Non Breast-Milk-Fed Very Preterm Infants Are at Increased Risk of Iron Deficiency at 4–6-Months Corrected Age: A Retrospective Population-Based Cohort Study
Abstract
1. Introduction
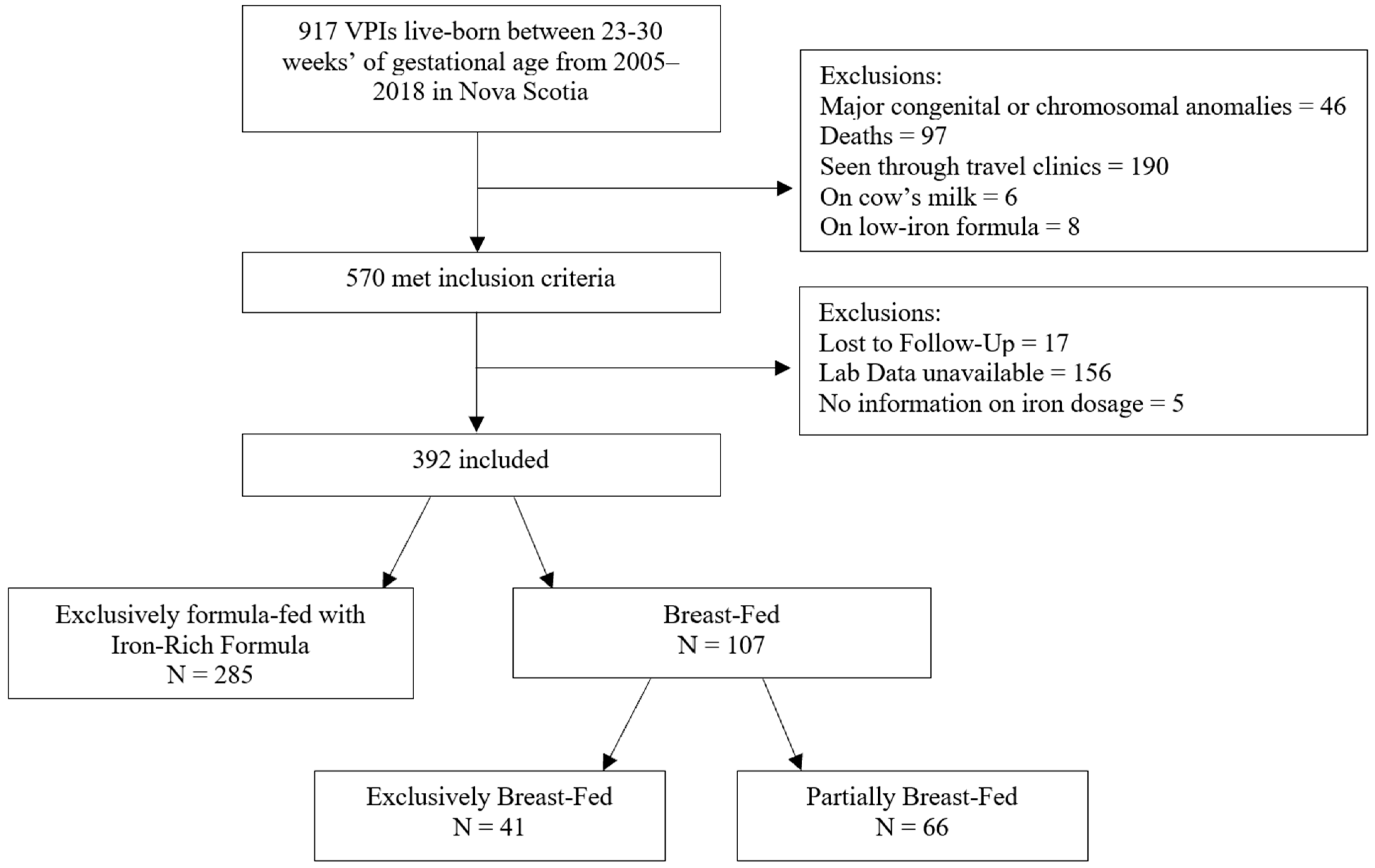
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozoff, B. Iron deficiency and child development. Food Nutr. Bull. 2007, 28, S560–S571. [Google Scholar] [CrossRef]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 2011, 69, S43–S48. [Google Scholar] [CrossRef] [PubMed]
- German, K.R.; Juul, S.E. Iron and Neurodevelopment in Preterm Infants: A Narrative Review. Nutrients 2021, 13, 3737. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhan, S.; Gong, T.; Lee, L. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia (Review). Cochrane Database Syst. Rev. 2013, 2013, CD001444. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.; Georgieff, M.K. Postdischarge iron requirements of the preterm infant. J. Pediatr. 2015, 167, S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M. Meeting the Iron Needs of Low and Very Low Birth Weight Infants. Ann. Nutr. Metab. 2017, 71, 16–23. [Google Scholar] [CrossRef]
- Ilardi, L.; Proto, A.; Ceroni, F.; Morniroli, D.; Martinelli, S.; Mosca, F.; Giannì, M.L. Overview of Important Micronutrients Supplementation in Preterm Infants after Discharge: A Call for Consensus. Life 2021, 11, 331. [Google Scholar] [CrossRef]
- McCarthy, E.K.; Dempsey, E.M.; Kiely, M.E. Iron supplementation in preterm and low-birth-weight infants: A systematic review of intervention studies. Nutr. Rev. 2019, 77, 865–877. [Google Scholar] [CrossRef]
- Moreno-Fernandez, J.; Ochoa, J.J.; Latunde-Dada, G.O.; Diaz-Castro, J. Iron deficiency and iron homeostasis in low birth weight preterm infants: A systematic review. Nutrients 2019, 11, 1090. [Google Scholar] [CrossRef]
- Ferri, C.; Procianoy, R.S.; Silveira, R.C. Prevalence and risk factors for iron-deficiency anemia in very-low-birth-weight preterm infants at 1 year of corrected age. J. Trop. Pediatr. 2014, 60, 53–60. [Google Scholar] [CrossRef][Green Version]
- Landry, C.; Dorling, J.; Kulkarni, K.; Campbell-Yeo, M.; Morrison, L.; Ledwidge, J.; Vincer, M.; Ghotra, S. Postdischarge Iron Status in Very Preterm Infants Receiving Prophylactic Iron Supplementation after Birth. J. Pediatr. 2022, 247, 74–80.e2. [Google Scholar] [CrossRef] [PubMed]
- Tudehope, D.I. Human milk and the nutritional needs of preterm infants. J. Pediatr. 2013, 162 (Suppl. S3), S17–S25. [Google Scholar] [CrossRef]
- Unger, S.L.; Fenton, T.R.; Jetty, R.; Critch, J.N.; O’connor, D.L. Iron requirements in the first 2 years of life. Paediatr. Child Health 2019, 24, 555. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.D.; Greer, F.R.; Committee on Nutrition. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Embleton, N.D.M.; Moltu, S.J.; Lapillonne, A.; Akker, C.H.v.D.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.M.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2022, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Canadian Medical Association. Nutrient needs and feeding of premature infants. Nutrition Committee, Canadian Paediatric Society. CMAJ 1995, 152, 1765–1785. [Google Scholar]
- MacQueen, B.C.; Baer, V.L.; Scott, D.M.; Ling, C.Y.; O’brien, E.A.; Boyer, C.; Henry, E.; Fleming, R.E.; Christensen, R.D. Iron Supplements for Infants at Risk for Iron Deficiency. Glob. Pediatr. Health 2017, 4, 2333794X17703836. [Google Scholar] [CrossRef]
- Saarinen, U.M.; Siimes, M.A. Iron absorption from infant milk formula and the optimal level of iron supplementation. Acta Paediatr. Scand. 1977, 66, 719–722. [Google Scholar] [CrossRef]
- van de Lagemaat, M.; Amesz, E.M.; Schaafsma, A.; Lafeber, H.N. Iron deficiency and anemia in iron-fortified formula and human milk-fed preterm infants until 6 months post-term. Eur. J. Nutr. 2014, 53, 1263–1271. [Google Scholar] [CrossRef]
- Saarinen, U.M.; Siimes, M.A.; Dallman, P.R. Iron absorption in infants: High bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J. Pediatr. 1977, 91, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, M.L.; Remy, R.R.d.l.F.S.; Iglesias, H.G.; López-Sastre, J.B.; Fernández-Colomer, B.; Pérez-Solís, D.; Sanz-Medel, A. Iron content and its speciation in human milk from mothers of preterm and full-term infants at early stages of lactation: A comparison with commercial infant milk formulas. Microchem. J. 2012, 105, 108–114. [Google Scholar] [CrossRef]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Verhaeghe, R.; George, K.; Westerman, M.; Olbina, G.; McCann, D.; Parrow, N.; Pincus, E.; Havranek, T.; Fleming, R.E. Hepcidin Status at 2 Months in Infants Fed Breast Milk Compared with Formula. Neonatology 2020, 117, 474–479. [Google Scholar] [CrossRef]
- Rivera, S.; Liu, L.; Nemeth, E.; Gabayan, V.; Sorensen, O.E.; Ganz, T. Hepcidin excess induces the sequestration of iron and exacerbates tumor-associated anemia. Blood 2005, 105, 1797–1802. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- van Santen, S.; Kroot, J.J.; Zijderveld, G.; Wiegerinck, E.T.; Spaanderman, M.E.; Swinkels, D.W. The iron regulatory hormone hepcidin is decreased in pregnancy: A prospective longitudinal study. Clin. Chem. Lab. Med. 2013, 51, 1395–1401. [Google Scholar] [CrossRef]
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef] [PubMed]
- Brickley, E.B.; Duffy, P.E.; Morrison, R.; Kabyemela, E.; Fried, M.; Spottiswoode, N.; Drakesmith, H.; Wood, A.M.; Kurtis, J.D. Cord blood hepcidin: Cross-sectional correlates and associations with anemia, malaria, and mortality in a Tanzanian birth cohort study. Am. J. Trop. Med. Hyg. 2016, 95, 817–826. [Google Scholar] [CrossRef]
- German, K.R.; Comstock, B.A.; Parikh, P.; Whittington, D.; Mayock, D.E.; Heagerty, P.J.; Bahr, T.M.; Juul, S.E. Do Extremely Low Gestational Age Neonates Regulate Iron Absorption via Hepcidin? J. Pediatr. 2022, 241, 62–67.e1. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Stratas, A.; Landry, C.; Morrison, L.; Kulkarni, K.; Campbell-Yeo, M.; Singh, B.; Higgins, M.; Ghotra, S. Formula Feeding Significantly Increases Risk of Iron Deficiency in Very Preterm Infants during the First 4-6 Months of Life. Blood 2022, 140 (Suppl. S1), 8196–8197. [Google Scholar] [CrossRef]
| Variables | Non-Breast-Milk-Fed Infants N = 285 n(%) | Breast-Milk-Fed Infants N = 107 n(%) | Odds Ratio or Mean Difference (95% CI) |
|---|---|---|---|
| Antenatal Variables | |||
| Maternal age, years, mean ± SD * | 29.2 ± 5.9 | 31.9 ± 4.8 | 2.7 (1.6 to 3.9) |
| Maternal anemia | 20 (7.1) | 2 (1.9) | 4.0 (0.91, 17.3) |
| Gestational hypertension | 51 (17.9) | 16 (14.9) | 1.2 (0.67, 2.3) |
| Smoking * | 88 (30.9) | 7 (6.5) | 6.6 (2.9, 14.7) |
| Maternal diabetes | 17 (6.0) | 5 (4.7) | 1.3 (0.47, 3.6) |
| Antepartum hemorrhage | 47 (16.5) | 20 (18.7) | 0.87 (0.49, 1.55) |
| Multiple gestation * | 82 (28.8) | 42 (39.3) | 0.63 (0.39, 0.99) |
| Mode of delivery | |||
| Vaginal | 116 (40.7) | 49 (45.8) | Ref |
| Cesarean section | 169 (59.3) | 58 (54.2) | 1.2 (0.79, 1.93) |
| Neonatal Variables | |||
| Gestational age, weeks, mean ± SD | 27.8 ± 1.9 | 28 ± 1.7 | 0.18 (−0.22 to 0.58) |
| Gestational age, weeks | |||
| 23–27 | 108 (37.9) | 36 (33.6) | 1.20 (0.75, 1.92) |
| 28–30 | 177 (62.1) | 71 (66.4) | Ref |
| Birth weight, grams, mean ± SD | 1133 ± 317 | 1172 ± 318 | 38.52 (−32 to 109) |
| Birth weight, grams <1100 ≥1100 | |||
| 133 (46.7) | 46 (43.0) | 1.2 (0.74, 1.82) | |
| 152 (53.3) | 61 (57.0) | Ref | |
| Length of hospital stay, days, mean ± SD | 78 ± 49 | 76 ± 34 | −2.14 (−10.87 to 6.59) |
| Need of any blood transfusions | 150 (52.6) | 61 (57.0) | 0.86 (0.55, 1.34) |
| Hemoglobin at discharge, g/L, mean ± SD | 111 ± 25 | 109 ± 20 | −2.67 (−7.62 to 2.29) |
| Ferritin at discharge, µg/L, mean ± SD | 93 ± 92 | 102 ± 93 | 8.55 (−17.37 to 34.46) |
| Feeding at NICU discharge Mixed (Breast and formula) Exclusive Breast-feeding Exclusive Formula-feeding | |||
| 134 (47.0) | 95 (88.9) | 0.04 (0.01–0.1) | |
| 10 (3.5) | 8 (7.5) | 0.04 (0.01–01) | |
| 141 (49.5) | 4 (3.7) | Ref | |
| Dose of iron at discharge, mg/kg/d, mean ± SD * | 3.0 ± 1.1 | 3.3 ± 1.2 | 0.28 (0.02 to 0.53) |
| Male sex | 155 (54.4) | 63 (58.9) | 0.84 (0.54, 1.3) |
| BPD requiring oxygen at 36 weeks | 64 (22.5) | 17 (15.9) | 1.5 (0.85, 2.8) |
| HS-PDA | 73 (25.6) | 24 (22.4) | 1.2 (0.70, 2.0) |
| Necrotizing enterocolitis | 9 (3.2) | 2 (1.9) | 1.7 (0.37, 8.1) |
| Culture positive sepsis | 56 (19.6) | 22 (20.6) | 0.95 (0.54, 1.64) |
| Intraventricular hemorrhage, any grade | 93 (32.6) | 33 (30.8) | 1.1 (0.67, 1.75) |
| Cystic brain injury | 14 (4.9) | 7 (6.5) | 0.74 (0.29, 1.9) |
| Sociodemographic Variables | |||
| Single parent * | 36 (12.6) | 4 (3.7) | 3.8 (1.3, 11.1) |
| Urban dweller (vs. rural) | 225 (78.9) | 89 (83.2) | 0.77 (0.43, 1.4) |
| Variable | Non-Breast-Milk-Fed Infants N = 285 n(%) | Breast-Milk-Fed Infants N = 107 n(%) | Odds Ratios/Mean Differences (95% CI) | p-Values |
|---|---|---|---|---|
| Corrected age at time of assessment, months (mean ± SD) | 4.9 ± 1.2 | 4.9 ± 1.1 | 0.07 (−0.18 to 0.33) | 0.58 |
| Formula intake (mL/kg/day) (mean ± SD) * | 132± 40 | 37 ± 44 | −95.25 (−104.89 to −85.60) | <0.001 |
| Iron obtained from formula, mg/kg/day, mean ± SD * | 1.66 ± 0.54 | 0.43 ± 0.54 | −1.22 (−1.35 to −1.10) | <0.001 |
| Iron supplements * | 165 (57.9) | 85 (79.4) | 0.36 (0.21, 0.60) | <0.001 |
| Iron intake from supplement, mg/kg/day, mean ± SD * | 0.93 ± 1.00 | 1.59 ± 1.09 | 0.66 (0.42 to 0.89) | <0.001 |
| Total iron intake, mg/kg/day, mean ± SD * | 2.59 ± 1.22 | 2.02 ± 1.23 | −0.57 (−0.85 to −0.30) | <0.001 |
| Anti-reflux medication | 105 (36.8) | 41 (38.3) | 0.94 (0.60, 1.49) | 0.79 |
| Iron deficiency * | 105 (36.8) | 22 (20.6) | 2.25 (1.33, 3.82) | 0.002 |
| Marker | Non-Breast-Milk-Fed Infants N = 285 mean ± SD | Breast-Milk-Fed Infants N = 107 mean ± SD | Mean Differences (95% CI) | p-Values |
|---|---|---|---|---|
| Ferritin, µg/L * | 26.8 ± 18.4 | 44.8 ± 38.1 | 17.9 (12.3–23.6) | <0.001 |
| Ferritin, µg/L * median (IQR) | 20.4 (17.3) | 31.3 (39.4) | <0.001 | |
| MCV, fL * | 78.8 ± 3.1 | 77.7 ± 3.8 | −1.1 (−1.8 to −0.4) | 0.004 |
| MCH, pg * | 27.4 ± 1.2 | 26.9 ± 1.6 | −0.6 (−0.8 to −0.3) | <0.001 |
| MCHC, g/L * | 348.4 ± 8.5 | 346.1 ± 10.6 | −2.3 (−4.4 to −0.3) | 0.02 |
| RDW, % | 12.6 ± 1.3 | 12.9 ± 0.8 | 0.2 (0.0–0.5) | 0.07 |
| RetCount, % | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.0 (−0.1 to 0.0) | 0.24 |
| Ret-He, pg | 30.4 ± 2.1 | 30.5 + 3.2 | 0.1 (−0.8 to 0.9) | 0.91 |
| Hemoglobin, g/L | 124.1 ± 9.3 | 122.3 ± 10.7 | −1.8 (−4.0 to 0.4) | 0.10 |
| Variables | ID N = 105 n (%) | Non-ID N = 180 n (%) | OR or Mean Difference (95% CI) |
|---|---|---|---|
| Antenatal Variables | |||
| Maternal age, years, mean ± SD | 30.1 ± 6.2 | 28.7 ± 5.8 | −1.43 (−2.9 to 0.03) |
| Maternal anemia | 7 (6.7) | 13 (7.2) | 0.92 (0.35, 2.37) |
| Gestational hypertension * | 27 (25.7) | 24 (13.3) | 2.25 (1.22, 4.16) |
| Smoking | 31 (29.5) | 57 (31.7) | 0.93 (0.55, 1.58) |
| Maternal diabetes | 6 (5.7) | 11 (6.1) | 0.93 (0.33, 2.59) |
| Antepartum hemorrhage | 19 (18.1) | 28 (15.6) | 1.20 (0.63, 2.27) |
| Multiple gestation | 30 (28.6) | 52 (28.9) | 1.00 (0.59, 1.70) |
| Mode of delivery * | |||
| Vaginal | 34 (32.4) | 82 (45.6) | Ref |
| Cesarean section | 71 (67.6) | 98 (54.4) | 1.75 (1.06, 2.89) |
| Neonatal Variables | |||
| Gestational age, weeks, mean ± SD * | 27.5 ± 2.0 | 28.0 ± 1.9 | 0.52 (0.05 to 0.99) |
| Gestational age, weeks | |||
| 23–27 | 45 (42.9) | 63 (35.0) | 1.39 (0.85, 2.28) |
| 28–30 | 60 (57.1) | 117 (65.0) | Ref |
| Birth weight, grams, mean ± SD * | 1069 ± 294 | 1170 ± 326 | 101.09 (27.01 to 175.17) |
| Birth weight, grams * | |||
| <1100 | 59 (56.2) | 74 (41.1) | 1.84 (1.13, 2.99) |
| ≥1100 | 46 (43.8) | 106 (58.9) | Ref |
| Length of hospital stay, days, mean ± SD | 80 ± 56 | 77 ± 46 | −2.89 (−15.89 to 10.12) |
| Need of any blood transfusions during neonatal stay * | 65 (61.9) | 85 (47.2) | 1.76 (1.08, 2.88) |
| Hemoglobin at discharge, g/L, mean ± SD | 109 ± 20 | 113 ± 28 | 3.94 (−1.73 to 9.60) |
| Ferritin at discharge, µg/L, mean ± SD | 85 ± 81 | 99 ± 98 | 13.97 (−12.44 to 40.37) |
| Dose of iron at discharge, mg/kg/d, mean ± SD | 3.0 ± 1.1 | 3.0 ± 1.1 | 0.00 (−0.26 to 0.27) |
| Male sex | 63 (60.0) | 92 (51.1) | 1.47 (0.90, 2.40) |
| BPD requiring oxygen at 36 weeks | 28 (26.7) | 36 (20.0) | 1.48 (0.84, 2.62) |
| HS-PDA | 31 (29.5) | 42 (23.3) | 1.38 (0.80, 2.37) |
| Necrotizing enterocolitis | 3 (2.9) | 6 (3.3) | 0.86 (0.21, 3.52) |
| Culture positive sepsis | 26 (24.8) | 30 (16.7) | 1.65 (0.91, 2.97) |
| Intraventricular hemorrhage, any grade | 32 (30.5) | 61 (33.9) | 0.86 (0.51, 1.44) |
| Cystic brain injury | 8 (7.6) | 6 (3.3) | 2.42 (0.82, 7.17) |
| Sociodemographic Variables | |||
| Single parent | 15 (14.3) | 21 (11.7) | 1.31 (0.64, 2.67) |
| Urban dweller (vs. rural) | 81 (77.1) | 144 (80.0) | 0.88 (0.49, 1.59) |
| Variable | ID N = 105 n (%) | Non-ID N = 180 n (%) | Odds Ratios and Mean Differences (95% CI) | p-Values |
|---|---|---|---|---|
| Corrected age at time of assessment, months (mean ± SD) * | 4.4 ± 0.88 | 5.2 ± 1.20 | 0.78 (0.53 to 1.02) | <0.001 |
| Post-discharge Preterm formula * | 59 (56.2) | 45 (25.0) | 3.85 (2.31–6.42) | <0.001 |
| Formula intake, mL/kg/day (mean ± SD) * | 142 ± 43 | 127 ± 36 | −14.75 (−24.62 to −4.87) | 0.004 |
| Iron obtained from formula, mg/kg/day, mean ± SD * | 1.83 ± 0.57 | 1.55 ± 0.49 | −0.27 (−0.40 to −0.14) | <0.001 |
| Iron supplements | 66 (62.9) | 99 (55.0) | 1.39 (0.85–2.27) | 0.20 |
| Iron intake from supplement, mg/kg/day, mean ± SD | 1.08 ± 1.05 | 0.85 ± 0.95 | −0.24 (−0.48 to 0.01) | 0.06 |
| Total iron intake, mg/kg/day, mean ± SD * | 2.92 ± 1.27 | 2.40 ± 1.16 | −0.52 (−0.82 to −0.22) | <0.001 |
| Anti-reflux medication | 44 (41.9) | 61 (33.9) | 1.41 (0.86–2.31) | 0.18 |
| Marker | Iron Deficient N = 105 Mean ± SD | Non-Iron Deficient N = 180 Mean ± SD | Mean Differences (95% CI) | p-Values |
|---|---|---|---|---|
| Ferritin, µg/L * | 15.7 ± 5.9 | 33.2 ± 20.0 | 17.5 (13.5–21.5) | <0.001 |
| Ferritin, µg/L * median (IQR) | 15.4 (6.7) | 26.4 (18.8) | <0.001 | |
| MCV, fL | 78.8 ± 3.1 | 78.7 ± 3.1 | 0.0 (−0.8–0.7) | 0.92 |
| MCH, pg | 27.4 ± 1.2 | 27.4 ± 1.2 | 0.0 (−0.3–0.3) | 0.81 |
| MCHC, g/L | 348.0 ± 8.3 | 348.6 ± 8.6 | 0.6 (−1.4–2.7) | 0.56 |
| RDW, % | 12.5 ± 1.9 | 12.7 ± 0.7 | 0.1 (−0.2–0.5) | 0.37 |
| RetCount, % | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.0 (−0.1–0.1) | 0.72 |
| Ret-He, pg * | 29.7 ± 2.0 | 30.7 ± 2.0 | 1.0 (0.2–1.9) | 0.02 |
| Hemoglobin, g/L | 124.2 ± 9.3 | 124.1 ± 9.2 | −0.1 (−2.3–2.2) | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Power, G.; Morrison, L.; Kulkarni, K.; Barr, H.; Campbell-Yeo, M.; Singh, B.; Stratas, A.; Landry, C.; Higgins, M.; Ghotra, S. Non Breast-Milk-Fed Very Preterm Infants Are at Increased Risk of Iron Deficiency at 4–6-Months Corrected Age: A Retrospective Population-Based Cohort Study. Nutrients 2024, 16, 407. https://doi.org/10.3390/nu16030407
Power G, Morrison L, Kulkarni K, Barr H, Campbell-Yeo M, Singh B, Stratas A, Landry C, Higgins M, Ghotra S. Non Breast-Milk-Fed Very Preterm Infants Are at Increased Risk of Iron Deficiency at 4–6-Months Corrected Age: A Retrospective Population-Based Cohort Study. Nutrients. 2024; 16(3):407. https://doi.org/10.3390/nu16030407
Chicago/Turabian StylePower, Grace, Lisa Morrison, Ketan Kulkarni, Hudson Barr, Marsha Campbell-Yeo, Balpreet Singh, Alexandra Stratas, Carmen Landry, Michelle Higgins, and Satvinder Ghotra. 2024. "Non Breast-Milk-Fed Very Preterm Infants Are at Increased Risk of Iron Deficiency at 4–6-Months Corrected Age: A Retrospective Population-Based Cohort Study" Nutrients 16, no. 3: 407. https://doi.org/10.3390/nu16030407
APA StylePower, G., Morrison, L., Kulkarni, K., Barr, H., Campbell-Yeo, M., Singh, B., Stratas, A., Landry, C., Higgins, M., & Ghotra, S. (2024). Non Breast-Milk-Fed Very Preterm Infants Are at Increased Risk of Iron Deficiency at 4–6-Months Corrected Age: A Retrospective Population-Based Cohort Study. Nutrients, 16(3), 407. https://doi.org/10.3390/nu16030407





