Higher Vitamin D Levels before Methotrexate Therapy Initiation Are Associated with Lower Subsequent Mortality in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Data Extraction and Statistical Analysis
3. Results
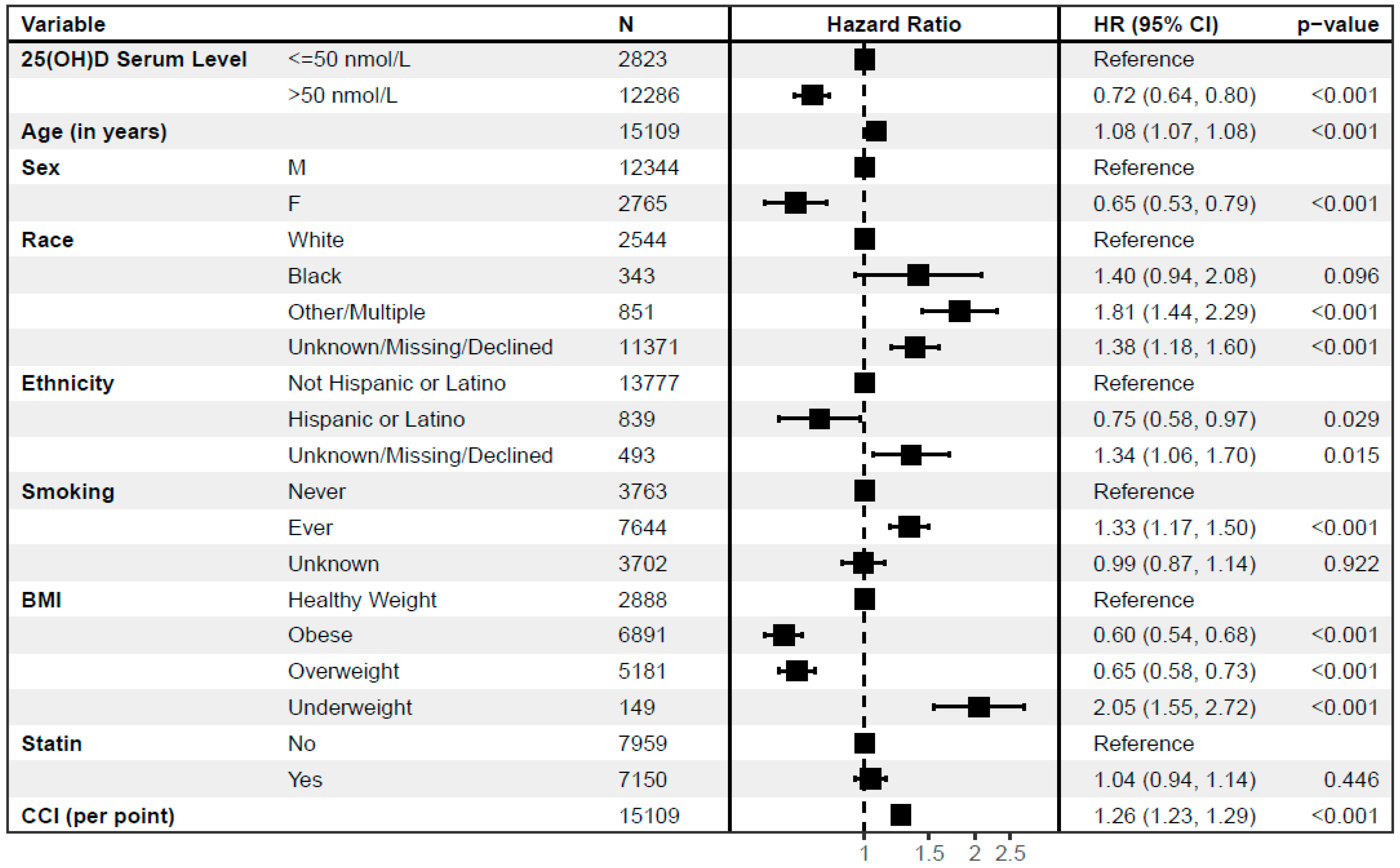
3.1. Subsequent Mortality Risk Is Lower in RA Patients with Higher 25(OH)D Levels before MTX Initiation
3.2. Proportion of Patients with 25(OH)D Levels > 50 nmol/L Increases before Methotrexate Initiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Mouterde, G.; Gamon, E.; Rincheval, N.; Lukas, C.; Seror, R.; Berenbaum, F.; Dupuy, A.M.; Daien, C.; Daures, J.P.; Combe, B. Association Between Vitamin D Deficiency and Disease Activity, Disability, and Radiographic Progression in Early Rheumatoid Arthritis: The ESPOIR Cohort. J. Rheumatol. 2020, 47, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Herly, M.; Stengaard-Pedersen, K.; Vestergaard, P.; Christensen, R.; Möller, S.; Østergaard, M.; Junker, P.; Hetland, M.L.; Hørslev-Petersen, K.; Ellingsen, T. Impact of season on the association between vitamin D levels at diagnosis and one-year remission in early Rheumatoid Arthritis. Sci. Rep. 2020, 10, 7371. [Google Scholar] [CrossRef] [PubMed]
- Herly, M.; Stengaard-Pedersen, K.; Vestergaard, P.; Ostergaard, M.; Junker, P.; Hetland, M.L.; Horslev-Petersen, K.; Ellingsen, T. The D-vitamin metabolite 1,25(OH)(2) D in serum is associated with disease activity and Anti-Citrullinated Protein Antibodies in active and treatment naive, early Rheumatoid Arthritis Patients. Scand. J. Immunol. 2018, 88, e12704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Hypponen, E. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2023, 52, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. The Effect of Vitamin D Supplementation on Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 596007. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch. Intern. Med. 2008, 168, 1629–1637. [Google Scholar] [CrossRef]
- Wang, T.Y.; Wang, H.W.; Jiang, M.Y. Prevalence of vitamin D deficiency and associated risk of all-cause and cause-specific mortality among middle-aged and older adults in the United States. Front. Nutr. 2023, 10, 1163737. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, M.; Yan, H.; Chen, J.; Wang, Y.; Mo, X. Association of serum total 25-hydroxy-vitamin D concentration and risk of all-cause, cardiovascular and malignancies-specific mortality in patients with hyperlipidemia in the United States. Front. Nutr. 2022, 9, 971720. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Daneshvar, M.; Jibril, A.T.; Sluyter, J.D.; Waterhouse, M.; Romero, B.D.; Neale, R.E.; Manson, J.E.; Shab-Bidar, S. Serum 25(OH)D Concentration, Vitamin D Supplementation, and Risk of Cardiovascular Disease and Mortality in Patients with Type 2 Diabetes or Prediabetes: A Systematic Review and Dose-Response Meta-Analysis. Am. J. Clin. Nutr. 2023, 118, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Stancakova Yaluri, A.; Tkac, I.; Tokarcikova, K.; Kozelova, Z.; Rasiova, M.; Javorsky, M.; Kozarova, M. Decreased 25-Hydroxy Vitamin D Level Is Associated with All-Cause Mortality in Patients with Type 2 Diabetes at High Cardiovascular Risk. Metabolites 2023, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, M.; Chen, L. Association of Serum 25-Hydroxyvitamin D Concentrations With All-Cause and Cause-Specific Mortality Among Adult Patients With Existing Cardiovascular Disease. Front. Nutr. 2021, 8, 740855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Yu, H.C.; Li, Y.; Zhang, Y.B.; Geng, T.T.; Lu, Q.; Liao, Y.F.; Guo, K.Q.; Du, L.; Ruan, H.L.; et al. Association between serum 25-hydroxy vitamin D concentrations and mortality among individuals with metabolic dysfunction-associated fatty liver disease: A prospective cohort study. Am. J. Clin. Nutr. 2022, 116, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Zhou, M.; Xiao, Q.; Zou, H.; Zhu, X. L-shaped association between serum 25-hydroxyvitamin D and all-cause mortality of individuals with rheumatoid arthritis. Rheumatology 2023, 62, 575–582. [Google Scholar] [CrossRef]
- Lange, A.; Kostadinova, L.; Damjanovska, S.; Gad, I.; Syed, S.; Siddiqui, H.; Yousif, P.; Kowal, C.M.; Shive, C.; Burant, C.; et al. Red Cell Distribution Width and Absolute Lymphocyte Count Associate With Biomarkers of Inflammation and Subsequent Mortality in Rheumatoid Arthritis. J. Rheumatol. 2023, 50, 166–174. [Google Scholar] [CrossRef]
- Patriota, P.; Guessous, I.; Rezzi, S.; Marques-Vidal, P. Vitamin D Levels Are Associated with Cardiovascular Disease Events but Not with Cardiovascular Disease or Overall Mortality: A Prospective Population-Based Study. Nutrients 2023, 15, 4046. [Google Scholar] [CrossRef]
- Pirrotta, F.; Cavati, G.; Mingiano, C.; Merlotti, D.; Nuti, R.; Gennari, L.; Palazzuoli, A. Vitamin D Deficiency and Cardiovascular Mortality: Retrospective Analysis “Siena Osteoporosis” Cohort. Nutrients 2023, 15, 3303. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Pallares-Carratala, V.; Turegano-Yedro, M.; Torres, F.; Sapena, V.; Martin-Gorgojo, A.; Martin-Moreno, J.M. Vitamin D Supplementation and Its Impact on Mortality and Cardiovascular Outcomes: Systematic Review and Meta-Analysis of 80 Randomized Clinical Trials. Nutrients 2023, 15, 1810. [Google Scholar] [CrossRef]
- Semb, A.G.; Ikdahl, E.; Wibetoe, G.; Crowson, C.; Rollefstad, S. Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat. Rev. Rheumatol. 2020, 16, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Sakalyte, R.; Denkovskij, J.; Bernotiene, E.; Stropuviene, S.; Mikulenaite, S.O.; Kvederas, G.; Porvaneckas, N.; Tutkus, V.; Venalis, A.; Butrimiene, I. The Expression of Inflammasomes NLRP1 and NLRP3, Toll-Like Receptors, and Vitamin D Receptor in Synovial Fibroblasts From Patients With Different Types of Knee Arthritis. Front. Immunol. 2021, 12, 767512. [Google Scholar] [CrossRef] [PubMed]
- Tardito, S.; Martinelli, G.; Soldano, S.; Paolino, S.; Pacini, G.; Patane, M.; Alessandri, E.; Smith, V.; Cutolo, M. Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 2019, 18, 102397. [Google Scholar] [CrossRef]
- Cutolo, M.; Soldano, S.; Sulli, A.; Smith, V.; Gotelli, E. Influence of Seasonal Vitamin D Changes on Clinical Manifestations of Rheumatoid Arthritis and Systemic Sclerosis. Front. Immunol. 2021, 12, 683665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhu, X.; Guo, Y.; Yang, Y.; Jiang, Y.; Liu, B. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J. Cell Physiol. 2019, 234, 6917–6926. [Google Scholar] [CrossRef]
- Li, D.; Jeffery, L.E.; Jenkinson, C.; Harrison, S.R.; Chun, R.F.; Adams, J.S.; Raza, K.; Hewison, M. Serum and synovial fluid vitamin D metabolites and rheumatoid arthritis. J. Steroid Biochem. Mol. Biol. 2019, 187, 1–8. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Buring, J.E.; Group, V.R. Principal results of the VITamin D and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin D trials. J. Steroid Biochem. Mol. Biol. 2020, 198, 105522. [Google Scholar] [CrossRef]
- Egger, S.; Smith, D.P.; Nair-Shalliker, V. Methodological considerations in D-health cancer mortality results. Lancet Diabetes Endocrinol. 2022, 10, 307. [Google Scholar] [CrossRef]
- Keum, N.; Chen, Q.Y.; Lee, D.H.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality by daily vs. infrequent large-bolus dosing strategies: A meta-analysis of randomised controlled trials. Br. J. Cancer 2022, 127, 872–878. [Google Scholar] [CrossRef]
- Gibbons, J.B.; Norton, E.C.; McCullough, J.S.; Meltzer, D.O.; Lavigne, J.; Fiedler, V.C.; Gibbons, R.D. Association between vitamin D supplementation and COVID-19 infection and mortality. Sci. Rep. 2022, 12, 19397. [Google Scholar] [CrossRef]
- Baker, J.F.; Billig, E.; Michaud, K.; Ibrahim, S.; Caplan, L.; Cannon, G.W.; Stokes, A.; Majithia, V.; Mikuls, T.R. Weight Loss, the Obesity Paradox, and the Risk of Death in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 1711–1717. [Google Scholar] [CrossRef] [PubMed]

| Overall N = 15,109 | 25(OH)D ≤ 50 nmol/L n = 2823 | 25(OH)D > 50 nmol/L n = 12,286 | p-Value | |
|---|---|---|---|---|
| Age (years) at Index (IQR) | 63 (55, 69) | 59 (51, 66) | 64 (56, 70) | <0.001 |
| Sex, n (%) | <0.001 | |||
| Female | 2765 (18%) | 638 (23%) | 2127 (17%) | |
| Male | 12,344 (82%) | 2185 (77%) | 10,159 (83%) | |
| Race, n (%) | <0.001 | |||
| Black | 2544 (17%) | 839 (30%) | 1705 (14%) | |
| Other/Multiple | 343 (2.3%) | 81 (2.9%) | 262 (2.1%) | |
| Unknown/Missing/Declined | 851 (5.6%) | 181 (6.4%) | 670 (5.5%) | |
| White | 11,371 (75%) | 1722 (61%) | 9649 (79%) | |
| Ethnicity, n (%) | 0.034 | |||
| Hispanic or Latino | 839 (5.6%) | 185 (6.6%) | 654 (5.3%) | |
| Not Hispanic or Latino | 13,777 (91%) | 2550 (90%) | 11,227 (91%) | |
| Unknown/Missing/Declined | 493 (3.3%) | 88 (3.1%) | 405 (3.3%) | |
| BMI, n (%) | <0.001 | |||
| <18.5 | 149 (1.0%) | 36 (1.3%) | 113 (0.9%) | |
| 18.5 to <25 | 2888 (19%) | 511 (18%) | 2377 (19%) | |
| 25 to <30 | 5181 (34%) | 822 (29%) | 4359 (35%) | |
| ≥30 | 6,891 (46%) | 1454 (52%) | 5437 (44%) | |
| Smoking, n (%) | 0.001 | |||
| Ever a Smoker | 7644 (51%) | 1510 (53%) | 6134 (50%) | |
| Never a Smoker | 3763 (25%) | 685 (24%) | 3078 (25%) | |
| Unknown | 3702 (25%) | 628 (22%) | 3074 (25%) | |
| Charlson Comorbidity Index, Median (IQR) | 1.00 (1.00, 3.00) | 1.00 (1.00, 2.00) | 1.00 (1.00, 3.00) | <0.001 |
| Statin, n (%) | <0.001 | |||
| No Statin | 7959 (53%) | 1706 (60%) | 6253 (51%) | |
| Statin Use | 7150 (47%) | 1117 (40%) | 6033 (49%) |
| Overall * N = 105 | 25(OH)D ≤ 50 nmol/L n = 18 | 25(OH)D > 50 nmol/L n = 87 | p-Value | |
|---|---|---|---|---|
| Age (years) at Index (IQR) | 66 (60, 70) | 63 (51, 67) | 67 (62, 70) | 0.03 |
| Sex, n (%) | 0.07 | |||
| Female | 10 (9.5%) | 4 (22%) | 6 (6.9%) | |
| Male | 95 (90%) | 14 (78%) | 81 (93%) | |
| Race, n (%) | 0.4 | |||
| Black | 7 (6.7%) | 2 (11%) | 5 (5.7%) | |
| Other/Multiple | 3 (2.9%) | 1 (5.6%) | 2 (2.3%) | |
| Unknown/Missing/Declined | 5 (4.8%) | 1 (5.6%) | 4 (4.6%) | |
| White | 90 (86%) | 14 (78%) | 76 (87%) | |
| Ethnicity, n (%) | 0.07 | |||
| Hispanic or Latino | 1 (1.0%) | 1 (5.6%) | 0 | |
| Not Hispanic or Latino | 102 (97%) | 16 (89%) | 86 (99%) | |
| Unknown/Missing/Declined | 2 (1.9%) | 1 (5.6%) | 1 (1.1%) | |
| BMI, n (%) | 0.3 | |||
| ≤18.5 | 0 | |||
| 18.5 to <25 | 14 (13%) | 2 (11%) | 12 (14%) | |
| 25 to <30 | 36 (34%) | 4 (22%) | 32 (37%) | |
| ≥30 | 53 (50%) | 11 (61%) | 42 (48%) | |
| Unknown | 2 (1.9%) | 1 (5.6%) | 1 (1.1%) | |
| Smoking, n (%) | 0.2 | |||
| Ever a Smoker | 84 (80%) | 13 (72%) | 71 (82%) | |
| Never a Smoker | 19 (18%) | 4 (22%) | 15 (17%) | |
| Unknown | 2 (1.9%) | 1 (5.6%) | 1 (1.1%) | |
| Statin, n (%) | 0.01 | |||
| No Statin | 43 (41%) | 12 (67%) | 31 (36%) | |
| Statin Use | 62 (59%) | 6 (33%) | 56 (64%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malakooti, S.K.; Siddiqui, H.; Wilson, B.; Bej, T.; O’Mara, M.; Desotelle, A.; Lange, A.; Shive, C.L.; Singer, N.G.; McComsey, G.A.; et al. Higher Vitamin D Levels before Methotrexate Therapy Initiation Are Associated with Lower Subsequent Mortality in Patients with Rheumatoid Arthritis. Nutrients 2024, 16, 401. https://doi.org/10.3390/nu16030401
Malakooti SK, Siddiqui H, Wilson B, Bej T, O’Mara M, Desotelle A, Lange A, Shive CL, Singer NG, McComsey GA, et al. Higher Vitamin D Levels before Methotrexate Therapy Initiation Are Associated with Lower Subsequent Mortality in Patients with Rheumatoid Arthritis. Nutrients. 2024; 16(3):401. https://doi.org/10.3390/nu16030401
Chicago/Turabian StyleMalakooti, Shahdi K., Hinnah Siddiqui, Brigid Wilson, Taissa Bej, Megan O’Mara, Alexandra Desotelle, Alyssa Lange, Carey L. Shive, Nora G. Singer, Grace A. McComsey, and et al. 2024. "Higher Vitamin D Levels before Methotrexate Therapy Initiation Are Associated with Lower Subsequent Mortality in Patients with Rheumatoid Arthritis" Nutrients 16, no. 3: 401. https://doi.org/10.3390/nu16030401
APA StyleMalakooti, S. K., Siddiqui, H., Wilson, B., Bej, T., O’Mara, M., Desotelle, A., Lange, A., Shive, C. L., Singer, N. G., McComsey, G. A., Kostadinova, L., Mattar, M., Zidar, D. A., & Anthony, D. D. (2024). Higher Vitamin D Levels before Methotrexate Therapy Initiation Are Associated with Lower Subsequent Mortality in Patients with Rheumatoid Arthritis. Nutrients, 16(3), 401. https://doi.org/10.3390/nu16030401





