercise reduces circulating levels of inflammatory oxylipins in postmenopausal women with knee osteoarthritis: Results from a pilot study. Front. Med. 2023, 10, 1210170. [Google Scholar] [CrossRef]
- Watkins, B.A.; Newman, J.W.; Kuchel, G.A.; Fiehn, O.; Kim, J. Dietary Docosahexaenoic Acid and Glucose Systemic Metabolic Changes in the Mouse. Nutrients 2023, 15, 2679. [Google Scholar] [CrossRef]
- Park, J. Network of biomarkers and their mediation effects on the associations between regular exercise and the incidence of cardiovascular & metabolic diseases. Sci. Rep. 2021, 11, 2679. [Google Scholar]
- Lee, E.C.; Fragala, M.S.; Kavouras, S.A.; Queen, R.M.; Pryor, J.L.; Casa, D.J. Biomarkers in Sports and Exercise: Tracking Health, Performance, and Recovery in Athletes. J. Strength. Cond. Res. 2017, 31, 2920–2937. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E.; et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef]
- Moreno-Sanchez, R.; Saavedra, E.; Rodriguez-Enriquez, S.; Olin-Sandoval, V. Metabolic control analysis: A tool for designing strategies to manipulate metabolic pathways. J. Biomed. Biotechnol. 2008, 2008, 597913. [Google Scholar] [CrossRef]
- Ranallo, R.F.; Rhodes, E.C. Lipid metabolism during exercise. Sports Med. 1998, 26, 29–42. [Google Scholar] [CrossRef]
- Muscella, A.; Stefano, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism During Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a myokine and exerkine: Drivers and signals of physiology and metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Siebenmann, C.; Sørensen, H.; Bonne, T.C.; Zaar, M.; Aachmann-Andersen, N.J.; Nordsborg, N.B.; Nielsen, H.B.; Secher, N.H.; Lundby, C.; Rasmussen, P. Cerebral lactate uptake during exercise is driven by the increased arterial lactate concentration. J. Appl. Physiol. 2021, 131, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Glowinski, J.; Premont, J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J. Neurosci. 1997, 17, 9060–9067. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Grist, J.T.; Serres, S.; Larkin, J.R.; Lau, A.Z.; Ray, K.; Fisher, K.R.; Hansen, E.; Tougaard, R.S.; Nielsen, P.M.; et al. (13)C Pyruvate Transport Across the Blood-Brain Barrier in Preclinical Hyperpolarised MRI. Sci. Rep. 2018, 8, 15082. [Google Scholar] [CrossRef]
- Robbins, J.M.; Gerszten, R.E. Exercise, exerkines, and cardiometabolic health: From individual players to a team sport. J. Clin. Investig. 2023, 133, e168121. [Google Scholar] [CrossRef]
- Watkins, B.A. Endocannabinoids, exercise, pain, and a path to health with aging. Mol. Asp. Med. 2018, 64, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.; Biedermann, S.V.; Bindila, L.; Lutz, B.; Fuss, J. Exercise-induced euphoria and anxiolysis do not depend on endogenous opioids in humans. Psychoneuroendocrinology 2021, 126, 105173. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Amatriain-Fernandez, S.; Murillo-Rodriguez, E.S.; Gronwald, T.; Machado, S.; Budde, H. Benefits of physical activity and physical exercise in the time of pandemic. Psychol. Trauma 2020, 12 (Suppl. S1), S264–S266. [Google Scholar] [CrossRef]
- Moosavi Sohroforouzani, A.; Shakerian, S.; Ghanbarzadeh, M.; Alaei, H. Treadmill exercise improves LPS-induced memory impairments via endocannabinoid receptors and cyclooxygenase enzymes. Behav. Brain Res. 2020, 380, 112440. [Google Scholar] [CrossRef]
- Schonke, M.; Martinez-Tellez, B.; Rensen, P.C. Role of the endocannabinoid system in the regulation of the skeletal muscle response to exercise. Curr. Opin. Pharmacol. 2020, 52, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Arrabal, S.; Lucena, M.A.; Canduela, M.J.; Ramos-Uriarte, A.; Rivera, P.; Serrano, A.; Pavón, F.J.; Decara, J.; Vargas, A.; Baixeras, E.; et al. Pharmacological Blockade of Cannabinoid CB1 Receptors in Diet-Induced Obesity Regulates Mitochondrial Dihydrolipoamide Dehydrogenase in Muscle. PLoS ONE 2015, 10, e0145244. [Google Scholar] [CrossRef] [PubMed]
- Signini, E.F.; Nieman, D.C.; Silva, C.D.; Sakaguchi, C.A.; Catai, A.M. Oxylipin Response to Acute and Chronic Exercise: A Systematic Review. Metabolites 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Watkins, B.A. Cannabinoid receptor antagonists and fatty acids alter endocannabinoid system gene expression and COX activity. J. Nutr. Biochem. 2014, 25, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Aguilera Vasquez, N.; Nielsen, D.E. The Endocannabinoid System and Eating Behaviours: A Review of the Current State of the Evidence. Curr. Nutr. Rep. 2022, 11, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ruiz, I.; Medina, M.A.; Martinez-Poveda, B. From Food to Genes: Transcriptional Regulation of Metabolism by Lipids and Carbohydrates. Nutrients 2021, 13, 1513. [Google Scholar] [CrossRef]
- Burri, L.; Thoresen, G.H.; Berge, R.K. The Role of PPARalpha Activation in Liver and Muscle. PPAR Res. 2010, 2010, 542359. [Google Scholar] [CrossRef]
- Ferrero, G.; Carpi, S.; Polini, B.; Pardini, B.; Nieri, P.; Impeduglia, A.; Grioni, S.; Tarallo, S.; Naccarati, A. Intake of Natural Compounds and Circulating microRNA Expression Levels: Their Relationship Investigated in Healthy Subjects with Different Dietary Habits. Front. Pharmacol. 2020, 11, 619200. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Saghafi-Asl, M.; Ostadrahimi, A. A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-alpha, on the management and prevention of obesity. Clin. Exp. Pharmacol. Physiol. 2020, 47, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Horia, E.; Watkins, B.A. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis 2007, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A. Comparison of endocannabinoid actions on metabolomic analysis of mouse and human myoblast cultures. FASEB J. 2014, 28 (Suppl. S1), 1036.1. [Google Scholar] [CrossRef]
- Kim, J.; Carlson, M.E.; Watkins, B.A. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Front. Physiol. 2014, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Carlson, M.E.; Kuchel, G.A.; Newman, J.W.; Watkins, B.A. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6J mice. Int. J. Obes. 2016, 40, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Kim, J.; Kenny, A.; Pedersen, T.L.; Pappan, K.L.; Newman, J.W. Circulating levels of endocannabinoids and oxylipins altered by dietary lipids in older women are likely associated with previously identified gene targets. Biochim. Biophys. Acta 2016, 1861, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Friedman, A.N.; Kim, J.; Borkowski, K.; Kaiser, S.; Fiehn, O.; Newman, J.W. Blood Levels of Endocannabinoids, Oxylipins, and Metabolites Are Altered in Hemodialysis Patients. Int. J. Mol. Sci. 2022, 23, 9781. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Stewart, N.R.; Ude, A.A.; Alderman, B.L. Brain insulin resistance and cognitive function: Influence of exercise. J. Appl. Physiol. 2022, 133, 1368–1380. [Google Scholar] [CrossRef]
- Watkins, B.A. Carbohydrate feeding and impact on global metabolomics in relation to insulin sensitivity in men with metabolic syndrome. FASEB J. 2014, 28 (Suppl. S1), 248.8. [Google Scholar] [CrossRef]
- Park, Y.; Watkins, B.A. Endocannabinoids and aging-Inflammation, neuroplasticity, mood and pain. Vitam. Horm. 2021, 115, 129–172. [Google Scholar]
- Muller, P.; Duderstadt, Y.; Lessmann, V.; Muller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation from Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, Y.; Watkins, B.A. Fat to treat fat: Emerging relationship between dietary PUFA, endocannabinoids, and obesity. Prostaglandins Other Lipid Mediat. 2013, 104–105, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Charytoniuk, T.; Zywno, H.; Konstantynowicz-Nowicka, K.; Berk, K.; Bzdega, W.; Chabowski, A. Can physical activity support the endocannabinoid system in the preventive and therapeutic approach to neurological disorders? Int. J. Mol. Sci. 2020, 21, 4221. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, B.; Hu, J.; Bian, X.; Lou, S. The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: A dual role as an energy supply substrate and a signaling molecule. Nutr. Metab. 2022, 19, 52. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Suga, T.; Takenaka, S.; Tanaka, D.; Takeuchi, T.; Hamaoka, T.; Isaka, T.; Hashimoto, T. Greater impact of acute high-intensity interval exercise on post-exercise executive function compared to moderate-intensity continuous exercise. Physiol. Behav. 2016, 155, 224–230. [Google Scholar] [CrossRef]
- Vints, W.A.J.; Levin, O.; Fujiyama, H.; Verbunt, J.; Masiulis, N. Exerkines and long-term synaptic potentiation: Mechanisms of exercise-induced neuroplasticity. Front. Neuroendocrinol. 2022, 66, 100993. [Google Scholar] [CrossRef]
- Wang, X.; Cai, Z.D.; Jiang, W.T.; Fang, Y.Y.; Sun, W.X.; Wang, X. Systematic review and meta-analysis of the effects of exercise on depression in adolescents. Child Adolesc. Psychiatry Ment. Health 2022, 16, 16. [Google Scholar] [CrossRef]
- Felix-Soriano, E.; Stanford, K.I. Exerkines and redox homeostasis. Redox Biol. 2023, 63, 102748. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, C.; Lv, Y.; Zhao, C.; Jiao, Z.; Wang, T. Circulating microRNAs in Response to Exercise Training in Healthy Adults. Front. Genet. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]

| Compound (Changes in Compounds, Treatments Compared to BSA) | C2C12 | 1° Mouse | 1° Human | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BSA | DHA | DHEA | AEA | NESS0327 | BSA | DHA | DHEA | AEA | NESS0327 | BSA | DHA | DHEA | AEA | NESS0327 | |
| docosahexaenoate | ↑ | ↑ | ↑ | ||||||||||||
| docosapentaenoate | ↑ | ↑ | ↑ | ||||||||||||
| docosahexaenoyl ethanolamide | ↑ | ||||||||||||||
| 6-keto prostaglandin F1alpha | ↓ | ↓ | |||||||||||||
| prostaglandin E2 | + | ↑ | |||||||||||||
| 11-HETE | ↓ | - | |||||||||||||
| 13-HODE + 9-HODE | ↓ | ↓ | |||||||||||||
| 1-docosahexaenoylglycerophosphoethanolamine * | ↑ | ↑ | ↑ | ||||||||||||
| 1-docosahexaenoylglycerophosphocholine (22:6n3) * | ↑ | ||||||||||||||
| 2-docosahexaenoylglycerophosphoethanolamine * | ↑ | ↑ | ↑ | + | + | ||||||||||
| 2-docosahexaenoylglycerophosphocholine * | ↑ | ↑ | ↑ | ↓ | |||||||||||
| 1-oleoylglycerophosphoethanolamine * | ↓ | ↓ | - | ||||||||||||
| 2-oleoylglycerophosphoethanolamine * | ↓ | ↓ | ↓ | ||||||||||||
| 2-palmitoylglycerophosphoethanolamine * | ↓ | - | ↓ | ||||||||||||
| 2-linoleoylglycerophosphoethanolamine * | ↓ | ↓ | - | ||||||||||||
| ethanolamine | + | ↑ | + | ||||||||||||
| choline | + | ||||||||||||||
| glycerophosphorylcholine (GPC) | ↑ | ↑ | |||||||||||||
| choline phosphate | - | - | ↓ | ↓ | ↑ | ||||||||||
| phosphoethanolamine | ↑ | ↑ | ↑ | ↑ | + | ||||||||||
| glycerol 3-phosphate (G3P) | ↑ | + | ↑ | ↑ | ↑ | + | ↑ | ||||||||
| glycerol | ↑ | + | |||||||||||||
| 1-docosahexaenoylglycerol (1-monodocosahexaenoin) | ↑ | ↑ | |||||||||||||
| 1-palmitoylglycerol (1-monopalmitin) | + | ||||||||||||||
| 1-myristoylglycerol (1-monomyristin) | ↑ | + | + | ||||||||||||
| 2-myristoylglycerol (2-monomyristin) | ↑ | ↑ | + | ||||||||||||
| 1-linoleoylglycerol (1-monolinolein) | ↑ | ↑ | ↑ | ||||||||||||
| 2-linoleoylglycerol (2-monolinolein) | ↑ | ↑ | ↑ | ||||||||||||
| glucose | ↑ | ↑ | ↑ | ↑ | |||||||||||
| glucose-6-phosphate (G6P) | ↑ | ↑ | ↑ | ↑ | |||||||||||
| fructose-6-phosphate (F6P) | ↑ | ↑ | ↑ | ↑ | |||||||||||
| 3-phosphoglycerate | ↑ | ↑ | ↑ | ||||||||||||
| 2-phosphoglycerate | ↑ | ↑ | ↑ | ||||||||||||
| phosphoenolpyruvate (PEP) | ↑ | ↑ | ↑ | ||||||||||||
| pyruvate | ↑ | + | + | ||||||||||||
| nicotinamide adenine dinucleotide (NAD+) | + | ↑ | |||||||||||||
| nicotinamide adenine dinucleotide reduced (NADH) | ↑ | + | |||||||||||||
| lactate | + | ↑ | ↑ | ↑ | |||||||||||
| citrate | ↓ | - | |||||||||||||
| succinate | ↑ | + | ↑ | ||||||||||||
| arginine | ↑ | ↑ | + | ||||||||||||
| ornithine | + | ↑ | ↑ | ||||||||||||
| putrescine | ↑ | ||||||||||||||
| spermidine | ↓ | ↓ | |||||||||||||
| spermine | ↓ | - | ↓ | - | |||||||||||
| 4-guanidinobutanoate | ↑ | - | |||||||||||||
| pantothenate | ↓ | + | ↑ | ↓ | ↓ | ||||||||||
| phosphopantetheine | ↑ | + | ↑ | ↑ | |||||||||||
| 3′-dephosphocoenzyme A | ↑ | ↑ | ↑ | ↑ | + | ↑ | |||||||||
| coenzyme A | ↑ | ↑ | ↑ | ||||||||||||
| Repeated Measures ANOVA Contrasts | Repeated Measures ANOVA Main Effect | ||||||
|---|---|---|---|---|---|---|---|
| Biochemical Name | 35 g Baseline | 125 g Baseline | 350 g Baseline | 125 g 35 g | 350 g 35 g | 350 g 125 g | |
| linoleate (18:2n6) | 0.89 | 0.8 | 0.72 | 0.89 | 0.81 | 0.9 | |
| linolenate [alpha or gamma; (18:3n3 or 6)] | 0.77 | 0.76 | 0.62 | 0.99 | 0.81 | 0.81 | |
| dihomo-linolenate (20:3n3 or n6) | 0.65 | 0.63 | 0.78 | 0.97 | 1.2 | 1.24 | |
| eicosapentaenoate (EPA; 20:5n3) | 0.91 | 0.95 | 0.85 | 1.04 | 0.93 | 0.89 | |
| docosapentaenoate (n3 DPA; 22:5n3) | 0.8 | 0.81 | 0.81 | 1.02 | 1.02 | 1 | |
| docosapentaenoate (n6 DPA; 22:5n6) | 0.68 | 0.66 | 0.69 | 0.97 | 1.02 | 1.05 | |
| docosahexaenoate (DHA; 22:6n3) | 0.9 | 1.07 | 0.99 | 1.19 | 1.1 | 0.93 | |
| myristate (14:0) | 0.94 | 0.8 | 0.68 | 0.84 | 0.73 | 0.86 | |
| myristoleate (14:1n5) | 0.98 | 0.93 | 0.7 | 0.94 | 0.71 | 0.75 | |
| pentadecanoate (15:0) | 1.01 | 0.88 | 0.71 | 0.87 | 0.7 | 0.81 | |
| palmitate (16:0) | 1.09 | 0.83 | 0.76 | 0.77 | 0.7 | 0.91 | |
| palmitoleate (16:1n7) | 0.89 | 0.79 | 0.59 | 0.89 | 0.66 | 0.74 | |
| margarate (17:0) | 1.14 | 0.88 | 0.79 | 0.78 | 0.7 | 0.9 | |
| 10-heptadecenoate (17:1n7) | 0.94 | 0.85 | 0.69 | 0.91 | 0.73 | 0.8 | |
| stearate (18:0) | 0.99 | 0.91 | 0.75 | 0.93 | 0.76 | 0.83 | |
| oleate (18:1n9) | 1.12 | 0.95 | 0.77 | 0.85 | 0.69 | 0.81 | |
| cis-vaccenate (18:1n7) | 1.04 | 0.92 | 0.76 | 0.88 | 0.73 | 0.83 | |
| stearidonate (18:4n3) | 0.62 | 0.63 | 0.64 | 1.02 | 1.04 | 1.03 | |
| nonadecanoate (19:0) | 1.18 | 1.01 | 0.78 | 0.86 | 0.66 | 0.77 | |
| 10-nonadecenoate (19:1n9) | 1.23 | 0.92 | 0.72 | 0.75 | 0.58 | 0.78 | |
| eicosenoate (20:1n9 or 11) | 1.14 | 0.97 | 0.7 | 0.85 | 0.61 | 0.72 | |
| dihomo-linoleate (20:2n6) | 1.01 | 0.83 | 0.75 | 0.82 | 0.75 | 0.91 | |
| arachidonate (20:4n6) | 0.99 | 0.92 | 0.91 | 0.93 | 0.92 | 0.98 | |
| adrenate (22:4n6) | 0.89 | 0.74 | 0.8 | 0.83 | 0.89 | 1.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watkins, B.A.; Smith, B.J.; Volpe, S.L.; Shen, C.-L. Exerkines, Nutrition, and Systemic Metabolism. Nutrients 2024, 16, 410. https://doi.org/10.3390/nu16030410
Watkins BA, Smith BJ, Volpe SL, Shen C-L. Exerkines, Nutrition, and Systemic Metabolism. Nutrients. 2024; 16(3):410. https://doi.org/10.3390/nu16030410
Chicago/Turabian StyleWatkins, Bruce A., Brenda J. Smith, Stella Lucia Volpe, and Chwan-Li Shen. 2024. "Exerkines, Nutrition, and Systemic Metabolism" Nutrients 16, no. 3: 410. https://doi.org/10.3390/nu16030410
APA StyleWatkins, B. A., Smith, B. J., Volpe, S. L., & Shen, C.-L. (2024). Exerkines, Nutrition, and Systemic Metabolism. Nutrients, 16(3), 410. https://doi.org/10.3390/nu16030410








