Serum 25-Hydroxyvitamin D Is Inversely Associated with Nasopharyngeal Carcinoma: A Hospital-Based Matched Case–Control Study in Malaysia
Abstract
1. Introduction
2. Methodology
2.1. Study Subjects and Locations
2.2. Ethical Approval
2.3. Test Principle of Total Serum 25(OH)D
2.4. Interviewer-Administered Questionnaire
2.5. Data Analysis
3. Results
3.1. Characteristics of Study Subjects
3.2. Distribution of 25(OH)D Serum Concentration across Clinical Characteristics of Nasopharyngeal Carcinoma Cases
3.3. Association between the Serum Concentration of 25(OH)D and Nasopharyngeal Carcinoma Adjusting for Confounding Factors
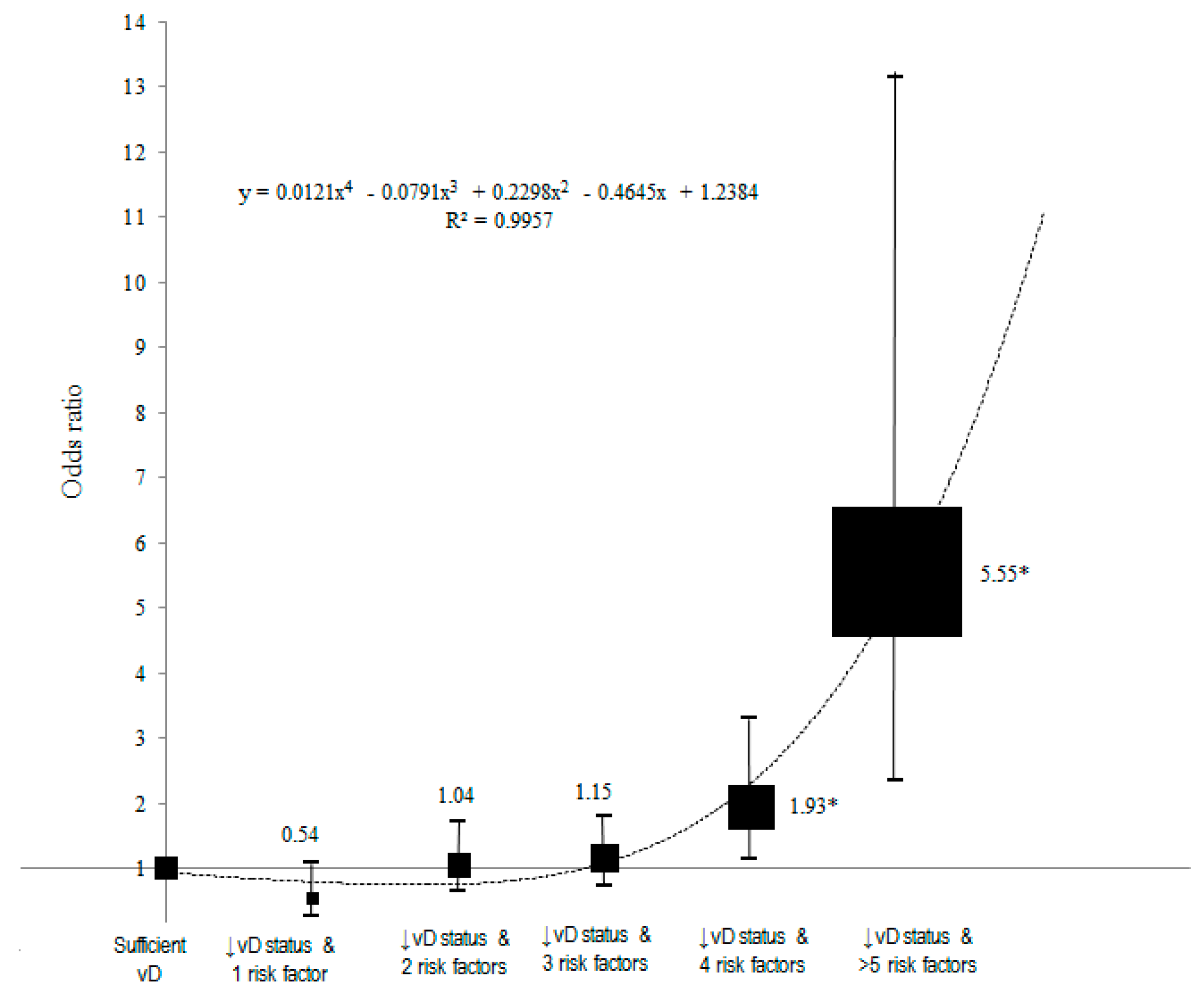
3.4. Dose–Response Analysis between Inadequate Serum Concentration of 25(OH)D with Accumulation of Risk Factors and Nasopharyngeal Carcinoma Using Polynomial Analysis
3.5. Five-Year Survival Time of Nasophrayngeal Carcinoma Cases across the Levels of Serum 25(OH)D Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, E.; O’Sullivan, B.; Kim, J.; Siu, L.; Bartlett, E. Magnetic Resonance Imaging of Nasopharyngeal Carcinoma. Expert Rev. Anticancer Ther. 2010, 10, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Globocan. Globocan. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-sheets.pdf (accessed on 16 December 2023).
- Cancer Registry Report National Cancer Society. Cancer Registry Report National Cancer Society. 2019. Available online: https://nci.moh.gov.my/index.php/ms/pengumuman/340-national-cancer-registry-report (accessed on 30 August 2023).
- Ji, J.; Jiang, D.; Xu, Z.; Yang, Y.; Qian, K.; Zhang, M. Continuous Quality Improvement of Nutrition Management during Radiotherapy in Patients with Nasopharyngeal Carcinoma. Nurs. Open 2021, 8, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association Between Vitamin D and Risk of Colorectal Cancer: A Systematic Review of Prospective Studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Boniol, M.; Haukka, J.; Byrnes, G.; Cox, B.; Sneyd, M.J.; Mullie, P.; Autier, P. Meta-analysis of Observational Studies of Serum 25-hydroxyvitamin D Levels and Colorectal, Breast and Prostate Cancer and Colorectal Adenoma. Int. J. Cancer 2011, 128, 1414–1424. [Google Scholar] [CrossRef]
- Albanes, D.; Mondul, A.M.; Yu, K.; Parisi, D.; Horst, R.L.; Virtamo, J.; Weinstein, S.J. Serum 25-Hydroxy Vitamin D and Prostate Cancer Risk in a Large Nested Case–Control Study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1850–1860. [Google Scholar] [CrossRef]
- Rausch, J.C.; Berger-Jenkins, E.; Nieto, A.R.; McCord, M.; Meyer, D. Effect of a School-Based Intervention on Parents’ Nutrition and Exercise Knowledge, Attitudes, and Behaviors. Am. J. Health Educ. 2015, 46, 33–39. [Google Scholar] [CrossRef]
- Badawi, A. Vitamins D, C, and E in the Prevention of Type 2 Diabetes Mellitus: Modulation of Inflammation and Oxidative Stress. Biol. Targets Ther. 2011, 5, 7–19. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Laillou, A.; Smith, G.; Schofield, D.; Moench-Pfanner, R. A Review of Vitamin D Fortification: Implications for Nutrition Programming in Southeast Asia. Food Nutr. Bull. 2013, 34 (Suppl. 2), S81–S89. [Google Scholar] [CrossRef]
- Mattila, P.H.; Valkonen, E.; Valaja, J. Effect of Different Vitamin D Supplementations in Poultry Feed on Vitamin D Content of Eggs and Chicken Meat. J. Agric. Food Chem. 2011, 59, 8298–8303. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review Article: Vitamin D and Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Hedelin, M.; Löf, M.; Olsson, M.; Lewander, T.; Nilsson, B.; Hultman, C.M.; Weiderpass, E. Dietary Intake of Fish, Omega-3, Omega-6 Polyunsaturated Fatty Acids and Vitamin D and the Prevalence of Psychotic-like Symptoms in a Cohort of 33,000 Women from the General Population. BMC Psychiatry 2010, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, W.; Weinstock, M. Factors Influencing Vitamin D Status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Brasky, T.M.; Neuhouser, M.L.; Cohn, D.E.; White, E. Associations of Long-Chain ω-3 Fatty Acids and Fish Intake with Endometrial Cancer Risk in the VITamins and Lifestyle Cohort. Am. J. Clin. Nutr. 2014, 99, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Fadzlina, A.; Harun, F.; Nurul Haniza, M.; Al Sadat, N.; Murray, L.; Cantwell, M.M.; Su, T.T.; Majid, H.A.; Jalaludin, M.Y. Metabolic Syndrome among 13 Year Old Adolescents: Prevalence and Risk Factors. BMC Public Health 2014, 14 (Suppl. 3), S7. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, H.G.; Murray, L.J.; Anderson, L.A.; Cantwell, M.M. Vitamin D, Calcium and Dairy Intake, and Risk of Oesophageal Adenocarcinoma and Its Precursor Conditions. Br. J. Nutr. 2011, 106, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Feldman, D. Mechanisms of the Anti-Cancer and Anti-Inflammatory Actions of Vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Yin, L.; Grandi, N.; Raum, E.; Haug, U.; Arndt, V.; Brenner, H. Meta-Analysis: Circulating Vitamin D and Ovarian Cancer Risk. Gynecol. Oncol. 2011, 121, 369–375. [Google Scholar] [CrossRef]
- Weinstein, S.J.; Stolzenberg-Solomon, R.Z.; Kopp, W.; Rager, H.; Virtamo, J.; Albanes, D. Impact of Circulating Vitamin D Binding Protein Levels on the Association between 25-Hydroxyvitamin D and Pancreatic Cancer Risk: A Nested Case–Control Study. Cancer Res. 2012, 72, 1190–1198. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Chang, E.T.; Abel, G.A.; Zhang, S.M.; Spiegelman, D.; Qureshi, A.A.; Laden, F. Sunlight Exposure, Vitamin D, and Risk of Non-Hodgkin Lymphoma in the Nurses’ Health Study. Cancer Causes Control 2011, 22, 1731–1741. [Google Scholar] [CrossRef][Green Version]
- Mai, Z.-M.; Ngan, R.K.-C.; Ng, W.-T.; Lin, J.-H.; Kwong, D.L.-W.; Yuen, K.-T.; Lee, C.K.; Leung, J.N.-S.; Ip, D.K.-M.; Chan, Y.-H.; et al. Low Vitamin D Exposure and Risk of Nasopharyngeal Carcinoma: Observational and Genetic Evidence from a Multicenter Case–Control Study. Clin. Nutr. 2021, 40, 5180–5188. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chan, A.T.C.; Le, Q.-T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal Carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef]
- Huang, X.; Cao, Z.; Zhang, Z.; Yang, Y.; Wang, J.; Fang, D. No Association between Vitamin D Receptor Gene Polymorphisms and Nasopharyngeal Carcinoma in a Chinese Han Population. Biosci. Trends 2011, 5, 99–103. [Google Scholar] [CrossRef]
- Vieth, R. Why the Minimum Desirable Serum 25-Hydroxyvitamin D Level Should Be 75 Nmol/L (30 Ng/Ml). Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 681–691. [Google Scholar] [CrossRef]
- Gagnon, C.; Lu, Z.X.; Magliano, D.J.; Dunstan, D.W.; Shaw, J.E.; Zimmet, P.Z.; Sikaris, K.; Ebeling, P.R.; Daly, R.M. Low Serum 25-Hydroxyvitamin D Is Associated with Increased Risk of the Development of the Metabolic Syndrome at Five Years: Results from a National, Population-Based Prospective Study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab). J. Clin. Endocrinol. Metab. 2012, 97, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Chang, C.-S.; Chang, Y.-F.; Wu, S.-J.; Chiu, C.-J.; Hou, M.-T.; Chen, C.-Y.; Liu, P.-Y.; Wu, C.-H. Inverse Relationship between Metabolic Syndrome and 25-Hydroxyvitamin D Concentration in Elderly People without Vitamin D Deficiency. Sci. Rep. 2018, 8, 17052. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.B.; Nahas-Neto, J.; Bueloni-Dias, F.; Poloni, P.F.; Orsatti, C.L.; Petri Nahas, E.A. Vitamin D Deficiency Is Associated with Metabolic Syndrome in Postmenopausal Women. Maturitas 2018, 107, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Chan, Y.M.; Ramachandran, V.; Shariff, Z.M.; Chin, Y.S.; Arumugam, M. Dietary Acid Load and Its Interaction with IGF1 (Rs35767 and Rs7136446) and IL6 (Rs1800796) Polymorphisms on Metabolic Traits among Postmenopausal Women. Nutrients 2021, 13, 2161. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yao, X.; Zhu, Z. Associations between Serum Calcium, 25(OH)D Level and Bone Mineral Density in Older Adults. J. Orthop. Surg. Res. 2019, 14, 458. [Google Scholar] [CrossRef]
- Fan, J.; Li, N.; Gong, X.; He, L. Serum 25-Hydroxyvitamin D, Bone Turnover Markers and Bone Mineral Density in Postmenopausal Women with Hip Fractures. Clin. Chim. Acta 2018, 477, 135–140. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Z.; Liu, J.; Xiao, X.; Wang, C.; Deng, M.; Chen, L. Correlation between Serum 25-OH Vitamin D Expression and Non-alcoholic Fatty Liver Disease. Exp. Ther. Med. 2020, 19, 1681–1686. [Google Scholar] [CrossRef]
- International World Cancer Research Fund, 2020. Preventing Cancer. Saving Lives. WCRF International. Available online: https://www.wcrf.org/ (accessed on 4 January 2024).
- Orell–Kotikangas, H.; Schwab, U.; Österlund, P.; Saarilahti, K.; Mäkitie, O.; Mäkitie, A.A. High Prevalence of Vitamin D Insufficiency in Patients with Head and Neck Cancer at Diagnosis. Head Neck 2012, 34, 1450–1455. [Google Scholar] [CrossRef]
- Yu, X.; Liu, B.; Zhang, N.; Wang, Q.; Cheng, G. Immune Response: A Missed Opportunity Between Vitamin D and Radiotherapy. Front. Cell Dev. Biol. 2021, 9, 646981. [Google Scholar] [CrossRef]
- Teleni, L.; Baker, J.; Koczwara, B.; Kimlin, M.G.; Walpole, E.; Tsai, K.; Isenring, E.A. Clinical Outcomes of Vitamin D Deficiency and Supplementation in Cancer Patients. Nutr. Rev. 2013, 71, 611–621. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Yeh, C.-N.; Hsu, J.-T.; Chen, L.-W.; Kuo, S.-F.; Sun, C.-C.; Huang, C.-C.; Pang, J.-H.S.; Flanagan, J.N.; Takano, M.; et al. MART-10, a Novel Vitamin D Analog, Inhibits Head and Neck Squamous Carcinoma Cells Growth through Cell Cycle Arrest at G0/G1 with Upregulation of P21 and P27 and Downregulation of Telomerase. J. Steroid Biochem. Mol. Biol. 2013, 138, 427–434. [Google Scholar] [CrossRef]
- Yuan, F.-N.F.; Valiyaparambil, J.; Woods, M.C.; Tran, H.; Pant, R.; Adams, J.S.; Mallya, S.M. Vitamin D Signaling Regulates Oral Keratinocyte Proliferation in Vitro and in Vivo. Int. J. Oncol. 2014, 44, 1625–1633. [Google Scholar] [CrossRef][Green Version]
- Sharma, V.; Fretwell, D.; Crees, Z.; Kerege, A.; Klopper, J.P. Thyroid Cancer Resistance to Vitamin D Receptor Activation Is Associated with 24-Hydroxylase Levels but Not the Ff FokI Polymorphism. Thyroid 2010, 20, 1103–1111. [Google Scholar] [CrossRef]
- Binkley, N.; Ramamurthy, R.; Krueger, D. Low Vitamin D Status: Definition, Prevalence, Consequences, and Correction. Endocrinol. Metab. Clin. N. Am. 2010, 39, 287–301. [Google Scholar] [CrossRef]
- Vanhevel, J.; Verlinden, L.; Doms, S.; Wildiers, H.; Verstuyf, A. The Role of Vitamin D in Breast Cancer Risk and Progression. Endocr. Relat. Cancer 2022, 29, R33–R55. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Pike, J.W. The Vitamin D Receptor Functions as a Transcription Regulator in the Absence of 1,25-Dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 2016, 164, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Pike, J.W.; Meyer, M.B. The Vitamin D Receptor: New Paradigms for the Regulation of Gene Expression by 1,25-Dihydroxyvitamin D3. Endocrinol. Metab. Clin. N. Am. 2010, 39, 255–269. [Google Scholar] [CrossRef] [PubMed]

| Variables | Case | Control | χ2 | p-Value | |||
|---|---|---|---|---|---|---|---|
| n (%) | Mean ± SD/ Median (IQR) b | n (%) | Mean ± SD/ Median (IQR) b | ||||
| Age of respondents (years) | 54.06 ± 10.94 | 54.24 ± 11.11 | |||||
| <40 | 28 (9.3) | 27 (9.0) | 1.97 | 0.741 | |||
| 40–49 | 77 (25.7) | 71 (23.7) | |||||
| 50–59 | 105 (35.0) | 96 (32.0) | |||||
| 60–69 | 73 (24.3) | 86 (28.7) | |||||
| ≥70 | 17 (5.7) | 20 (6.7) | |||||
| Gender | |||||||
| Male | 231 (77.0) | 231 (77.0) | - | - | |||
| Female | 69 (23.0) | 69 (23.0) | |||||
| Ethnicity | |||||||
| Malay | 85 (28.3) | 85 (28.3) | - | - | |||
| Chinese | 213 (71.0) | 213 (71.0) | |||||
| Indian | 2 (0.7) | 2 (0.7) | |||||
| Body mass index (kg/m2) | 21.62 (6.06) b | 25.93 (6.56) b | −9.55 a | <0.001 * | |||
| Underweight | <18.5 | 60 (20.0) | 13 (4.3) | 69.26 | <0.001 * | ||
| Normal | 18.5–24.9 | 158 (52.7) | 119 (39.7) | ||||
| Overweight | 25–29.9 | 60 (20.0) | 100 (33.3) | ||||
| Obese | ≥30 | 22 (7.3) | 68 (22.7) | ||||
| Physical activity intensity (MET; hour/week) | 85.3 (184.50) b | 69.7 (128.50) b | 2.03 a | 0.043 * | |||
| Smoking status | |||||||
| Ever smoke | 155 (51.7) | 126 (42.0) | 5.63 | 0.018 * | |||
| Never smoke | 145 (48.3) | 174 (58.0) | |||||
| Alcohol consumption | |||||||
| Ever consumed | 137 (45.7) | 114 (38.0) | |||||
| Never consumed | 163 (54.3) | 186 (62.0) | |||||
| Consumption of food high in vitamin D | |||||||
| Beef_Liver | |||||||
| <once a week | 65 (21.7) | 58 (19.3) | 0.50 | 0.479 | |||
| ≥once a week | 235 (78.3) | 242 (80.7) | |||||
| Pork | |||||||
| <once a week | 193 (64.3) | 202 (67.3) | 0.60 | 0.439 | |||
| ≥once a week | 107 (35.7) | 98 (32.7) | |||||
| Fish | |||||||
| <once a week | 291 (97.0) | 296 (98.7) | 1.97 | 0.161 | |||
| ≥once a week | 9 (3.0) | 4 (1.3) | |||||
| Egg | |||||||
| <once a week | 274 (91.3) | 292 (97.3) | 10.10 | 0.001 * | |||
| ≥once a week | 26 (8.7) | 8 (2.7) | |||||
| Soy products | |||||||
| <once a week | 250 (83.3) | 277 (92.3) | 11.37 | 0.001 * | |||
| ≥once a week | 50 (16.7) | 23 (7.7) | |||||
| Dairy products | |||||||
| <once a week | 248 (82.7) | 254 (84.7) | 0.44 | 0.508 | |||
| ≥once a week | 52 (17.3) | 46 (15.3) | |||||
| Salted fish consumption | |||||||
| Ever | 228 (76.0) | 173 (57.7) | 22.75 | <0.001 * | |||
| Never | 72 (24.0) | 127 (42.3) | |||||
| Consumption of salted fish per day (g) | 1.88 (4.41) b | 0.66 (1.88) b | −5.45 a | <0.001 * | |||
| Family history of NPC | |||||||
| Yes | 37 (12.3) | 6 (2.0) | 24.07 | <0.001 * | |||
| No | 263 (87.7) | 294 (98.0) | |||||
| Clinical Characteristics | Mean ± SD | F/t | p-Value |
|---|---|---|---|
| Cancer indicators ++ | |||
| Neck swelling | 26.19 ± 7.73 | 1.751 e | 0.081 |
| Nasal symptoms | 25.57 ± 7.49 | −0.393 e | 0.695 |
| Aural symptoms | 25.35 ± 7.72 | 0.086 e | 0.931 |
| Facial numbness | 24.85 ± 7.35 | −0.329 e | 0.743 |
| Other symptoms | 25.17 ± 7.41 | −0.211 e | 0.833 |
| Histopathological grading a | |||
| WHO type I = keratinising squamous-cell carcinoma | 25.92 ± 6.94 | 0.209 f | 0.648 |
| WHO type II = non-keratinising epidermoid carcinoma | 25.44 ± 7.97 | ||
| WHO type III = undifferentiated carcinoma | 25.56 ± 7.71 | ||
| AJCC staging for NPC | |||
| T= Tumor b | |||
| 0: no evidence of primary tumor | 39.26 ± 1.23 | 1.063 f | 0.381 |
| 1: tumor confined to nasopharynx | 24.93 ± 7.93 | ||
| 2a: tumor extends to soft tissue of oropharynx and/or Nasal cavity without parapharyngeal extension | 25.12 ± 7.66 | ||
| 2b: tumor extends to soft tissue of oropharynx and/or Nasal cavity with parapharyngeal extension | 26.61 ± 8.08 | ||
| 3: tumor invading bony structures and/or paranasal sinuses | 25.60 ± 6.74 | ||
| 4: tumor with intracranial extension and/or involvement of cranial nerve | |||
| N = Regional nodes b | |||
| 0: none | 25.30 ± 7.72 | 0.191 f | 0.943 |
| 1: unilateral nodal metastasis < 6 cm in greatest diameter | 25.36 ± 7.83 | ||
| 2: bilateral nodal metastasis < 6 cm in greatest diameter | 25.58 ± 8.11 | ||
| 3a: cervical lymph nodes > 6 cm | 24.31 ± 5.91 | ||
| 3b: any cervical nodes in superclavicular fossa | 25.77 ± 5.91 | ||
| M = Metastasis c | |||
| 0: no | 25.22 ± 7.60 | 1.219 g | 0.224 |
| 1: yes | 28.36 ± 7.86 | ||
| Staging b | |||
| 1 | 24.58 ± 9.00 | 0.468 f | 0.800 |
| 2 | 24.75 ± 8.23 | ||
| 3 | 25.91 ± 7.83 | ||
| 4A | 25.13 ± 7.38 | ||
| 4B | 24.32 ± 6.05 | ||
| 4C | 26.93 ± 8.68 | ||
| Staging investigations procedures ¥,d | |||
| Endoscopy | 25.11 ± 7.53 | −1.099 e | 0.272 |
| CT scan | 25.56 ± 7.56 | 1.582 e | 0.295 |
| MRI scan | 26.84 ± 7.17 | 1.569 e | 0.118 |
| Bone scan | 26.58 ± 7.06 | 1.387 e | 0.166 |
| Chest radiography | 24.60 ± 6.56 | −0.611 e | 0.541 |
| USS liver | 25.12 ± 7.72 | −0.209 e | 0.834 |
| PET scans | 24.99 ± 6.40 | −0.349 e | 0.295 |
| Treatment | |||
| Radiotherapy | |||
| Yes | 25.64 ± 7.63 | 2.125 g | 0.034 * |
| No | 21.21 ± 7.09 | ||
| Type of radiotherapy (n = 281) | |||
| External beam radiotherapy (EBRT) | 25.69 ± 7.56 | 1.019 f | 0.398 |
| Palliative radiotherapy | 20.03 ± 12.47 | ||
| Autologous SCT | 21.92 ± 6.74 | ||
| Deep X-ray therapy | 37.37 ± 2.13 | ||
| Unknown | 26.31 ± 7.40 | ||
| Chemotherapy | |||
| Yes | 25.33 ± 7.68 | 0.130 g | 0.897 |
| No | 25.18 ± 7.71 | ||
| Type of chemotherapy (N = 253) | |||
| Induction | 26.06 ± 7.07 | 0.475 f | 0.752 |
| Adjuvant | 24.49 ± 4.36 | ||
| Concurrent | 24.91 ± 8.45 | ||
| Palliative | 25.35 ± 4.20 | ||
| Unknown | 23.12 ± 5.35 | ||
| Surgery | |||
| Yes | 27.75 ± 7.98 | 1.312 g | 0.191 |
| No | 25.17 ± 7.65 | ||
| Type of surgery (N = 16) | |||
| Debulking nasopharynx | 34.11 ± 4.39 | 0.509 f | 0.731 |
| Radical neck dissection | 28.73 ± 7.50 | ||
| Nasopharyngectomy | 27.67 ± 10.70 | ||
| Unknown | 28.47 ± 11.19 | ||
| Response to treatment | |||
| Complete remission | 25.04 ± 7.70 | −1.209 g | 0.228 |
| Diagnosed with residual disease | 26.36 ± 7.55 | ||
| Survival status | |||
| Alive | 25.50 ± 7.77 | 0.581 g | 0.562 |
| Not alive | 24.97 ± 7.52 |
| Factors | Case n (%) | Control n (%) | COR + (95% CI) | p-Value | B | SE | AOR ++ (95% CI) a | p-Value |
|---|---|---|---|---|---|---|---|---|
| Serum 25(OH)D (Mean ± SD) | 25.3 ± 7.7 | 27.0 ± 9.2 | 0.78 (0.66, 0.95) | 0.011 * | −0.029 | 0.013 | 0.73 (0.57, 0.94) | 0.016 * |
| Body mass index | ||||||||
| <18 kg/m2 | 60 (20.0) | 13 (4.3) | 6.22 (3.08, 12.58) | <0.001 * | 1.74 | 0.38 | 5.82 (2.79, 12.14) | <0.001 * |
| ≥18 kg/m2 | 240 (80.0) | 287 (95.7) | 1 b | 1 b | ||||
| Salted fish consumption | ||||||||
| Ever | 228 (76.0) | 173 (57.7) | 2.34 (1.62, 3.38) | <0.001 * | 0.88 | 0.22 | 2.40 (1.58, 3.65) | <0.001 * |
| Never | 72 (24.0) | 127 (42.3) | 1 b | 1 b | ||||
| Family history of NPC | ||||||||
| Yes | 37 (12.3) | 6 (2.0) | 6.17 (2.60, 14.61) | <0.001 * | 1.92 | 0.47 | 6.59 (2.63, 16.56) | <0.001 * |
| No | 263 (87.7) | 294 (98.0) | 1 b | 1 b | ||||
| Smoking | ||||||||
| Ever smoked | 155 (51.7) | 126 (42.0) | 1.69 (1.15, 2.48) | 0.007 * | 0.31 | 0.23 | 1.35 (0.86, 2.11) | 0.174 |
| Never smoked | 145 (48.3) | 174 (58.0) | 1 b | 1 b | ||||
| Alcohol consumption | ||||||||
| Ever consumed | 137 (45.7) | 114 (38.0) | 1.55 (1.05, 2.28) | 0.027 * | 0.19 | 0.24 | 1.22 (0.77, 1.93) | 0.413 |
| Never consumed | 163 (54.3) | 186 (62.0) | 1 b | 1 b | ||||
| Physical activity | ||||||||
| Low intensity (MET < 10 hours/week) | 256 (85.3) | 251 (83.7) | 1.13 (0.73, 1.75) | 0.579 | na | na | na | na |
| High intensity (MET ≥ 10 hours/week) | 44 (14.7) | 49 (16.3) | 1 b | |||||
| Consumption of food high in Vitamin D | ||||||||
| <once a week | 128 (42.7) | 128 (42.7) | 1.00 (0.72, 1.40) | 1.000 | na | na | na | na |
| ≥once a week | 172 (57.3) | 172 (57.3) | 1 b | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulaganathan, V.; Lye, M.S.; Loh, S.P.; Yap, Y.Y.; Kandiah, M.; Augundhooa, D.; Bhattacharya, T.; Al-Olayan, E.; Wang, C. Serum 25-Hydroxyvitamin D Is Inversely Associated with Nasopharyngeal Carcinoma: A Hospital-Based Matched Case–Control Study in Malaysia. Nutrients 2024, 16, 397. https://doi.org/10.3390/nu16030397
Ulaganathan V, Lye MS, Loh SP, Yap YY, Kandiah M, Augundhooa D, Bhattacharya T, Al-Olayan E, Wang C. Serum 25-Hydroxyvitamin D Is Inversely Associated with Nasopharyngeal Carcinoma: A Hospital-Based Matched Case–Control Study in Malaysia. Nutrients. 2024; 16(3):397. https://doi.org/10.3390/nu16030397
Chicago/Turabian StyleUlaganathan, Vaidehi, Munn Sann Lye, Su Peng Loh, Yoke Yeow Yap, Mirnalini Kandiah, Digsha Augundhooa, Tanima Bhattacharya, Ebtesam Al-Olayan, and Chuanyi Wang. 2024. "Serum 25-Hydroxyvitamin D Is Inversely Associated with Nasopharyngeal Carcinoma: A Hospital-Based Matched Case–Control Study in Malaysia" Nutrients 16, no. 3: 397. https://doi.org/10.3390/nu16030397
APA StyleUlaganathan, V., Lye, M. S., Loh, S. P., Yap, Y. Y., Kandiah, M., Augundhooa, D., Bhattacharya, T., Al-Olayan, E., & Wang, C. (2024). Serum 25-Hydroxyvitamin D Is Inversely Associated with Nasopharyngeal Carcinoma: A Hospital-Based Matched Case–Control Study in Malaysia. Nutrients, 16(3), 397. https://doi.org/10.3390/nu16030397








