Abstract
The most common cancer in Thailand is colorectal cancer (CRC). A lack of knowledge and misleading information from social media have contributed to cancer deaths from malnutrition. A web application is a tool that provides easy access to scientific nutritional information via an online platform. In this study, our goal was to compare the nutritional status of CRC patients using different nutrition-based educational tools with nutrition counseling, namely the Nutrition Educational Prototype based on Smartphone Web Applications (NEPSA) and standard hospital leaflets. Anthropometric and biochemical analyses and a dietary assessment, especially calories and protein, were measured during three visits. This study finally included 28 CRC patients who were undergoing chemotherapy and malnutrition with a body mass index (BMI) of <20 kg/m2. Thirteen participants received NEPSA while the remaining fifteen participants received a standard hospital leaflet. The results showed that NEPSAs improved nutritional outcomes by encouraging weight gain, increasing BMI, hemoglobin, hematocrit, and albumin levels, and consuming more calories and protein. NEPSA should be implemented to enhance the nutrition outcomes from anthropometric, biochemical, and dietary perspectives from nutrition advice among CRC patients. There could be positive impacts at the national level regarding equal accessibility to Thailand’s nutrition information.
1. Introduction
Colorectal cancer is the most common type of cancer in both males and females worldwide, including Thailand [1,2]. The number of cases increased from 3416 to 6068 between 2014 and 2018, a rise of approximately 45.2% over five years in Thailand [1]. Chemotherapy and radiotherapy can cause gastrointestinal problems such as loss of appetite, taste perception change, nausea, vomiting, constipation, and diarrhea [3]. These symptoms can lead to poor dietary intake-induced malnutrition [3,4]. Various factors including cytokines, metabolic changes, and emotional distress [3,4,5,6,7], contribute to malnutrition or cancer cachexia, a major cause of death in CRC [5]. Lack of nutritional knowledge contributes to malnutrition and complicates treatment, leading to longer hospital stays, contracting infections more easily, or complications [4,8,9]. The doctor can refer malnourished patients to a dietitian, but it is often too late; patients will likely have a poor quality of life and experience difficulty following nutritional recommendations [10]. Other studies have found that nutrition knowledge is linked to healthy eating practices [9,10,11,12,13].
Health education is a cost-effective way to improve malnutrition in cancer patients [11,12,13,14,15,16]. It increases dietary intake and helps with self-confidence and food choices [15]. Nowadays, people can easily find unclear nutrition information on social media. According to a technology use survey in Thailand, nearly 40% of smartphone owners have searched for health information [17]. The impact of information impact on society is crucial for cancer groups because it influences the risk of malnutrition and recurrence, as previously mentioned. Thailand’s public health system faces a new challenge: combining nutritional education with smartphone apps. Previous studies have shown that smartphone applications are helpful for cancer patients [18,19,20,21,22]. They are easy to access and have convenient features. Thus, an alternative and interesting nutrition-based educational tool in this digital era can provide reliable nutritional information for cancer patients undergoing chemotherapy, resulting in less toxic and cost-effective treatments afterward.
Cancer patients face a knowledge gap due to a lack of dietetic services and limited time. The patient also faces certain aforementioned obstacles during chemotherapy. The internet cannot always provide reliable information based on nutrition guidelines [23]. Therefore, combining nutrition guidelines using science and technology is a tangible solution. At the national level, this should improve nutrition and democratize nutritional information.
2. Materials and Methods
2.1. Study Design
A quasi-experimental study (pre-test and post-test designs) with a non-equivalent control group was conducted from August 2021 to October 2022 at the Faculty of Medicine, Thammasat University Hospital, Pathum Thani, Thailand. A convenient sample was calculated from a similar research design using a t-test [24]. Match-group comparison was designed in this study. At least 11 participants from each group were settled, including 20% of those who did not participate.
This study recruited 453 CRC patients in that period from the oncology unit. Only 32 participants passed the eligibility assessment for inclusion criteria: those who were diagnosed with CRC between 30 and 75 years old with a BMI of <20 kg/m2 who planned to receive intravenous chemotherapy, were able to eat orally, can communicate in Thai, and had a smartphone or tablet with screen time of at least 1 h per day. We excluded those with complications such as gut obstruction, brain dysfunction, gastrointestinal dysfunction, or metastasis. Additionally, we excluded those who experienced critical events. Two participants were excluded from the initial screening because their families disagreed based on negative experiences in previous studies.
In total, 30 participants were included in this study. Two participants left the study due to disease progression. They were unable to eat orally and had to rely on a nasogastric tube (NG). Additionally, they had a palliative care plan at the time of their first visit. Thus, we conducted our statistical analysis based on 28 participants, as shown in Figure 1. The intervention group was supplied with a Nutrition Educational Prototype based on a Smartphone Web Application (NEPSA) as a tool along with nutrition counseling, which included six modules:
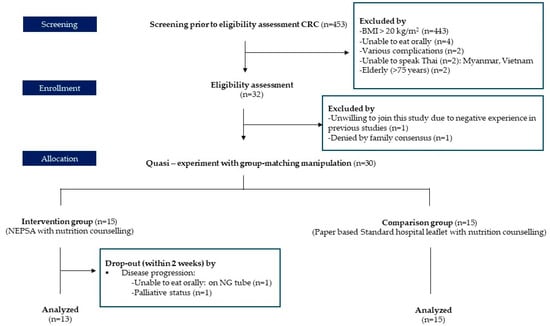
Figure 1.
Intervention enrollment.
- Nutritional news: scientific information combatting misleading information shown on social media.
- Self-weekly body weight recorder: motivates patients to monitor their body weight with a self-weekly body weight recorder.
- Food group selection: proper nutritional information on how to make appropriate food choices during chemotherapy based on food groups, e.g., carbohydrates, protein, fat, fruit, and vegetables.
- Fruit and vegetable washing techniques: three easy washing methods followed by public health concerns that can be practiced at the household level.
- Therapeutic recipes with diet tricks for reducing chemotherapy side effects: 50 therapeutic recipes with cooking videos. The menu function offers cooking tips for decreasing chemotherapy side effects, including recipes for reducing nausea, preventing diarrhea, stimulating appetite, and other related issues.
- Medical food selection: informs on commercial oral nutritional supplements (ONS) available in Thailand and lists the beneficial properties of each formula with automatic scoop calculation features.
Additionally, the comparison group received nutrition counseling using standard hospital leaflet as a tool with six modules, as described below:
- Food group selection: proper nutritional information on making appropriate food choices during chemotherapy based on food groups, e.g., carbohydrates, protein, fat, fruit, vegetables, and water intake.
- Meal plan examples with a table of calorie requirements: 1500 kcal/1800 kcal and 2000 kcal with specific portion sizes of food groups: starch/protein (meat)/milk or dairy product/oil/fruit and vegetables.
- ONS: suggests medical formulas that provide immunonutrients to cancer patients who do not achieve nutritional goals.
- Eating guidelines when suffering from chemotherapy side effects: information on reducing treatment side effects, such as poor appetite, nausea and vomiting, mucositis, chewing and swallowing difficulty, constipation, diarrhea, neutropenia, and changes in taste.
- Food avoidance in cancer patients.
- Diet tricks: nutritional tricks to reach nutritional goals with small, frequent meals containing calorie-dense foods and a protein-based diet.
Both the intervention and comparison groups received personal nutrition counselling from an experienced registered dietitian (RD) at their first visit by followed ESPEN guidelines for cancer patients [23]:
- A calorie-dense and regular protein diet with small, multiple meals were distributed to participants individually.
- Carbohydrate suggestions followed by gastrointestinal symptoms and underlying diseases.
- Lean protein from breast chicken, fish, white egg, tofu, and skinless meat were suggested to be cooked well-done in proper portion sizes.
- Mainly monounsaturated fatty acid (MUFA) oil was suggested as a trick for adding more calories to limited portions.
- Immunonutrient supplementation (omega-3, arginine, and nucleotides) during the day was suggested (as a snack or after resistance exercise) to provide adequate calories and protein.
- In terms of physical activity, increasing resistance exercise in addition to aerobic exercise was suggested to maintain muscle mass and prevent muscle atrophy.
2.2. Data Collection
This study was conducted in three visits according to the oncologist’s chemotherapy appointment plans. Oncologists’ treatment guidelines were modified to reduce hospital visits during the COVID-19 situation. Two chemotherapy regimens, FOLFOX and CAPOX, which have 14- and 21-day cycles, respectively, were suggested depending on oncologists’ recommendations. Bioelectric impedance analysis (BIA) with TANITA–330 was used for anthropometric assessment. The machine was calibrated before every intervention visit to ensure reliable numeric values. Hemoglobin, hematocrit, total protein, globulin, albumin, and ESR were analyzed. Medical technicians collected blood once as per the oncologist’s instructions to avoid any invasive procedures on the participants. Dietary assessment using 24-h recall was conducted following the ethics committee’s suggestion of reducing the burden on participants. An experienced registered dietitian conducted the anthropometric and dietary assessments.
2.3. Ethical Approval
This study was conducted during the COVID-19 pandemic under Thammasat University Hospital regulations, which are in accordance with the Declaration of Helsinki, and approved by the Ethical Review Committee for Human Research of the Faculty of Public Health, Mahidol University (protocol no. 111/2020 COA No. MUPH 2020–148) on 23 November 2020. Thammasat University’s Human Research Ethics Committee at the Faculty of Medicine approved the research protocol MTU-EC-OO-4-284/63 on 27 May 2021. Written informed consent was obtained from all study participants.
2.4. Data Analyses
SPSS version 18 (SPSS (Thailand) Co., Ltd., Bangkok, Thailand) Mahidol University licensed for Windows was used for statistical analyses. A Shapiro–Wilk test assessed the normal distribution of all indicators. Descriptive data were presented as the mean ± SD and percentage. A chi-square test was used to compare demographic data between the experimental and comparison groups. Paired and independent t-tests were used to evaluate the differences in normally distributed variables within and between the groups, respectively. The Mann–Whitney U and signed rank tests were used to evaluate the differences in distribution within and between the groups, respectively. Repeated-measures ANOVA using Bonferroni’s test was used to evaluate differences in normally distributed variables within the group. Friedman’s and Wallis’ analyses of variance were used within and between the groups, respectively, during three intervention visits. Statistical significance was considered when p < 0.05. Friedman’s analysis of variance was considered statistically significant when p < 0.017.
3. Results
3.1. Baseline Charateristics
In total, 28 participants were analyzed in this study. The groups did not significantly differ in characteristics, including gender, age, marital status, educational level, monthly income, residence, cancer staging, and chemotherapy formula. Most of participants in our study were CRC stage 4, namely 84.62% in intervention group and 80% in the comparison group. The mean age of the intervention group was 63.77 ± 2.26 while that of comparison group was 61.07 ± 2.12. Both groups contained 100% non-alcohol users and non-smokers. Table 1 shows a comparison between the study groups.

Table 1.
Baseline characteristics (n = 28).
3.2. Anthropometric Assessment
The baseline characteristics of anthropometric parameters were not significantly different between groups. During the second intervention visit (Visit2), the intervention group showed significant improvements in all measurements, whereas the comparison group did not present any significant changes. After implementation, the intervention group’s body weight, BMI, fat mass, and muscle mass increased significantly compared to baseline. However, the comparison group’s anthropometric parameters did not change significantly, with only body weight and BMI significantly improved compared to baseline at the end of the intervention, as shown in Table 2.

Table 2.
Comparison of anthropometric parameters (n = 28).
3.3. Biochemical Assessment
Biochemical parameters were not significantly different from baseline between groups. During implementation (Visit2 of the intervention), only hemoglobin, hematocrit, and albumin levels in the intervention group had significantly improved compared to baseline. At the end of implementation, hemoglobin, hematocrit, total protein, albumin levels, and ESR in the intervention group had significantly improved compared to baseline. No biochemical parameters significantly changed in the comparison group. Only total protein, globulin, and albumin levels were significantly changed between groups after implementation, as shown in Table 3.

Table 3.
Comparison of biochemical parameters (n = 28).
3.4. Dietary Assessment
The INMUCAL 4.0 program (Institution of Nutrition, Mahidol University licensed) is a standard Thai program developed by the Institution of Nutrition, Mahidol University, Thailand, for calculating nutritional values. It is generally used to analyze the nutritional value of macronutrients, micronutrient, and provide other Thai traditional food nutrition facts. Important nutritional values in clinical cancer practice include total calories and protein intake.
Calorie and protein intakes were not significantly different from baseline between groups. Calorie and protein intakes had increased significantly by visiting dependents within the intervention group. Only protein intake was significantly elevated at the end of implementation in the comparison group. However, no significant differences were detected at the end of implementation when comparing the calorie intake between the intervention and comparison groups.
In terms of protein intake, the intervention group was significantly higher than the comparison group. It presented itself as significantly higher by visiting dependents. Total protein intake in the intervention group was elevated at 32.24 ± 4.68 g, whereas the comparison group increased by only 14.42 ± 4.09 g. A considerable change in protein intake, of up to 74.35%, was detected at the end of implementation in the intervention group, as shown in Table 4.

Table 4.
Comparison of calorie and protein intake (n = 28).
4. Discussion
From the anthropometric results, NEPSA could improve body weight and BMI more efficiently than the comparison group within three visits of implementation. This finding demonstrates the beneficial health outcome of providing appropriate nutritional transformation via the mHealth platform to fragile populations who must receive proper nutritional consultation to reduce the risk of cancer cachexia [25,26,27]. Cancer cachexia, known to reduce skeletal muscle mass, always occurs in malnourished cancer patients because cancer-induced inflammation is associated with weight loss. Gaining muscle mass is difficult for cancer patients because the synthesis of skeletal muscle protein is suppressed by inflammation [28]. Surprisingly, our study showed an increase in muscle mass only in the intervention group when comparing the second and third visits to baseline, as shown in Table 2. Decreasing inflammation (ESR) levels in the body, as shown to occur in Table 3, may help improve protein synthesis in muscle mass [29]. In addition, a sufficient intake of calories and protein, as shown in Table 4, can substantially impact total body weight. Physical activity recommendations following ESPEN guidelines, also mentioned at the baseline visit, include daily walking, gradually increasing resistance exercise, and supplementing immunonutrition with ONS, which may be another method of supporting muscle mass gain [23]. Reduced hemoglobin and hematocrit were generally observed in CAPOX and FOLFOX regimens due to cancer-induced inflammation [30,31,32,33]. According to previous studies, improving hemoglobin and hematocrit levels may positively impact quality of life, clinical status, and survival rates [34], and we also point out similar trends in our study. The participants in the NEPSA group had elevated hemoglobin and hematocrit level as shown in Table 3. Additionally, albumin levels are a crucial biochemical factor in nutrition [35]. Insufficient nutritional support, especially calorie and protein intake, is directly associated with hypoalbuminemia, which is an indicator of malnutrition [36,37]. Our study results showed that raising albumin levels was also significantly associated with reduced ESR. In the intervention group at the end of the study, following a similar trend as the previous observation when albumin was higher, inflammation was lessened after the patients received adequate nutritional support from calories and protein [38,39].
Regarding dietary assessment, calorie intake was raised by 35.17% compared to the first visit in the NEPSA group. By contrast, a 23.62% increase in calorie intake was observed in the comparison group after three intervention visits. These findings reflected a change in eating behaviors caused by NEPSA’s six nutrition modules, which provide proper nutritional information related to 50 therapeutic recipes, diet tips to relieve chemotherapy side effects, cooking preparation, fruit and vegetable washing techniques, and foods for lifestyle modification during chemotherapy. The previous BENECA mHealth app was an innovative tool for determining reliable calorie balance and promoting practical lifestyle changes, which the authors of our study also approved [40]. Experts used a smartphone application to facilitate and deliver nutrition and dietary counseling for Malaysian cancer survivors, as well as pain management for adolescents with cancer [41]. By making nutritional information more accessible, this platform can promote health equality, thereby improving cancer patients’ quality of life [40,41,42,43,44,45,46]. In theory, nutrition literacy refers to the ability to make informed decisions about food and its impact on health through knowledge, skill, and attitude [47]. Promoting nutrition literacy among cancer patients is a public health-based approach to reducing malnutrition and improving anthropometric and biochemical outcomes, as shown in our study.
A strength of this study was the NEPSA development process, which surveyed the needs of CRC patients in phase I of our study. NEPSA can be used on all mobile operating systems (Android and iOS), which reduces accessibility or selection bias. Match-group comparison was designed at the beginning of intervention to reduce confounding variables in the quasi-experiment [48]. A professional multidisciplinary team comprising oncologists, nutritionists, oncology nurses, dietitians, and app developers collaborated on this project. Ethical and moral guidelines were strictly applied in this study to reduce researcher bias during implementation. In Thai hospitals, old-fashioned nutritional tools like paper-based leaflets are commonly used. During the COVID-19 pandemics, social distancing policies were promoted throughout the outbreak. NEPSA has the potential to work a responsive, online tool that can facilitate access to nutrition information and support policies. Conversely, a limitation was the small sample size, which was associated with the inclusion criteria for malnutrition in CRC patients (BMI < 20 kg/m2) because most of CRC patients are obese. Nonetheless, our study’s sample size was higher than previous studies [24,48]. Our enrollment period lasted over 7 months: this was associated to our study design with group-matching manipulate limitation. Furthermore, this study was a quasi-experiment that cannot control for confounding factors, such as participants’ free-living lifestyles.
For further investigation, we suggest recruiting subjects by sarcopenia risk or percentage of weight loss instead of BMI because finding such participants with CRC will be less difficult. The rehabilitation program, quality of life, and psychological well-being could also be considered.
5. Conclusions
Based on our study findings, we can conclude that NEPSA improves nutritional outcomes more efficiently than standard hospital leaflets, as indicated by increases in body weight, BMI, hemoglobin, hematocrit, calorie intake, and protein intake. In addition, NEPSA reduced inflammation by decreasing ESR levels and increasing albumin levels, which was recognized to confer an improvement in nutrition status after three visits. Promoting nutrition literacy in cancer patients helps reduce malnutrition by improving anthropometric and biochemical outcomes in CRC patients. It may also serve as an alternative nutrition educational tool based on mHealth to support Thailand’s digital platform at the national level.
Author Contributions
Conceptualization and methodology, P.N., C.H., and S.W.; software development planning, P.N. and C.L.; validation, C.H. and S.W.; data curation, P.N.; writing—original draft preparation, P.N.; writing—review and editing, P.N and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the Research and Researchers for Industries (RRi) under Thailand Science Research and Innovation, grant number PHD61I0019, and Nestle Research Award 2017 from Nestlé (Thai) Ltd. and Nutrition Association of Thailand under the Patronage of Her Royal Highness Princess Maha Chakri Sirindhorn, grant number N4/2560 for the financial support throughout of this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Review Committee for Human Research of the Faculty of Public Health, Mahidol University (protocol code 111/2020 (COA. No. MUPH 2020-148) and Thammasat University’s Human Research Ethics Committee in the Faculty of Medicine approved research protocol MTU-EC-OO-4-284/63 on 27 May 2021.
Informed Consent Statement
Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to express the sincerely thankful to the oncology team at Thammasat University Hospital, led by Hataiwan Rattanabunjerdkul, Panuch Eiamprapaporn, and Panchanin Patanayindee, for the professional collaboration during data collection.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Health Statistic A.D. 2021. Ministry of Public Health ISSN 0857-3093. Available online: http://bps.moph.go.th/new_bps/sites/default/files/stratistics64.pdf (accessed on 3 June 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Olson, K.; Turner, A.R.; Courneya, K.S.; Field, C.; Man, G.; Cree, M.; Hanson, J. Possible links between behavioral and physiological indices of tiredness, fatigue, and exhaustion in advanced cancer. Support Care Cancer 2008, 16, 241–249. [Google Scholar] [CrossRef]
- Norman, K.; Kirchner, H.; Lochs, H.; Pirlich, M. Malnutrition affects quality of life in gastroenterology patients. World J. Gastroenterol. 2006, 12, 3380. [Google Scholar] [CrossRef] [PubMed]
- Von, M.M. Cancer-associated malnutrition: An introduction. Eur. J. Oncol. Nurs. 2005, 9, S35–S38. [Google Scholar]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Rehse, B.; Pukrop, R. Effects of psychosocial interventions on quality of life in adult cancer patients: Meta analysis of 37 published controlled outcome studies. Patient Educ. Couns. 2003, 50, 179–186. [Google Scholar] [CrossRef]
- McGrath, P. Reflections on nutritional issues associated with cancer therapy. Cancer Pract. 2002, 10, 94–101. [Google Scholar] [CrossRef]
- Baldwin, C. The effectiveness of nutritional interventions in malnutrition and cachexia. Proc. Nutr. Soc. 2015, 74, 397–404. [Google Scholar] [CrossRef]
- Moskovitz, D.N.; Kim, Y.I. Does perioperative immunonutrition reduce postoperative complications in patients with gastrointestinal cancer undergoing operations? Nutr. Rev. 2004, 62, 443–447. [Google Scholar] [CrossRef]
- Morley, J.E.; Mooradian, A.D.; Silver, A.J.; Heber, D.; Alfin-Slater, R.B. Nutrition in the elderly. Ann. Intern. Med. 1988, 109, 890–904. [Google Scholar] [CrossRef]
- Fukuda, Y.; Yamamoto, K.; Hirao, M.; Nishikawa, K.; Maeda, S.; Haraguchi, N.; Tsujinaka, T. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann. Surg. Oncol. 2015, 22, 778–785. [Google Scholar] [CrossRef]
- Sierpina, V.; Levine, L.; McKee, J.; Campbell, C.; Lian, S.; Frenkel, M. Nutrition, Metabolism, and Integrative Approaches in Cancer Survivors. In Proceedings of the Seminar in Oncology Nursing, WB Saunders, Philadelphia, PA, USA, 1 February 2015. [Google Scholar]
- Wolf, P.G.; Manero, J.; Harold, K.B.; Chojnacki, M.; Kaczmarek, J.; Liguori, C.; Arthur, A. Educational video intervention improves knowledge and self-efficacy in identifying malnutrition among healthcare providers in a cancer center: A pilot study. Support Care Cancer 2020, 28, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, J.B. Educational needs of self-care in cachectic cancer patients and caregivers. Curr. Opin. Oncol. 2023, 35, 254–260. [Google Scholar] [CrossRef]
- Thompson, J.; Silliman, K.; Clifford, D.E. Impact of an early education multimedia intervention in managing nutrition-related chemotherapy side effects: A pilot study. Springerplus 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Ministry of Digital Economy and Society. Household Survey on the Use of Information and Communication Technology Use of Information and Communication Technology of Population, 3rd ed.; National Statistic Office: Bangkok, Thailand, 2017; p. 148.
- Gell, N.M.; Grover, K.W.; Humble, M.; Sexton, M.; Dittus, K. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support Care Cancer 2017, 25, 1291–1300. [Google Scholar] [CrossRef]
- Cai, T.; Huang, Y.; Zhang, Y.; Lu, Z.; Huang, Q.; Yuan, C. Mobile health applications for the care of patients with breast cancer: A scoping review. Int. J. Nurs. Sci. 2021, 8, 470–476. [Google Scholar] [CrossRef]
- Smith, S.A.; Whitehead, M.S.; Sheats, J.; Mastromonico, J.; Yoo, W.; Coughlin, S.S. A community-engaged approach to developing a mobile cancer prevention app: The mCPA study protocol. JMIR Res. Protoc. 2016, 5, e5290. [Google Scholar] [CrossRef]
- Nasi, G.; Cucciniello, M.; Guerrazzi, C. The role of mobile technologies in health care processes: The case of cancer supportive care. J. Med. Internet Res. 2015, 17, e26. [Google Scholar] [CrossRef]
- Kushi, L.H.; Doyle, C.; McCullough, M.; Rock, C.L.; Demark-Wahnefried, W.; Bandera, E.V.; American Cancer Society 2010 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2012, 62, 30–67. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bischoff, S.C. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Orlemann, T.; Reljic, D.; Zenker, B.; Meyer, J.; Eskofier, B.; Thiemt, J.; Zopf, Y. A novel mobile phone app (OncoFood) to record and optimize the dietary behavior of oncologic patients: Pilot study. JMIR Cancer 2018, 4, e10703. [Google Scholar] [CrossRef]
- Gupta, D.; Lis, C.G.; Granick, J.; Grutsch, J.F.; Vashi, P.G.; Lammersfeld, C.A. Malnutrition was associated with poor quality of life in colorectal cancer: A retrospective analysis. Clin. Epidemiol. 2006, 59, 704–709. [Google Scholar] [CrossRef]
- Van, C.E.; Arends, J. The causes and consequences of cancer-associated malnutrition. Eur. J. Oncol. Nurs. 2005, 9, S51–S63. [Google Scholar]
- Aaldriks, A.A.; van der Geest, L.G.; Giltay, E.J.; le Cessie, S.; Portielje, J.E.; Tanis, B.C.; Maartense, E. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J. Geriatr. Oncol. 2013, 4, 218–226. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxid. Med. Cell. Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef]
- Antoun, S.; Raynard, B. Muscle protein anabolism in advanced cancer patients: Response to protein and amino acids support, and to physical activity. Ann. Oncol. 2018, 29, ii10–ii17. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, J.; Zhu, Y.; Luo, L.; He, T.; Hu, H.; Teng, Z. Abdominal obesity and colorectal cancer risk: Systematic review and meta-analysis of prospective studies. Biosci. Rep. 2017, 37, BSR20170945. [Google Scholar] [CrossRef]
- Madeddu, C.; Neri, M.; Sanna, E.; Oppi, S.; Macciò, A. Experimental drugs for chemotherapy-and cancer-related anemia. J. Exp. Pharmacol. 2021, 13, 593–611. [Google Scholar] [CrossRef]
- Aapro, M.; Österborg, A.; Gascón, P.; Ludwig, H.; Beguin, Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of iv iron. Ann. Oncol. 2012, 23, 1954–1962. [Google Scholar] [CrossRef]
- Taylor, S.; Ho, C.; Hu, F.; Barnett, J.; Melosky, B. Anemia and FOLFOX chemotherapy for colorectal carcinoma. J. Clin. Oncol. 2005, 23 (Suppl. S16), 3684. [Google Scholar] [CrossRef]
- Cella, D.; Dobrez, D.; Glaspy, J. Control of cancer-related anemia with erythropoietic agents: A review of evidence for improved quality of life and clinical outcomes. Ann. Oncol. 2003, 14, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Keller, U. Nutritional laboratory markers in malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Mobarhan, S. The role of albumin in nutritional support. J. Am. Coll. Nutr. 1988, 7, 445–452. [Google Scholar] [CrossRef]
- Akkiz, H.; Carr, B.I.; Bag, H.G.; Karaoğullarından, Ü.; Yalçın, K.; Ekin, N.; Delik, A. Serum levels of inflammatory markers CRP, ESR and albumin in relation to survival for patients with hepatocellular carcinoma. Int. J. Clin. Pract. 2021, 75, e13593. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Poor Nutritional Status and Inflammation: Serum Albumin: Relationship to Inflammation and Nutrition. In Seminars in Dialysis; Wiley: Oxford, UK, 2004. [Google Scholar]
- Lozano-Lozano, M.; Cantarero-Villanueva, I.; Martin-Martin, L.; Galiano-Castillo, N.; Sanchez, M.J.; Fernández-Lao, C.; Arroyo-Morales, M. A mobile system to improve quality of life via energy balance in breast cancer survivors (BENECA mHealth): Prospective test-retest quasi-experimental feasibility study. JMIR mHealth uHealth 2019, 7, e14136. [Google Scholar] [CrossRef]
- Salihah, N.; Lua, P.L.; Ahmad, A.; Shahril, M.R. CandiTm”: A Malaysian-tailored dietary smartphone app for cancer patients and survivors. Malays J. Public Health Med. 2017, 2, 32–40. [Google Scholar]
- Scarry, A.; Rice, J.; O’Connor, E.M.; Tierney, A.C. Usage of mobile applications or mobile health technology to improve diet quality in adults. Nutrients 2022, 14, 2437. [Google Scholar] [CrossRef]
- Nyman, M.H.; Frank, C.; Langius-Eklöf, A.; Blomberg, K.; Sundberg, K.; Wengström, Y. Patients’ perspective on participation in care with or without the support of a smartphone app during radiotherapy for prostate cancer: Qualitative study. JMIR mHealth uHealth 2017, 5, e6829. [Google Scholar]
- Veale, G.; Dogan, H.; Murphy, J. Development and Usability Evaluation of a Nutrition and Lifestyle Guidance Application for People Living with and Beyond Cancer. In Proceedings of the 8th International Conference Design, User Experience, and Usability, Application Domains, DUXU, Orlando, FL, USA, 26–31 July 2019. [Google Scholar]
- Sundberg, K.; Wengström, Y.; Blomberg, K.; Hälleberg-Nyman, M.; Frank, C.; Langius-Eklöf, A. Early detection and management of symptoms using an interactive smartphone application (Interaktor) during radiotherapy for prostate cancer. Support Care Cancer 2017, 25, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, K.; Eklöf, A.L.; Blomberg, K.; Isaksson, A.K.; Wengström, Y. Feasibility of an interactive ICT-platform for early assessment and management of patient-reported symptoms during radiotherapy for prostate cancer. Eur. J. Oncol. Nurs. 2015, 19, 523–528. [Google Scholar] [CrossRef]
- Velardo, S. The nuances of health literacy, nutrition literacy, and food literacy. J. Nutr. Educ. Behav. 2015, 47, 385–389.e381. [Google Scholar] [CrossRef] [PubMed]
- Handley, M.A.; Lyles, C.R.; McCulloch, C.; Cattamanchi, A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu. Rev. Public Health 2018, 39, 5–25. [Google Scholar] [CrossRef]
- Jibb, L.A.; Stevens, B.J.; Nathan, P.C.; Seto, E.; Cafazzo, J.A.; Stinson, J.N. A smartphone-based pain management app for adolescents with cancer: Establishing system requirements and a pain care algorithm based on literature review, interviews, and consensus. JMIR Res. Protoc. 2014, 3, e3041. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).