Daily Consumption of Golden Berry (Physalis peruviana) Has Been Shown to Halt the Progression of Insulin Resistance and Obesity in Obese Rats with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Diets and Animals
2.3. Gene Expression
2.4. Analysis UPLC/ESI-Q-Orbitrap
2.5. Discovery of Biological Association Networks
2.6. Statistical Analysis
3. Results
3.1. Anatomical Measurements
3.2. Biochemical Parameters
3.3. Impact of Dietary Variations on Organ and Adipose Tissue Weights in Wistar Rats: The Role of Golden Berry Supplementation
3.4. Comparative Analysis of Key Gene Expressions Influenced by Dietary Variations
3.5. Metabolic Profiling and Biomarker Discovery in Rat Urine Post-Golden Berry Consumption: An OSC-PLS-DA Approach
3.6. Identification and Analysis of Key Urinary Metabolites in Rats following Golden Berry Consumption
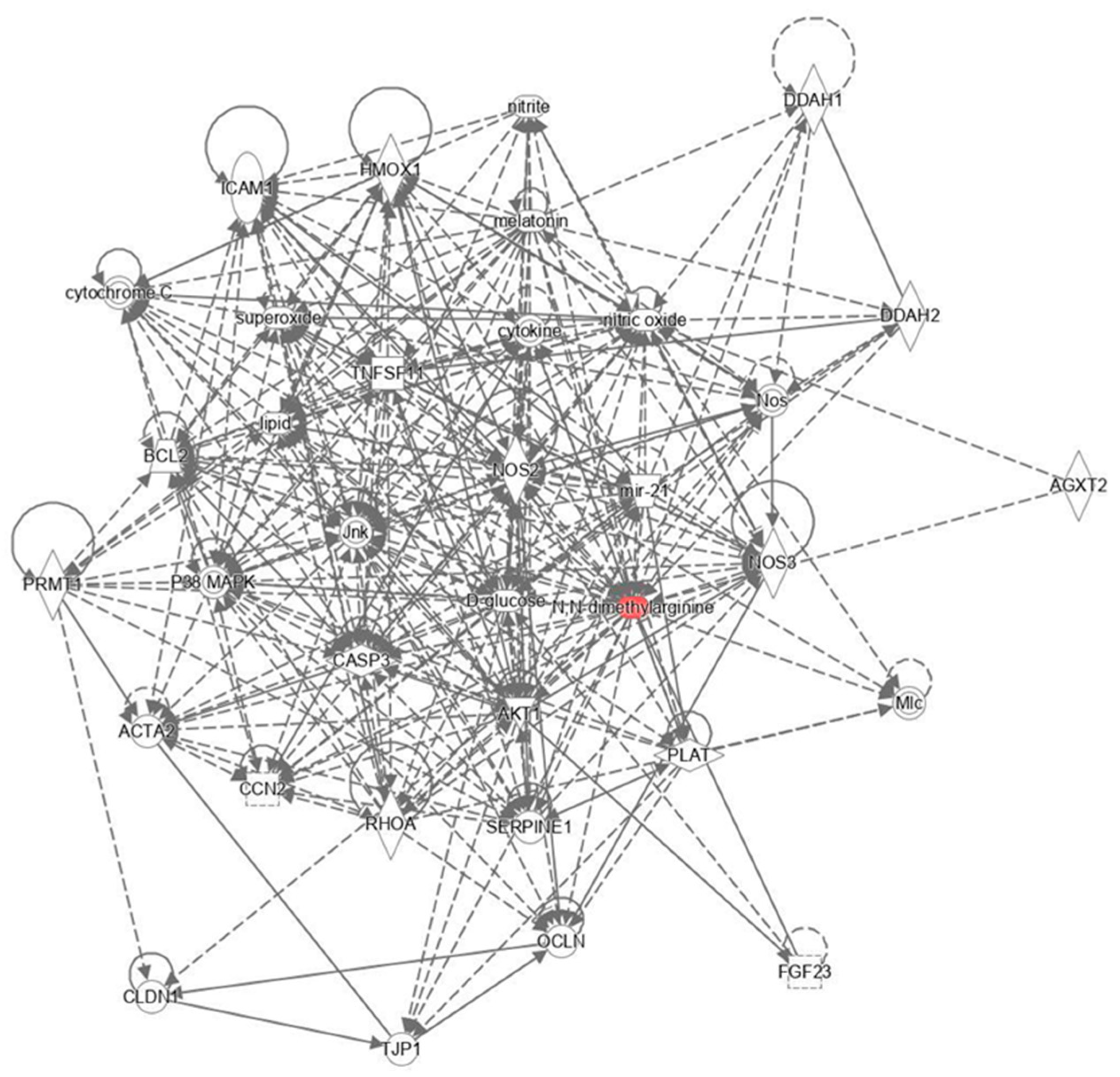
3.7. Elucidating Metabolic Networks and Biological Pathways Influenced by Golden Berry Consumption using Ingenuity Pathway Analysis
4. Discussion
4.1. Golden Berry’s Role in Counteracting Obesity and Supporting Weight Management
4.2. Beneficial Biochemical Effects of Golden Berry in Diet: Improved Glucose and Lipid Metabolism
4.3. Golden Berry’s Impact on Reducing Organ Weights and Preventing Metabolic Diseases
4.4. Influence of Golden Berry on the Reactivity of White and Brown Adipose Tissue
4.5. Golden Berry’s Influence on Gene Expression in Metabolic Regulation and Health
4.5.1. INSR Gene Expression Modulation by Golden Berry
4.5.2. FasN Expression and the Impact of Golden Berry
4.5.3. PPARγ Expression and Its Role in Metabolic Regulation: The Impact of Golden Berry Supplementation
4.5.4. Golden Berry’s Influence on LPL Gene Expression and Metabolic Health
4.6. Metabolomic Insights: Golden Berry’s Effect on Metabolic Biomarkers and Potential in Diabetes Management
4.6.1. Evaluation of Metabolic Impact through Acylcarnitines Post-Golden Berry Consumption
4.6.2. Acetylated Dimethylarginine and Golden Berry’s Metabolic Impact
4.6.3. Golden Berry’s Influence on Metabolic Pathways Linked to Diabetes
4.7. Golden Berry’s Multifaceted Impact on Metabolic Health and the Potential for Personalized Nutritional Therapy
4.8. Expanding the Horizon: New Insights into Golden Berry’s Role in Metabolic Regulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates in pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.; Mangelsdorf, D.J.; Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef]
- Fazal, F.; Saleem, T.; Ur Rehman, M.E.; Haider, T.; Khalid, A.R.; Tanveer, U.; Mustafa, H.; Tanveer, J.; Noor, A. The rising cost of healthcare and its contribution to the worsening disease burden in developing countries. Ann. Med. Surg. 2022, 82, 104683. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Chekol Abebe, E.; Behaile Teklemariam, A.; Tilahun Mulu, A.; Agidew, M.M.; Teshome Azezew, M.; Zewde, E.A.; Agegnehu, T.A. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci. 2022, 9, 1002710. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic consequences of obesity and type 2 diabetes: Balancing genes and environment for personalized care. Cell 2021, 184, 1530–1544. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.-T. Oil Goldenberry (Physalis peruviana L.). J. Agric. Food Chem. 2003, 51, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, F.; Corrales-Agudelo, V.; Moreno-Castellanos, N.; Ángel-Martín, A.; Henao-Rojas, J.C.; Muñoz-Durango, K.; Poucheret, P. Plasma Metabolome Profiling by High-Performance Chemical Isotope-Labelling LC-MS after Acute and Medium-Term Intervention with Golden Berry Fruit (Physalis peruviana L.), Confirming Its Impact on Insulin-Associated Signaling Pathways. Nutrients 2021, 13, 3125. [Google Scholar] [CrossRef]
- Novelli, E.L.B.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.X.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli, J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Imbernon, M.; Whyte, L.; Diaz-Arteaga, A.; Russell, W.R.; Moreno, N.R.; Vazquez, M.J.; Gonzalez, C.R.; Ruiz, A.; Lopez, M.; Malagon, M.M.; et al. Regulation of GPR55 in rat white adipose tissue and serum LPI by nutritional status, gestation, gender and pituitary factors. Mol. Cell. Endocrinol. 2014, 383, 159–169. [Google Scholar] [CrossRef] [PubMed]
- ICONTEC. Fresh Fruits Cape Gooseberry. Specifications. Colombian Standard. NTC 4580. Bogota, Colombia. p. 15. 1999. Available online: https://kontii.files.wordpress.com/2012/10/ntc-4580.pdf (accessed on 16 January 2024).
- Bonilla-Carvajal, K.; Stashenko, E.E.; Moreno-Castellanos, N. Lippia alba chemotype carvone essential oil (Verbenaceae) regulates lipid mobilization and adipogenesis in adipocytes. Curr. Issues Mol. Biol. 2022, 44, 5741–5755. [Google Scholar] [CrossRef]
- Thonusin, C.; IglayReger, H.B.; Soni, T.; Rothberg, A.E.; Burant, C.F.; Evans, C.R. Evaluation of intensity drift correction strategies using MetaboDrift, a normalization tool for multi-batch metabolomics data. J. Chromatogr. A 2017, 1523, 265–274. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. BioSystems 2015, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, J.B.; Mohamed, M.; Bakar, A.B.A. A systematic review on different models of obesity induction in animals: Advantages and limitations. J. Adv. Vet. Anim. Res. 2019, 7, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Hassanien MF, R. Physalis peruviana: A Rich Source of Bioactive Phytochemicals for Functional Foods and Pharmaceuticals. Food Rev. Int. 2011, 27, 259–273. [Google Scholar] [CrossRef]
- Mayorga, H.; Knapp, H.; Winterhalter, P.; Duque, C. Glycosidically Bound Flavor Compounds of Cape Gooseberry (Physalis peruviana L.). J. Agric. Food Chem. 2001, 49, 1904–1908. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Fokunang, C.N.; Mushagalusa, F.K.; Tembe, E.; Ngoupayo, J.; Ngameni, B.; Njinkio, L.N.; Kadima, J.N.; Kechia, F.A.; Tiedeu, B.; Mbacham, W.F.; et al. Phytochemical and zootechnical studies of Physalis peruviana L. leaves exposured to streptozotocin-induced diabetic rats. J. Pharmacogn. Phytother. 2017, 9, 123–130. [Google Scholar] [CrossRef]
- Valenzuela, A.; Ronco, A.M. Fitoesteroles y fitoestanoles: Aliados naturales para la protección de la salud cardiovascular. Rev. Chil. Nutr. 2004, 31 (Suppl. S1), 161–169. [Google Scholar] [CrossRef]
- Reyes-Beltrán, M.E.D.; Guanilo-Reyes, C.K.; Ibáñez-Cárdenas, M.W.; García-Collao, C.E.; Idrogo-Alfaro, J.J.; Huamán-Saavedra, J.J. Efecto del consumo de Physalis peruviana L. (aguaymanto) sobre el perfil lipídico de pacientes con hipercolesterolemia. Acta Médica Peru. 2015, 32, 195–201. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1728-59172015000400002&lng=es&tlng=es (accessed on 24 April 2023). [CrossRef]
- Balcázar, H.G.; de Heer, H.; Rosenthal, L.; Aguirre, M.; Flores, L.; Puentes, F.A.; Cardenas, V.M.; Duarte, M.O.; Ortiz, M.; Schulz, L.O. A promotores de salud intervention to reduce cardiovascular disease risk in a high-risk Hispanic border population, 2005–2008. Prev. Chronic Dis. 2010, 7, A28. [Google Scholar]
- Erman, F.; Kirecci, O.; Ozsahin, A.; Erman, O.; Kaya, T.; Yilmaz, O. Effects of Physalis peruviana and Lupinus albus on malondialdehyde, glutathione, cholesterol, vitamins and fatty acid levels in kidney and liver tissues of diabetic rats. Prog. Nutr. 2018, 20 (Suppl. S1), 218–230. [Google Scholar] [CrossRef]
- Bazalar Pereda, M.S.; Nazareno, M.A.; Viturro, C.I. Nutritional and antioxidant properties of Physalis peruviana L. fruits from the Argentinean Northern Andean region. Plant Foods Hum. Nutr. 2019, 74, 68–75. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Gao, B.; Gershwin, M.E. Liver Immunology. Compr. Physiol. 2013, 3, 567. [Google Scholar] [CrossRef]
- Ramadan, M.F. Physalis peruviana pomace suppresses highcholesterol diet-induced hypercholesterolemia in rats. Grasas Aceites 2012, 63, 411–422. [Google Scholar] [CrossRef]
- Fuente, F.P.; Nocetti, D.; Sacristán, C.; Ruiz, P.; Guerrero, J.; Jorquera, G.; Uribe, E.; Bucarey, J.L.; Espinosa, A.; Puente, L. Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice. Nutrients 2020, 12, 700. [Google Scholar] [CrossRef]
- Garcia-Compean, D.; Jaquez-Quintana, J.O.; Gonzalez-Gonzalez, J.A.; Maldonado-Garza, H. Liver cirrhosis and diabetes: Risk factors, pathophysiology, clinical implications and management. World J. Gastroenterol. 2009, 15, 280–288. [Google Scholar] [CrossRef]
- Moussa, S.A.A.; Ibrahim, F.A.A.; Elbaset, M.A.; Aziz, S.W.; Morsy, F.A.; Abdellatif, N.; Attia, A.; El Toumy, S.A.; Salib, J.Y.; Bashandy, S.A.E. Goldenberry (Physalis peruviana) alleviates hepatic oxidative stress and metabolic syndrome in obese rats. J. Appl. Pharm. Sci. 2022, 12, 138–150. [Google Scholar] [CrossRef]
- Obregón-La Rosa, A.J.; Contreras-López, E.; Flores Juárez, E.; Gonzales Barrón, Ú.; Muñoz, A.M.; Ramos-Escudero, F. Nutritional and antioxidant profile of the Physalis fruit grown in three Andean regions of Peru. Rocz. Panstw. Zakl. Hig. 2023, 74, 49–57. [Google Scholar] [CrossRef]
- Eibl, G.; Cruz-Monserrate, Z.; Korc, M.; Petrov, M.S.; Goodarzi, M.O.; Fisher, W.E.; Habtezion, A.; Lugea, A.; Pandol, S.J.; Hart, P.A.; et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J. Acad. Nutr. Diet. 2018, 118, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Bol, V.V.; Reusens, B.M.; Remacle, C.A. Postnatal Catch-up Growth After Fetal Protein Restriction Programs Proliferation of Rat Preadipocytes. Obesity 2008, 16, 2760–2763. [Google Scholar] [CrossRef] [PubMed]
- Makhijani, P.; Basso, P.J.; Chan, Y.T.; Chen, N.; Baechle, J.; Khan, S.; Furman, D.; Tsai, S.; Winer, D.A. Regulation of the immune system by the insulin receptor in health and disease. Front. Endocrinol. 2023, 14, 1128622. [Google Scholar] [CrossRef]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhao, P.; Pang, K.; Ma, Y.; Huang, H.; Zhou, T.; Yang, X. Antidiabetic Effect of a Flavonoid-Rich Extract from Sophora alopecuroides L. in HFD- and STZ- Induced Diabetic Mice through PKC/GLUT4 Pathway and Regulating PPARα and PPARγ expression. J. Ethnopharmacol. 2020, 2020, 113654. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, S.; Morimoto, M.; Liu, E.; Koike, Y.; Higaki, Y.; Taura, K.; Mamba, K.; Itamoto, K.; Watanabe, T.; Tsutsumi, K.; et al. Overexpression of lipoprotein lipase improves insulin resistance induced by a high-fat diet in transgenic rabbits. Diabetologia 2004, 47, 1202–1209. [Google Scholar] [CrossRef][Green Version]
- Kupska, M.; Wasilewski, T.; Jędrkiewicz, R.; Gromadzka, J. Determination of Terpene Profiles in Potential Superfruits. Int. J. Food Prop. 2016, 19, 2726–2738. [Google Scholar] [CrossRef]
- Yilmaztekin, M. Characterization of Potent Aroma Compounds of Cape Gooseberry (Physalis peruviana L.) Fruits Grown in Antalya Through the Determination of Odor Activity Values. Int. J. Food Prop. 2013, 17, 469–480. [Google Scholar] [CrossRef][Green Version]
- Vaillant, F.; Llano, S.; Ángel, A.; Moreno, N. Main urinary biomarkers of golden berries (Physalis peruviana) following acute and short-term nutritional intervention in healthy human volunteers. Food Res. Int. 2023, 173, 113443. [Google Scholar] [CrossRef]
- Sierra, J.A.; Escobar, J.S.; Corrales-Agudelo, V.; Guzman, O.J.; Mejia, E.P.; Rojas, J.C.; Quintero, A.; Vaillant, F.; Durango, K. Consumption of golden berries (Physalis peruviana L.) might reduce biomarkers of oxidative stress and alter gut permeability in men without changing inflammation status or the gut microbiota. Food Res. Int. 2022, 162, 111949. [Google Scholar] [CrossRef]
- Jeremias, A.; Soodini, G.; Gelfand, E.; Xu, Y.; Stanton, R.C.; Horton, E.S.; Cohen, D.J. Effects of N-acetyl-cysteine on endothelial function and inflammation in patients with type 2 diabetes mellitus. Heart Int. 2009, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Unnithan, A.S.; Jiang, Y.; Rumble, J.L.; Pulugulla, S.H.; Posimo, J.M.; Gleixner, A.M.; Leak, R.K. N-acetyl cysteine prevents synergistic, severe toxicity from two hits of oxidative stress. Neurosci. Lett. 2014, 560, 71–76. [Google Scholar] [CrossRef]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Tan, V.P.; Miyamoto, S. Nutrient-sensing mTORC1: Integration of metabolic and autophagic signals. J. Mol. Cell. Cardiol. 2016, 95, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.; Garcia-Aloy, M.; Vázquez-Manjarrez, N.; Soria-Florido, M.T.; Llorach, R.; Mattivi, F.; Manach, C. Food intake biomarkers for berries and grapes. Genes Nutr. 2020, 15, 17. [Google Scholar] [CrossRef] [PubMed]

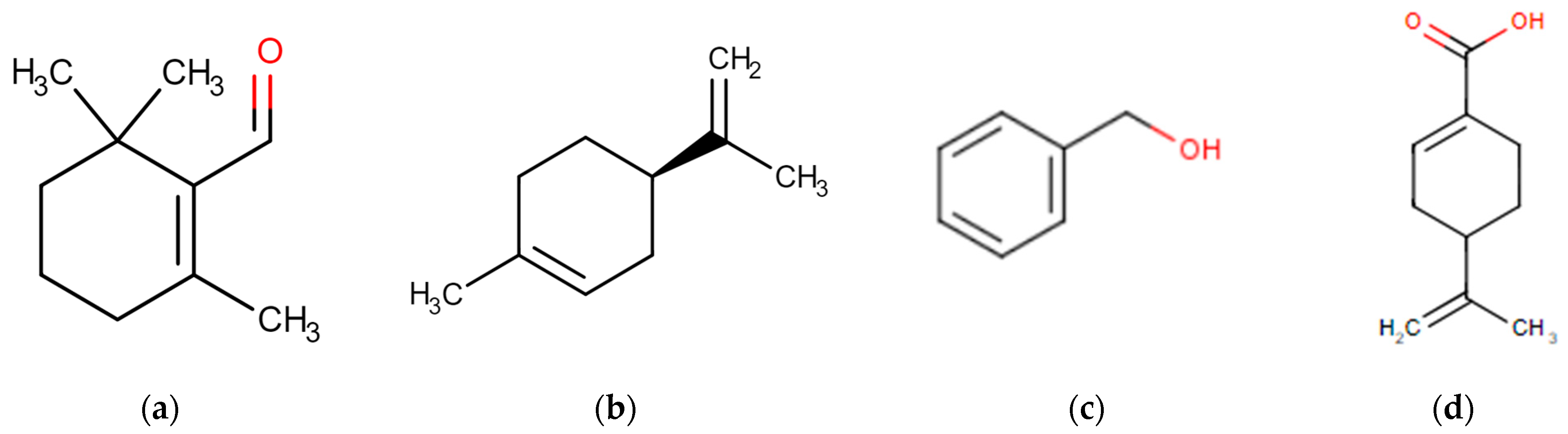
| Diet Type | Protein (%) | Fat (%) | Carbohydrates (%) | Kcal/g |
|---|---|---|---|---|
| SD | 20 | 16 | 64 | 4.8 |
| HFD | 19 | 43 | 38 | 5.37 |
| GB | Supplemented with 8% (w/w) fresh Golden Berry fruit, finely chopped into small pieces, and added to the diet. | |||
| SEX | Anatomical Parameters | SD | SD-GB | HFD | HFD-GB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| MALES | Weight | 370.3 ± 17.89 | 397.2 ± 17.49 | 395 ± 20.88 | 414.4 ± 16.5 | 418.8 ± 1.448 | 527 ± 18.45 b,d,e | 422.2 ± 9.42 | 457.1 ± 9.44 f |
| BMI | 0.218 ± 0.006 | 0.221 ± 0.014 | 0.206 ± 0.002 | 0.195 ± 0.006 | 0.218 ± 0.003 | 0.343 ± 0.035 b,d,e | 0.258 ± 0.005 | 0.258 ± 0.003 f | |
| FEMALES | Weight | 286 ± 9.77 | 311.8 ± 8.46 | 271.1 ± 3.79 | 296.1 ± 1.10 | 279.3 ± 0.54 | 342.1 ± 8.53 d,e | 281.4 ± 10.41 | 310.5 ± 2.53 f,g |
| BMI | 0.191 ± 0.012 | 0.191 ± 0.012 | 0.182 ± 0.002 | 0.181 ± 0.004 | 0.184 ± 0.002 | 0.217 ± 0.007 b,d,e | 0.212 ± 0.009 | 0.219 ± 0.003 b,d | |
| SEX | Biochemical parameters | SD | SD-GB | HFD | HFD-GB | ||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| MALES | GLY | 177 ± 959 | 209 ± 10.17 | 176.5 ± 11.96 | 208.3 ± 10.38 | 131 ± 9.64 | 253.3 ± 4.63 b,d | 129.5 ± 7.03 | 99.25 ± 7.98 b,d,f |
| CHO | 31 ± 3.97 | 40.14 ± 5.87 | 31.17 ± 4.21 | 38.67 ± 5.68 | 29.33 ± 3.48 | 119 ± 9.41 b,d,e | 27.5 ± 2.78 | 26 ± 2 f | |
| TG | 53.23 ± 3.97 | 66 ± 1.41 | 58.67 ± 2.33 | 29.67 ± 1.45 b | 39.61 ± 4.82 | 91.4 ± 9.51 b,d,e | 58.67 ± 3.93 | 35 ± 1.85 b,f | |
| LDL | 198.5 ± 38.78 | 284.8 ± 25.08 a | 77.6 ± 0.76 | 236.4 ± 16.48 c | 91.6 ± 10.29 | 168 ± 21.3 b,e | 78.93 ± 1.07 | 254.5 ± 11.01 g | |
| HDL | 240.1 ± 39.88 | 346.9 ± 21.12 a | 200.3 ± 39.71 | 278.7 ± 15.01 | 132.8 ± 6.55 | 302.5 ± 12.19 e | 122.9 ± 2.23 | 275.1 ± 7.52 g | |
| FEMALES | GLY | 190.2 ± 2.65 | 176 ± 10.97 | 171.6 ± 15.96 | 186.7 ± 16.22 | 144.9 ± 14.04 | 231 ± 10.73 b,e | 165.3 ± 17.45 | 191 ± 13.61 f |
| CHO | 26.33 ± 1.20 | 27.67 ± 0.88 | 24.5 ± 1.26 | 25.67 ± 1.76 | 17.67 ± 1.45 | 53 ± 3.79 b,d,e | 27 ± 1.16 | 32.75 ± 1.38 f | |
| TG | 72.75 ± 1.49 | 72.2 ± 9.48 | 65.8 ± 5.01 | 35.33 ± 1.67 b,d | 74.25 ± 2.39 | 125.3 ± 8.29 b,d,e | 88.2 ± 10.61 | 57.33 ± 3.33 f | |
| LDL | 262.9 ± 3.37 | 260.1 ± 8.60 | 117.1 ± 43.51 | 279.6 ± 5.03 c | 101.1 ± 4.02 | 209.3 ± 10.28 e | 33.33 ± 4.35 | 215.7 ± 4.90 g | |
| HDL | 215.7 ± 35.49 | 340 ± 15.49 a | 159.8 ± 32.39 | 304 ± 7.78 c | 240.1 ± 56.74 | 312.4 ± 9.52 d | 76.29 ± 1.47 | 284.9 ± 7.71 g | |
| SEX | Organ | SD (g/kg) | SD-GB (g/kg) | HFD (g/kg) | HFD-GB (g/kg) |
|---|---|---|---|---|---|
| MALES | Lv | 29.25 ± 4.80 | 25.14 ± 3.93 | 30.61 ± 16.77 a,b | 28.59 ± 12.63 a,b,c |
| Pc | 1.72 ± 0.27 | 1.51 ± 0.70 | 1.63 ± 0.99 | 1.63 ± 0.61 | |
| VAT | 15.70 ± 0.0569 | 8.9 ± 0.1263 | 39.0 ± 0.9886 a,b | 24.19 ± 0.3004 a,b,c | |
| BAT | 4.11 ± 0.0546 | 4.76 ± 0.0320 | 0.93 ± 0.0356 a,b | 2.19 ± 0.0121 a,b,c | |
| SAT | 18.32 ± 0.0832 | 10.76 ± 0.1515 | 40.13 ± 0.2659 a,b | 32.46 ± 0.6124 a,b,c | |
| FEMALES | Lv | 26.49 ± 8.97 | 25.24 ± 99.72 | 31.54 ± 17.05 a,b | 29.04 ± 15.41 a |
| Pc | 1.64 ± 0.50 | 1.55 ± 16.27 | 2.37 ± 8.49 a | 2.11 ± 1.69 a | |
| VAT | 13.67 ± 0.0815 | 12.71 ± 0.0498 | 53.46 ± 0.917 a,b | 45.15 ± 0.9088 a,b,c | |
| BAT | 3.70 ± 0.0105 | 5.66 ± 0.0210 | 1.39 ± 0.0132 a,b | 3.02 ± 0.0119 a,b,c | |
| SAT | 17.33 ± 0.0717 | 13.30 ± 0.1447 | 49.80 ± 0.4652 a | 44.89 ± 0.6132 a |
| Obs m/z [M + H] | Rt (min) | FORMULA | VIP Score | Z | Tentative Identification | HMDB | Error (ppm) | MS2 Measured (%peak) | p-Value | Average Relative Intensity | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TU vs. TS | B/A | Before | After GC | ||||||||
| 432.3470 | 12.55 | C27H45NO3 | 3.29 | 1 | Unknown | 98.0965 (100); 81.07 (10) | 9.5 × 10−3 | <10 | 5,008,467 | ||
| 430.3314 | 12.46 | 2.95 | 1 | Unknown | 98.0965 (100); 81.07 (10) | <10 | 300,000 | ||||
| 380.1914 | 11.73 | C10H16O2-glu-H2O-NH3 | 2.62 | 1 | Geranic acid | 0303972 | 2 | 345.1534 (100); 169.1219 (60) 151.1114 (30) | 3.2 × 10−4 | <10 | 188 |
| 364.1963 | 13.24 | C10H16O-glu-H2O-NH3 | 2.01 | 1 | p-menth-4(8)-ene-1,2-diol | 0035706 | 3 | 329.1594 (20); 153.1270 (100) 135.1165 (100) | 1.3 × 10−2 | <10 | 190,928 |
| 184.0968 | 11.59 | C7H8O-Glcycine | 2.62 | 1 | Benzyl-alcohol glycine | 0003119 | 109.0640 (80) 81.07 (100) | 31 × 10−2 | <10 | 77,000 | |
| 158.1175 | 12.44 | C8H15NO2 | 2.02 | 1 | Unknown | 0004827 | 3 | 4.1 × 10−3 | 20,000 | 5000 | |
| 130.0651 | 14.29 | C9H7N | 1.80 | 1 | Unknown | 0004827 | 0 | 103.05 (100); | 8.4 × 10−4 | 94,000 | 50,000 |
| 167.1067 | 15.12 | C10H14O2 | 1.75 | 1 | Perillic acid | 0000070 | 3 | 149.008 (30) 121.028 (20) 93.07 (80) 79.05 (100) | 3.4 × 10−2 | 5000 | 15,000 |
| 374.2526 | 12.53 | C19H35NO6 | 1.72 | 1 | Dodecanedioyl-L-carnitine | 0013327 | 4 | 231.1585 (50); 130.05 (40);85.02 (35) | 1 × 10−3 | 44,264 | 29,738 |
| 164.0367 | 12.51 | C5H9NO3S | 1.58 | 1 | Unknown | 0001890 | 5 | 122.0269 (100); 76.02 (70) | 2 × 10−2 | 60,000 | 30,000 |
| 245.1595 | 1.04 | C8H18N4O2 + [C2H2O] | 1.55 | 1 | Dimethylarginine acetlylated | 0001539 | 3 | 203.14 (30); 158.08 (100); 115.08 (70); 70.06 (50) | 2 × 10−3 | 5000 | 8000 |
| 146.0600 | 14.29 | C9H7NO2-[O] | 1.36 | 1 | Indolecarboxylic acid | 0002285 | 1 | 118.65 (100) 91.054 (70) 65.039 (60) 128.049 (30) | 2.4 × 10−2 | 50,000 | 25,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ángel-Martín, A.; Vaillant, F.; Moreno-Castellanos, N. Daily Consumption of Golden Berry (Physalis peruviana) Has Been Shown to Halt the Progression of Insulin Resistance and Obesity in Obese Rats with Metabolic Syndrome. Nutrients 2024, 16, 365. https://doi.org/10.3390/nu16030365
Ángel-Martín A, Vaillant F, Moreno-Castellanos N. Daily Consumption of Golden Berry (Physalis peruviana) Has Been Shown to Halt the Progression of Insulin Resistance and Obesity in Obese Rats with Metabolic Syndrome. Nutrients. 2024; 16(3):365. https://doi.org/10.3390/nu16030365
Chicago/Turabian StyleÁngel-Martín, Alberto, Fabrice Vaillant, and Natalia Moreno-Castellanos. 2024. "Daily Consumption of Golden Berry (Physalis peruviana) Has Been Shown to Halt the Progression of Insulin Resistance and Obesity in Obese Rats with Metabolic Syndrome" Nutrients 16, no. 3: 365. https://doi.org/10.3390/nu16030365
APA StyleÁngel-Martín, A., Vaillant, F., & Moreno-Castellanos, N. (2024). Daily Consumption of Golden Berry (Physalis peruviana) Has Been Shown to Halt the Progression of Insulin Resistance and Obesity in Obese Rats with Metabolic Syndrome. Nutrients, 16(3), 365. https://doi.org/10.3390/nu16030365







