Female Athlete Triad and Relative Energy Deficiency in Sport (REDs): Nutritional Management
Abstract
1. Introduction
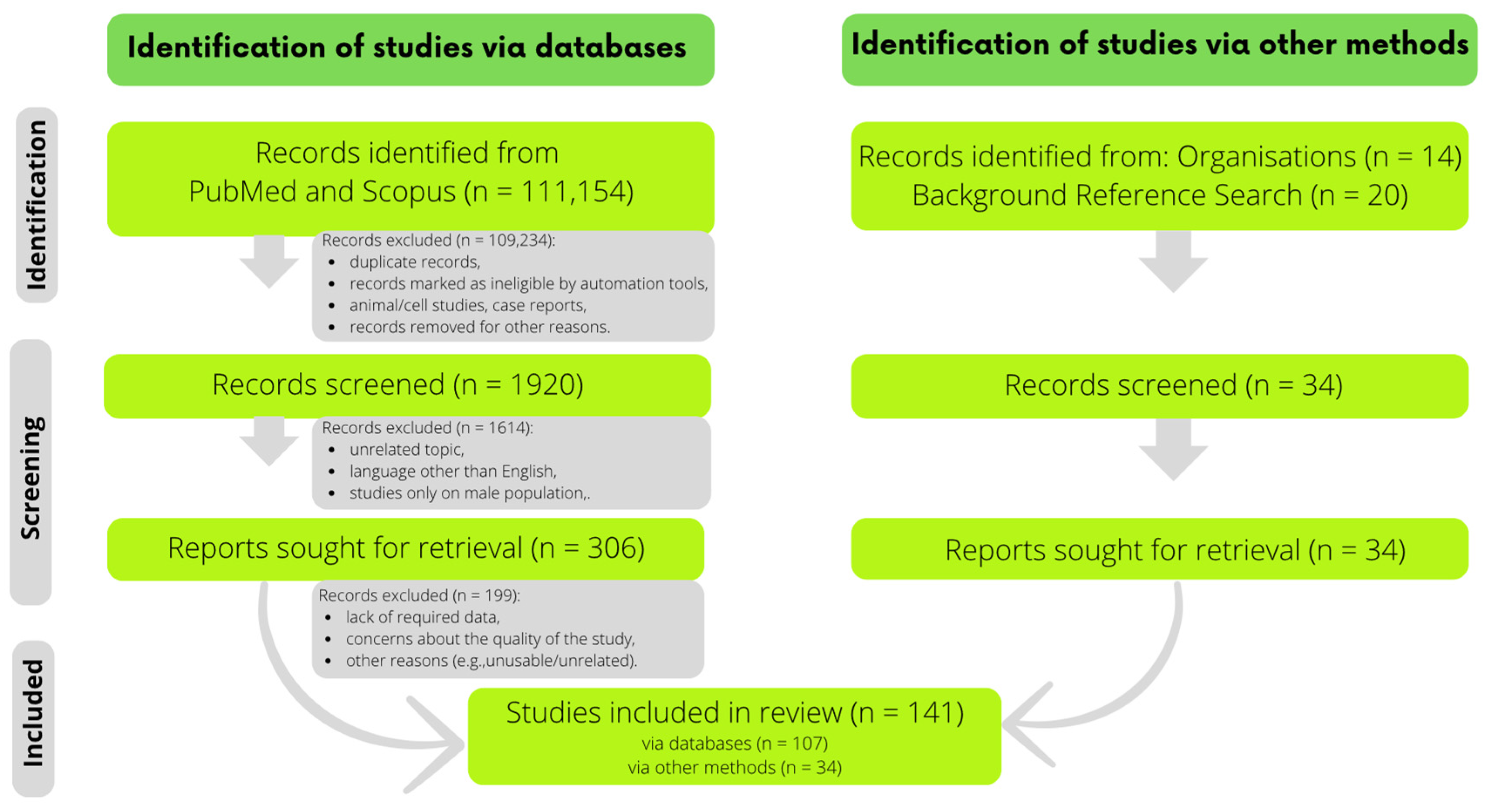
2. Materials and Methods
3. Results
3.1. Low Energy Availability
3.2. Bone Mineral Density
3.3. Menstrual Disorders
3.4. Consumption of Energy, Macronutrients, and Specific Micronutrients among Female Athletes
3.4.1. Exercise Energy Expenditure (EEE) and Energy Availability (EA)
3.4.2. Macronutrients
3.4.3. Selected Micronutrients
| Macro- Nutrients | Norms | Results | Sports Discipline | |||
|---|---|---|---|---|---|---|
| % of Energy or g/Day | g/kg BW | % of Energy | g/Day | g/kg BW | ||
| Proteins | 50 g/day | 1.2–2.0 | 17 * | 56 | n/d | disabled athletes [61] |
| n/d | 1.2 | 14 * ArtGym 13 * Swim | 55 * ArtGym 73 * Swim | 1.0 ± 0.2 ArtGym 1.2 ± 0.2 Swim | artistic gymnastics, swimming [46] | |
| n/d | n/d | 16 * ExAnov 14 * ExOvul 14 * ExLPD | 58 * ExAnov 68 * ExOvul 70 * ExLPD | n/d | running [42] | |
| n/d | n/d | 17 | n/d | n/d | ballet [69] | |
| n/d | 1.2–2.0 | 15 * | 71 | 1.4 ± 0.6 | running [45] | |
| 83 g/day | 1.4–1.7 | 19 * | 72 | 1.2 ± 0.44 | soccer [59] | |
| n/d | 1.2–1.7 | 13 * | 81 | 1.3 ± 0.3 | dancing [47] | |
| n/d | n/d | 13 AM 15 EU | 68 * AM 83 * EU | n/d | endurance sports [14] | |
| n/d | 1.2–2 | 21 | 98 | 1.5 ± 0.5 | soccer [56] | |
| 71 g/day | 1.2–1.7 | 17 | 116 ± 26 | 2.0 ± 0.5 | endurance sports [43] | |
| n/d | 1.3–1.7 | n/d | 80 Interm 77 Advanced 82 Elite | 1.3 (1.2–1.5) Interm 1.4 (1.2–1.6) Advanced 1.4 (1.2–1.9) Elite | climbing [50] | |
| Carbohydrates | n/d | 8–12 | 47 * ArtGym 54 * Swim | 181 * ArtGym 305 * Swim | 3.3 ± 0.8 ArtGym 5.1 ± 1.6 Swim | artistic gymnastics, swimming [46] |
| n/d | 5–7 Up to 1 h exercise/day 6–10 1–3 h exercise/day | 41 * | 192 | 3.0 ± 0.8 | soccer [56] | |
| 296 g/day | 5–7 | 53 * | 199 | 3.3 ± 1.2 | soccer [59] | |
| 130 g/day | n/d | 49 * | 164 | n/d | disabled athletes [61] | |
| n/d | n/d | 56 | 218* | n/d | ballet [69] | |
| n/d | n/d | 62 * ExAnov 60 * ExOvul 57 * ExLPD | 229 * ExAnov 285 * ExOvul 288 * ExLPD | n/d | running [42] | |
| n/d | 6–10 | 53 * | 255 | 4.9 ± 2.1 | running [45] | |
| n/d | n/d | 52 EU 57 AM | 286 * EU 299 * AM | n/d | endurance sports [14] | |
| n/d | 5–7 | 52 * | 313 | 5.0 ± 1.0 | dancing [47] | |
| 353 g/day | 6–10 | 53 | 369 | 6.4 ± 1.6 | endurance sports [43] | |
| n/d | 3–7 | n/d | 282 Interm 228 Advanced 253 Elite | 4.6 (4.0–5.4) Interm 4.2 (3.4–5.3) Advanced 4.2 (4.2–5.8) Elite | climbing [50] | |
| Fats | 15–30% | n/d | 34 * | 52 * | n/d | disabled athletes [61] |
| n/d | n/d | 26 | 45 * | n/d | ballet [69] | |
| 49.2 g/day | n/d | 28 | 47 | 0.78 ± 0.39 | soccer [59] | |
| n/d | n/d | 22 * ExAnov 26 * ExOvul 29 * ExLPD | 36 * ExAnov 54 * ExOvul 64 * ExLPD | n/d | running [42] | |
| 30–40% | n/d | 40 ArtGym 38 Swim | n/d | n/d | artistic gymnastics, swimming [46] | |
| n/d | n/d | 28 EU,AM | 68 EU 65 AM | n/d | endurance sports [14] | |
| n/d | n/d | 32 * | 69 | n/d | running [45] | |
| 20–35% | n/d | 35 | 72 | n/d | soccer [56] | |
| <30% | n/d | 34 | 92 | 1.5 ± 0.4 | dancing [47] | |
| 20–35% | n/d | 30 | 93 | 1.6 ± 0.5 | endurance sports [43] | |
| n/d | n/d | n/d | 70 Interm 60 Advanced 68 Elite | 1.2 (1.0–1.6) Interm 1.1 (0.9–1.3) Advanced 1.2 (0.8–1.2) Elite | climbing [50] | |
| Micronutrient | Norm | Results | Sports Discipline | ||
|---|---|---|---|---|---|
| Vit. D (µg) | 10 [61] 15 [45,60] 20 [46] = 15 | 1.69 | soccer [59] | ||
| 2.5 (1.3–5.9) Interm | 2.66 (0.9–4.1) Elite | 3.9 (1.3–7.2) Advanced | climbing [50] | ||
| 2.6 | disabled athletes [61] | ||||
| 2.8 ± 2.2 HCycl | 3.2 ± 3.1 Swim | 3.4 ± 3.1 Rowers | endurance sports [62] | ||
| 3.4 ± 2.7 LDRun | 3.7 ± 2.5 Skiers | 3.7 ± 3.0 BAthl | |||
| 4.5 ± 0.4 | running [45] | ||||
| 5.5 ± 9.6 Gym | 3.5 ± 3.5 Swim | artistic gymnastics, swimming [46] | |||
| 8.3 ± 7.2 | running [48] | ||||
| Ca (mg) | 700 [61] 800 19–50 y/1000 >50 y /1100 10–18 y [59,60] 1200 [46] 1300 [45] = 1000 | 608 | disabled athletes [61] | ||
| 629 ± 274 Gym | 806 ± 228 Swim | artistic gymnastics, swimming [46] | |||
| 646 ± 290 | soccer [59] | ||||
| 703 (605–817) Advanced | 706 (537–1097) Interm | 857 (753–1107) Elite | climbing [50] | ||
| 706 (332–1542) DE+ (15–19 y) | 819 (93–1738) DE+ (11–14 y) | swimming [66] | |||
| 843 (269–2305) DE− (11–14 y) | 909 (329–2563) DE– (15–19 y) | ||||
| 925 ± 545 | soccer [56] | ||||
| 1000 ± 504 Swim | 1066 ± 407 BAthl | 1117 ± 543 Skiers | endurance sports [62] | ||
| 1163 ± 484 HCycl | 1398 ± 399 Rowers | 1532 ± 1342 LDRun | |||
| 1013 ± 448 ArtGym | 1052 ± 577 RhytGym | 1680 ± 304 Ballet | gymnastics, ballet [65] | ||
| 1046 ± 58.9 | running [45] | ||||
| 1395 ± 684 | running [48] | ||||
| P (mg) | 580 10–18 y/1050 >18 y [59,60] 700 [61] 1250 [45,46] = 900 | 702 | disabled athletes [61] | ||
| 924 ± 192 ArtGym | 1236 ± 188 Swim | artistic gymnastics, swimming [46] | |||
| 1165 ± 357 | soccer [59] | ||||
| 1203 (1044–1451) Advanced | 1370 (1192–1546) Interm | 1535 (1400–1739) Elite | climbing [50] | ||
| 1256 ± 563 RhytGym | 1290 ± 567 ArtGym | 1353 ± 312 Ballet | gymnastics, ballet [65] | ||
| 1341 ± 72 | running [45] | ||||
| 1569 ± 549 | soccer [56] | ||||
| 1646 ± 321 LDRun | 1740 ± 732 Skiers | 1816 ± 552 HCycl | endurance sports [62] | ||
| 1841 ± 520 BAthl | 1865 ± 650 Swim | 2103 ± 546 Rowers | |||
| Ca/P | 0.96 [46] 1 [61] 1.04 [45] 1.05/1.37 [59] = 1 | 0.555 | soccer [59] | ||
| 0.589 | soccer [56] | ||||
| 0.536 Swim | 0.579 BAthl | 0.641 HCycl | endurance sports [62] | ||
| 0.642 Skiers | 0.665 Rowers | 0.931 LDRun | |||
| 0.680 ArtGym | 0.652 Swim | artistic gymnastics, swimming [46] | |||
| 0.780 | running [45] | ||||
| 0.785 ArtGym | 0.838 RhytGym | 0.863 Ballet | gymnastics, ballet [65] | ||
| 0.867 | disabled athletes [61] | ||||
| Mg (mg) | 280 [61] 255/300 [59] 350 [46] 360 [45] = 310 | 245 | soccer [59] | ||
| 292 ± 80 ArtGym | 333 ± 79 Swim | artistic gymnastics, swimming [46] | |||
| 301 ± 115 RhytGym | 309 ± 64 Ballet | 347 ± 183 ArtGym | gymnastics, ballet [65] | ||
| 317 (245–357) Advanced | 383 (322–505) Interm | 473 (411–510) Elite | climbing [50] | ||
| 351 ± 18 | running [45] | ||||
| 368 ± 138 | soccer [56] | ||||
| 448 ± 191 HCycl | 464 ± 150 BAthl | 480 ± 226 Skiers | endurance sports [62] | ||
| 493 ± 163 Rowers | 503 ± 221 Swim | 595 ± 335 LDRun | |||
| Zn (mg) | 7 [46,59] 8 [61] 9 [45] = 8 | 6 (1–14) DE+ (15–19 y) | 7 (1–16) DE+ (11–14 y) | swimming [66] | |
| 7 (2–17) DE− (11–14 y) | 10 (2–120) DE− (15–19 y) | ||||
| 6 | disabled athletes [61] | ||||
| 7 ± 2 ArtGym | 9 ± 6 Swim | artistic gymnastics, swimming [46] | |||
| 8 ± 3 | soccer [59] | ||||
| 10 (7–12) Advanced | 11 (10–12) Interm | 12 (12–13) Elite | climbing [50] | ||
| 10 ± 2 Ballet | 11 ± 9 ArtGym | 12 ± 7 RhytGym | gymnastics, ballet [65] | ||
| 12 ± 1 | running [45] | ||||
| 12 ± 4 | soccer [56] | ||||
| 13 ± 2 LDRun | 14 ± 5 Skiers | 15 ± 4 BAthl | endurance sports [62] | ||
| 15 ± 5 HCycl | 16 ± 6 Swim | 19 ± 6 Rowers | |||
| Fe (mg) | 8 [59] 8/14 [61] 15 [45,46] = 11 | 8 | disabled athletes [61] | ||
| 9 | soccer [59] | ||||
| 9 ± 4 ArtGym | 14 ± 7 Swim | artistic gymnastics, swimming [46] | |||
| 11 ± 3 Ballet | 15 ± 7 ArtGym | 15 ± 9 RhytGym | gymnastics, ballet [65] | ||
| 12 ± 3 | soccer [56] | ||||
| 12 (11–16) Advanced | 17 (14–17) Interm | 20 (18–21) Elite | climbing [50] | ||
| 13 (4–23) DE+, DE− (11–14 y) | 13 (6–27) DE+ (15–19 y) | 16 (7–35) DE− (15–19 y) | swimming [66] | ||
| 16 ± 1 | running [45] | ||||
| 17 ± 5 | middle- and long-distance running, race walking [51] | ||||
| 20 ± 4 LDRun | 24 ± 7 BAthl | 26 ± 11 Skiers | endurance sports [62] | ||
| 27 ± 10 Swim | 27 ± 12 HCycl | 27 ± 7 Rowers | |||
3.5. Nutritional Management of TRIAD/REDs
3.5.1. Overall Approach
3.5.2. Energy Requirement
3.5.3. Macronutrient Requirements
3.5.4. Micronutrient Requirements
| Micronutrient | Dietary Sources | Bioavailability Enhancement | Bioavailability Impairment | Best Bioavailable Forms |
|---|---|---|---|---|
| Vit. D [60,83,84,85,86,87,88,89,90,92] | fat-rich fish (salmon, herring, mackerel), eggs, milk, dairy products and cheese | fat-rich meals, vitamin E | polyunsaturated fatty acids, phytosterols, long-chain fatty acids | “exposure to sunlight”, cholecalciferol |
| Ca [60,83,97,98,99,100,101,102] | milk, milk-based foods, kale, parsley leaves, spinach, bean seeds | lactose, vitamin D, phosphopeptides | oxalic acid (spinach, rhubarb, beans), phytic acid (seeds, nuts, grains, certain raw beans and soy isolates), insoluble fibre fractions, high phosphorus content | calcium carbonate, gluconate |
| P [83,107,108,109,110,111] | rennet cheese, buckwheat groats, fish, offal, meat, wholegrain bread, legumes, eggs | phosphorus from animal products, activation of phytases in plant products (sprouting process, soaking legumes, using sourdough to bake bread) | calcium, vitamin D deficiency | organic phosphate esters, ionised inorganic forms |
| Mg [60,68,83,114,115,116,117,118] | cereals, legumes, nuts, cocoa, dark chocolate, rennet cheese, potatoes, bananas, drinking water | fermentation of soluble fibre fractions, acidic pH, vitamin B6- pyridoxine | phytic acid, phosphates, calcium | magnesium carbonate, citrate, aspartate, hydroaspartate, lactate |
| Zn [60,99,121,123,124,125,126] | meat, liver, rennet cheese, dark bread, buckwheat groats, eggs | citric acid, animal protein | phytic acid, oxalic acid, insoluble fibre fractions, alcohol, iron, cadmium | zinc gluconate, citrate, picolinate |
| Fe [60,68,128,133,134,135,136,137,138,139,140,141] | meat, liver, kidney, parsley, legumes, eggs | ascorbic acid, lactic acid, fermented products | phytic acid, oxalic acid, insoluble fibre fractions, plant protein, polyphenols | haem iron, ferrous sulphate, gluconate, ferric citrate, sulphate |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G.; et al. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference Held in San Francisco, CA, May 2012, and 2nd: International Conference Held in Indianapolis, IN, May 2013. Clin. J. Sport Med. 2014, 24, 96–119. [Google Scholar] [CrossRef]
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; AnHackney, C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 2023, 57, 1073–1097. [Google Scholar] [CrossRef]
- Holtzman, B.; Ackerman, K.E. Recommendations and Nutritional Considerations for Female Athletes: Health and Performance. Sports Med. 2021, 51, 43–57. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Portolés, O.; Sotos-Prieto, M.; Fernández-Carrión, R.; Ramirez-Sabio, J.B.; Zanón-Moreno, V.; Mattei, J.; Sorlí, J.V.; Ordovas, J.M. A Guide to Applying the Sex-Gender Perspective to Nutritional Genomics. Nutrients 2018, 11, 4. [Google Scholar] [CrossRef]
- Purcell, L.K. Sport nutrition for young athletes. Paediatr. Child. Health 2013, 18, 200–205. [Google Scholar] [CrossRef]
- Close, G.L.; Sale, C.; Baar, K.; Bermon, S. Nutrition for the Prevention and Treatment of Injuries in Track and Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 189–197. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Rehabilitation Nutrition for Injury Recovery of Athletes: The Role of Macronutrient Intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; McDonnell, S.J.; Corish, C.A. Low Energy Availability in Athletes: A Review of Prevalence, Dietary Patterns, Physiological Health, and Sports Performance. Sports Med. 2018, 48, 73–96. [Google Scholar] [CrossRef]
- De Souza, M.J.; Koltun, K.J.; Etter, C.V.; Southmayd, E.A. Current Status of the Female Athlete Triad: Update and Future Directions. Curr. Osteoporos. Rep. 2017, 15, 577–587. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; Available online: http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 (accessed on 25 August 2022).
- Muia, E.N.; Wright, H.H.; Onywera, V.O.; Kuria, E.N. Adolescent elite Kenyan runners are at risk for energy deficiency, menstrual dysfunction and disordered eating. J. Sports Sci. 2016, 34, 598–606. [Google Scholar] [CrossRef]
- Gibbs, J.C.; Williams, N.I.; Mallinson, R.J.; Reed, J.L.; Rickard, A.D.; DE Souza, M.J. Effect of high dietary restraint on energy availability and menstrual status. Med. Sci. Sports Exerc. 2013, 45, 1790–1797. [Google Scholar] [CrossRef]
- Reed, J.L.; De Souza, M.J.; Williams, N.I. Changes in energy availability across the season in Division I female soccer players. J. Sports Sci. 2013, 31, 314–324. [Google Scholar] [CrossRef]
- Schaal, K.; Van Loan, M.D.; Casazza, G.A. Reduced catecholamine response to exercise in amenorrheic athletes. Med. Sci. Sports Exerc. 2011, 43, 34–43. [Google Scholar] [CrossRef]
- Johnson, R.K. Dietary Intake—How Do We Measure What People Are Really Eating? Obes. Res. 2002, 10, 63S–68S. [Google Scholar] [CrossRef]
- Probst, Y.; Zammit, G. Predictors for Reporting of Dietary Assessment Methods in Food-based Randomized Controlled Trials over a Ten-year Period. Crit. Rev. Food Sci. Nutr. 2016, 56, 2069–2090. [Google Scholar] [CrossRef]
- Bailey, R.L. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar] [CrossRef]
- Subar, A.F.; Freedman, L.S.; Tooze, J.A.; Kirkpatrick, S.I.; Boushey, C.; Neuhouser, M.L.; Thompson, F.E.; Potischman, N.; Guenther, P.M.; Tarasuk, V.; et al. Addressing Current Criticism Regarding the Value of Self-Report Dietary Data. J. Nutr. 2015, 145, 2639–2645. [Google Scholar] [CrossRef]
- Freedman, L.S.; Midthune, D.; Arab, L.; Prentice, R.L.; Subar, A.F.; Willett, W.; Neuhouser, M.L.; Tinker, L.F.; Kipnis, V. Combining a Food Frequency Questionnaire With 24-Hour Recalls to Increase the Precision of Estimation of Usual Dietary Intakes-Evidence From the Validation Studies Pooling Project. Am. J. Epidemiol. 2018, 187, 2227–2232. [Google Scholar] [CrossRef]
- Dave, S.C.; Fisher, M. Relative energy deficiency in sport (RED–S). Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101242. [Google Scholar] [CrossRef]
- Rogers, M.A.; Appaneal, R.N.; Hughes, D.; Vlahovich, N.; Waddington, G.; Burke, L.M.; Drew, M. Prevalence of impaired physiological function consistent with Relative Energy Deficiency in Sport (RED-S): An Australian elite and pre-elite cohort. Br. J. Sports Med. 2021, 55, 38. [Google Scholar] [CrossRef]
- Areta, J.L.; Taylor, H.L.; Koehler, K. Low energy availability: History, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur. J. Appl. Physiol. 2021, 121, 1–21. [Google Scholar] [CrossRef]
- Dervish, R.A.; Wilson, L.J.; Curtis, C. Investigating the prevalence of low energy availability, disordered eating and eating disorders in competitive and recreational female endurance runners. Eur. J. Sport Sci. 2022, 23, 869–876. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Faber, J.; Ritz, C.; Sjödin, A.; Sundgot-Borgen, J. The LEAF questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. Br. J. Sports Med. 2014, 48, 540–545. [Google Scholar] [CrossRef]
- McNulty, K.Y.; Adams, C.H.; Anderson, J.M.; Affenito, S.G. Development and validation of a screening tool to identify eating disorders in female athletes. J. Am. Diet. Assoc. 2001, 101, 886–892, quiz 893–884. [Google Scholar] [CrossRef]
- Martinsen, M.; Holme, I.; Pensgaard, A.M.; Torstveit, M.K.; Sundgot-Borgen, J. The development of the brief eating disorder in athletes questionnaire. Med. Sci. Sports Exerc. 2014, 46, 1666–1675. [Google Scholar] [CrossRef]
- Sharps, F.R.J.; Wilson, L.J.; Graham, C.A.-M.; Curtis, C. Prevalence of disordered eating, eating disorders and risk of low energy availability in professional, competitive and recreational female athletes based in the United Kingdom. Eur. J. Sport Sci. 2021, 22, 1445–1451. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Areta, J.L. Low energy availability in female athletes: From the lab to the field. Eur. J. Sport Sci. 2022, 22, 709–719. [Google Scholar] [CrossRef]
- Saunier, J.; Chapurlat, R. Stress fracture in athletes. Jt. Bone Spine 2018, 85, 307–310. [Google Scholar] [CrossRef]
- Da Rocha Lemos Costa, T.M.; Borba, V.Z.C.; Correa, R.G.P.; Moreira, C.A. Stress fractures. Arch. Endocrinol. Metab. 2022, 66, 765–773. [Google Scholar] [CrossRef]
- Abbott, A.; Bird, M.L.; Wild, E.; Brown, S.M.; Stewart, G.; Mulcahey, M.K. Part I: Epidemiology and risk factors for stress fractures in female athletes. Phys. Sportsmed. 2020, 48, 17–24. [Google Scholar] [CrossRef]
- Barrack, M.T.; Gibbs, J.C.; De Souza, M.J.; Williams, N.I.; Nichols, J.F.; Rauh, M.J.; Nattiv, A. Higher Incidence of Bone Stress Injuries With Increasing Female Athlete Triad–Related Risk Factors: A Prospective Multisite Study of Exercising Girls and Women. Am. J. Clin. 2014, 42, 949–958. [Google Scholar] [CrossRef]
- Lappe, J.; Cullen, D.; Haynatzki, G.; Recker, R.; Ahlf, R.; Thompson, K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Miner. Res. 2008, 23, 741–749. [Google Scholar] [CrossRef]
- Moran, J.M. Nutrition and Women’s Bone Health. Nutrients 2022, 14, 763. [Google Scholar] [CrossRef]
- Zhang, Q. New Insights into Nutrients for Bone Health and Disease. Nutrients 2023, 15, 2648. [Google Scholar] [CrossRef]
- Matzkin, E.; Curry, E.J.; Whitlock, K. Female Athlete Triad: Past, Present, and Future. J. Am. Acad. Orthop. Surg. 2015, 23, 424–432. [Google Scholar] [CrossRef]
- Podfigurna, A.; Meczekalski, B. Functional Hypothalamic Amenorrhea: A Stress-Based Disease. Endocrines 2021, 2, 203–211. [Google Scholar] [CrossRef]
- Barrack, M.T.; Rauh, M.J.; Nichols, J.F. Prevalence of and traits associated with low BMD among female adolescent runners. Med. Sci. Sports Exerc. 2008, 40, 2015–2021. [Google Scholar] [CrossRef]
- Williams, N.I.; Helmreich, D.L.; Parfitt, D.B.; Caston-Balderrama, A.; Cameron, J.L. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J. Clin. Endocrinol. Metab. 2001, 86, 5184–5193. [Google Scholar] [CrossRef]
- De Souza, M.J.; Williams, N.I. Beyond hypoestrogenism in amenorrheic athletes: Energy deficiency as a contributing factor for bone loss. Curr. Sports Med. Rep. 2005, 4, 38–44. [Google Scholar] [CrossRef]
- De Souza, M.J.; Mallinson, R.J.; Strock, N.C.A.; Koltun, K.J.; Olmsted, M.P.; Ricker, E.A.; Scheid, J.L.; Allaway, H.C.; Mallinson, D.J.; Don, P.K.; et al. Randomised controlled trial of the effects of increased energy intake on menstrual recovery in exercising women with menstrual disturbances: The ‘REFUEL’ study. Hum. Reprod. 2021, 36, 2285–2297. [Google Scholar] [CrossRef]
- De Souza, M.J.; Miller, B.E.; Loucks, A.B.; Luciano, A.A.; Pescatello, L.S.; Campbell, C.G.; Lasley, B.L. High frequency of luteal phase deficiency and anovulation in recreational women runners: Blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J. Clin. Endocrinol. Metab. 1998, 83, 4220–4232. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Møller, S.S.; Faber, J.; Sundgot-Borgen, J.; Sjödin, A. Low-energy density and high fiber intake are dietary concerns in female endurance athletes. Scand. J. Med. Sci. Sports 2016, 26, 1060–1071. [Google Scholar] [CrossRef]
- Loucks, A.B.; Thuma, J.R. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 2003, 88, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.A.; Barrack, M.T.; Gray, V.B.; Cotter, J.A.; Van Loan, M.D.; Rauh, M.J.; McGowan, R.; Nichols, J.F. Adolescent Endurance Runners Exhibit Suboptimal Energy Availability and Intakes of Key Nutrients. J. Am. Nutr. Assoc. 2022, 41, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Jakše, B.; Jakše, B.; Mis, N.F.; Jug, B.; Šajber, D.; Godnov, U.; Čuk, I. Nutritional Status and Cardiovascular Health in Female Adolescent Elite-Level Artistic Gymnasts and Swimmers: A Cross-Sectional Study of 31 Athletes. J. Nutr. Metab. 2021, 2021, 8810548. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Howatson, G.; Quin, E.; Redding, E.; Stevenson, E.J. Energy intake and energy expenditure of pre-professional female contemporary dancers. PLoS ONE 2017, 12, e0171998. [Google Scholar] [CrossRef] [PubMed]
- McCormack, W.P.; Shoepe, T.C.; LaBrie, J.; Almstedt, H.C. Bone mineral density, energy availability, and dietary restraint in collegiate cross-country runners and non-running controls. Eur. J. Appl. Physiol. 2019, 119, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Day, J.; Wengreen, H.; Heath, E. Prevalence of Low Energy Availability in Collegiate Female Runners and Implementation of Nutrition Education Intervention. J. Nutr. Sci. Res. 2016, 1, 1. [Google Scholar] [CrossRef]
- Chmielewska, A.; Regulska-Ilow, B. The Evaluation of Energy Availability and Dietary Nutrient Intake of Sport Climbers at Different Climbing Levels. Int. J. Environ. Res. Public Health 2023, 20, 5176. [Google Scholar] [CrossRef]
- Heikura, I.A.; Burke, L.M.; Bergland, D.; Uusitalo, A.L.; Mero, A.A.; Stellingwerff, T. Impact of Energy Availability, Health, and Sex on Hemoglobin-Mass Responses Following Live-High-Train-High Altitude Training in Elite Female and Male Distance Athletes. Int. J. Sports Physiol. Perform. 2018, 13, 1090–1096. [Google Scholar] [CrossRef]
- Melin, A.; Tornberg, Å.B.; Skouby, S.; Møller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sidelmann, J.J.; Aziz, M.; Sjödin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports 2015, 25, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Hausswirth, C.; Le Meur, Y. Physiological and Nutritional Aspects of Post-Exercise Recovery. Sports Med. 2011, 41, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.S.; Sanford, T.C.; Davidson, M.M.; Yakushko, O.F.; Beck, N.C. Nutrient Intakes and Dietary Behaviors of Male and Female Collegiate Athletes. Int. J. Sport. Nutr. Exerc. Metab. 2004, 14, 389–405. [Google Scholar] [CrossRef]
- Gibson, J.C.; Stuart-Hill, L.; Martin, S.; Gaul, C. Nutrition Status of Junior Elite Canadian Female Soccer Athletes. Int. J. Sport. Nutr. Exerc. Metab. 2011, 21, 507–514. [Google Scholar] [CrossRef]
- Condo, D.; Lohman, R.; Kelly, M.; Carr, A. Nutritional Intake, Sports Nutrition Knowledge and Energy Availability in Female Australian Rules Football Players. Nutrients 2019, 11, 971. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27. [Google Scholar] [CrossRef]
- Dobrowolski, H.; Włodarek, D. Dietary Intake of Polish Female Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 1134. [Google Scholar] [CrossRef]
- Jarosz, M.R.E.; Stoś, K.; Charzewska, J. Nutrition Standards for the Population of Poland and Their Application. Available online: https://ncez.pzh.gov.pl/abc-zywienia/normy-zywienia-2020/ (accessed on 20 March 2022).
- Jeoung, B.; Kim, J. Analysis and Evaluation of Nutritional Intake and Nutrition Quotient of Korean Athletes with Disabilities in the Tokyo Paralympic Games. Nutrients 2021, 13, 3631. [Google Scholar] [CrossRef]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Žagminas, K.; Šurkienė, G.; Švedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravičius, J.A. Nutritional habits among high-performance endurance athletes. Medicina 2015, 51, 351–362. [Google Scholar] [CrossRef]
- Kemi, V.E.; Rita, H.J.; Kärkkäinen, M.U.; Viljakainen, H.T.; Laaksonen, M.M.; Outila, T.A.; Lamberg-Allardt, C.J. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: A cross-sectional study on healthy premenopausal women. Public Health Nutr. 2009, 12, 1885–1892. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Porter, A.K.; Viggeswarapu, M.; Roberts, J.L.; Beck, G.R., Jr. Effects of phosphorus and calcium to phosphorus consumption ratio on mineral metabolism and cardiometabolic health. J. Nutr. Biochem. 2020, 80, 108374. [Google Scholar] [CrossRef]
- Soric, M.; Misigoj-Durakovic, M.; Pedisic, Z. Dietary intake and body composition of prepubescent female aesthetic athletes. Int. J. Sport. Nutr. Exerc. Metab. 2008, 18, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, N.F.; Schtscherbyna, A.; Soares, E.A.; Ribeiro, B.G. Disordered eating among adolescent female swimmers: Dietary, biochemical, and body composition factors. Nutrition 2013, 29, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Alaunyte, I.; Stojceska, V.; Plunkett, A. Iron and the female athlete: A review of dietary treatment methods for improving iron status and exercise performance. J. Int. Soc. Sports Nutr. 2015, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P. Trace Minerals of Concern for Female Athletes: Iron and Zinc. In Nutrition and the Female Athlete: From Research to Practice; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Doyle-Lucas, A.F.; Akers, J.D.; Davy, B.M. Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J. Dance Med. Sci. 2010, 14, 146–154. [Google Scholar] [CrossRef]
- Ihle, R.; Loucks, A.B. Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 2004, 19, 1231–1240. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P.; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef] [PubMed]
- Manore, M.M. Weight management in the performance athlete. Nestle Nutr. Inst. Workshop Ser. 2013, 75, 123–133. [Google Scholar] [CrossRef]
- Shaw, G.; Boyd, K.T.; Burke, L.M.; Koivisto, A. Nutrition for swimming. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 360–372. [Google Scholar] [CrossRef]
- Sims, S.T.; Kerksick, C.M.; Smith-Ryan, A.E.; de Jonge, X.A.J.; Hirsch, K.R.; Arent, S.M.; Hewlings, S.J.; Kleiner, S.M.; Bustillo, E.; Tartar, J.L.; et al. International society of sports nutrition position stand: Nutritional concerns of the female athlete. J. Int. Soc. Sports Nutr. 2023, 20, 2204066. [Google Scholar] [CrossRef]
- Phillips, S.M.; Van Loon, L.J.C. Dietary protein for athletes: From requirements to optimum adaptation. J. Sports Sci. 2011, 29, S29–S38. [Google Scholar] [CrossRef]
- Wall, B.T.; Morton, J.P.; Van Loon, L.J.C. Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. Eur. J Sport Sci. 2015, 15, 53–62. [Google Scholar] [CrossRef]
- Houltham, S.D.; Rowlands, D.S. A snapshot of nitrogen balance in endurance-trained women. Appl. Physiol. Nutr. Metab. 2014, 39, 219–225. [Google Scholar] [CrossRef]
- Rodriguez, N.R.; Vislocky, L.M.; Gaine, P.C. Dietary protein, endurance exercise, and human skeletal-muscle protein turnover. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 40–45. [Google Scholar] [CrossRef]
- Rossi, K.A. Nutritional Aspects of the Female Athlete. Clin. Sports Med. 2017, 36, 627–653. [Google Scholar] [CrossRef]
- Paul, D.R.; Mulroy, S.M.; Horner, J.A.; Jacobs, K.A.; Lamb, D.R. Carbohydrate-Loading during the Follicular Phase of the Menstrual Cycle: Effects on Muscle Glycogen and Exercise Performance. Int. J. Sport. Nutr. Exerc. Metab. 2001, 11, 430–441. [Google Scholar] [CrossRef]
- Ruiz-Castellano, C.; Espinar, S.; Contreras, C.; Mata, F.; Aragon, A.A.; Martínez-Sanz, J.M. Achieving an Optimal Fat Loss Phase in Resistance-Trained Athletes: A Narrative Review. Nutrients 2021, 13, 3255. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Redzic, M.; Thomas, D.T. The Effects of Season-Long Vitamin D Supplementation on Collegiate Swimmers and Divers. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 431–440. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council (NHMRC). Nutrient Reference Values for Australian and New Zealand. Available online: https://www.nhmrc.gov.au/sites/default/files/images/nutrient-refererence-dietary-intakes.pdf (accessed on 20 August 2022).
- Nakamura, K.; Nashimoto, M.; Okuda, Y.; Ota, T.; Yamamoto, M. Fish as a major source of vitamin D in the Japanese diet. Nutrition 2002, 18, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Bischofova, S.; Dofkova, M.; Blahova, J.; Kavrik, R.; Nevrla, J.; Rehurkova, I.; Ruprich, J. Dietary Intake of Vitamin D in the Czech Population: A Comparison with Dietary Reference Values, Main Food Sources Identified by a Total Diet Study. Nutrients 2018, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, F.V.; Lang, M.A.B.; Scopel, L.; Marcondes, N.A.; Araújo, M.G.A.; Faulhaber, G.A.M.; Furlanetto, T.W. Effect of fat on serum 25-hydroxyvitamin D levels after a single oral dose of vitamin D in young healthy adults: A double-blind randomized placebo-controlled study. Eur. J. Nutr. 2015, 54, 391–396. [Google Scholar] [CrossRef]
- Argao, E.A.; Heubi, J.E.; Hollis, B.W.; Tsang, R.C. d-Alpha-tocopheryl polyethylene glycol-1000 succinate enhances the absorption of vitamin D in chronic cholestatic liver disease of infancy and childhood. Pediatr. Res. 1992, 31, 146–150. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Lichtenstein, A.H.; Dolnikowski, G.; Palermo, N.J.; Rasmussen, H. Dietary fat increases vitamin D-3 absorption. J. Acad. Nutr. Diet. 2015, 115, 225–230. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D supplementation: Cholecalciferol, calcifediol, and calcitriol. Eur. J. Clin. Nutr. 2020, 74, 1493–1497. [Google Scholar] [CrossRef]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; da Silva, D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin D and its metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef]
- Barrack, M.T.; Van Loan, M.D.; Rauh, M.J.; Nichols, J.F. Physiologic and behavioral indicators of energy deficiency in female adolescent runners with elevated bone turnover. Am. J. Clin. 2010, 92, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; DiMarco, N.M.; Langley, S. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Am. Diet. Assoc. 2009, 109, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Spence, K. Nutrients Needed for Optimal Bone Health in the Female Athlete. In Nutrition and the Female Athlete: From Research to Practice; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Deldicque, L.; Francaux, M. Recommendations for Healthy Nutrition in Female Endurance Runners: An Update. Front. Nutr. 2015, 2, 17. [Google Scholar] [CrossRef]
- Shkembi, B.; Huppertz, T. Calcium Absorption from Food Products: Food Matrix Effects. Nutrients 2021, 14, 180. [Google Scholar] [CrossRef]
- Cormick, G.; Belizán, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- Alpers, D.H. Intraluminal bioavailability of divalent cations. Curr. Opin. Gastroenterol. 2013, 29, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef]
- Szalast-Pietrzak, A.; Marzec, Z.; Kopciał, E.; Wiater, S. Influence of food products on bioavailability of calcium from dietary supplements used in osteoporosis. Probl. Hig. Epid. 2017, 98, 345–349. [Google Scholar]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, R.Y.; Brouwers, J.R.; Geusens, P.P.; Lems, W.F.; van den Bergh, J.P. Calcium and vitamin D supplementation: State of the art for daily practice. Food Nutr. Res. 2014, 58, 21796. [Google Scholar] [CrossRef] [PubMed]
- Song, L. Calcium and Bone Metabolism Indices. Adv. Clin. Chem. 2017, 82, 1–46. [Google Scholar] [CrossRef]
- Malliaropoulos, N.; Tsitas, K.; Porfiriadou, A.; Papalada, A.R.; Ames, P.; Del Buono, A.; Lippi, G.; Maffulli, N. Blood phosphorus and magnesium levels in 130 elite track and field athletes. Asian J. Sports Med. 2013, 4, 49–53. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on Dietary Reference Values for phosphorus. EFSA J. 2013, 13, 4185. [Google Scholar]
- Cupisti, A.; Kalantar-Zadeh, K. Management of natural and added dietary phosphorus burden in kidney disease. Semin. Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef]
- Cases, A.; Cigarrán-Guldrís, S.; Mas, S.; Gonzalez-Parra, E. Vegetable-Based Diets for Chronic Kidney Disease? It Is Time to Reconsider. Nutrients 2019, 11, 1263. [Google Scholar] [CrossRef]
- Trautvetter, U.; Ditscheid, B.; Jahreis, G.; Glei, M. Habitual Intakes, Food Sources and Excretions of Phosphorus and Calcium in Three German Study Collectives. Nutrients 2018, 10, 171. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Contributions to total phosphorus intake: All sources considered. Semin. Dial. 2013, 26, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar] [PubMed]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef]
- Blancquaert, L.; Vervaet, C.; Derave, W. Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients 2019, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Karmańska, A.; Stańczak, A.; Karwowski, B. Magnez aktualny stan wiedzy. Bromat. Chem. Toksyko 2015, 48, 677–689. [Google Scholar]
- James, J.D.; James, H.O.K.; William, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- De Souza, M.C.; Walker, A.F.; Robinson, P.A.; Bolland, K. A synergistic effect of a daily supplement for 1 month of 200 mg magnesium plus 50 mg vitamin B6 for the relief of anxiety-related premenstrual symptoms: A randomized, double-blind, crossover study. J. Womens Health Gend. Based Med. 2000, 9, 131–139. [Google Scholar] [CrossRef]
- Pardo, M.R.; Garicano Vilar, E.; San Mauro Martín, I.; Camina Martín, M.A. Bioavailability of magnesium food supplements: A systematic review. Nutrition 2021, 89, 111294. [Google Scholar] [CrossRef]
- DeRuisseau, K.C.; Cheuvront, S.N.; Haymes, E.M.; Sharp, R.G. Sweat iron and zinc losses during prolonged exercise. Int. J. Sport. Nutr. Exerc. Metab. 2002, 12, 428–437. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Vicente-García, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 2020, 35, 101529. [Google Scholar] [CrossRef] [PubMed]
- Prosser, N.R.; Heath, A.L.; Williams, S.M.; Gibson, R.S. Influence of an iron intervention on the zinc status of young adult New Zealand women with mild iron deficiency. Br. J. Nutr. 2010, 104, 742–750. [Google Scholar] [CrossRef]
- Eskici, G.; Gunay, M.; Baltaci, A.K.; Mogulkoc, R. The effect of zinc supplementation on the urinary excretion of elements in female athletes. Pak. J. Pharm. Sci. 2016, 29, 125–129. [Google Scholar] [PubMed]
- Ferguson, E.L.; Gibson, R.S.; Thompson, L.U.; Ounpuu, S. Dietary calcium, phytate, and zinc intakes and the calcium, phytate, and zinc molar ratios of the diets of a selected group of East African children. Am. J. Clin. Nutr. 1989, 50, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478s–1483s. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, L.; Almgren, A.; Sandström, B.; Hurrell, R.F. Zinc absorption in adult humans: The effect of iron fortification. Br. J. Nutr. 1995, 74, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Wegmüller, R.; Tay, F.; Zeder, C.; Brnic, M.; Hurrell, R.F. Zinc absorption by young adults from supplemental zinc citrate is comparable with that from zinc gluconate and higher than from zinc oxide. J. Nutr. 2014, 144, 132–136. [Google Scholar] [CrossRef]
- DellaValle, D.M.; Haas, J.D. Iron status is associated with endurance performance and training in female rowers. Med. Sci. Sports Exerc. 2012, 44, 1552–1559. [Google Scholar] [CrossRef]
- McClung, J. Iron, Zinc, and Physical Performance. Biol. Trace Elem. Res. 2019, 188, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Rubeor, A.; Goojha, C.; Manning, J.; White, J. Does iron supplementation improve performance in iron-deficient nonanemic athletes? Sports Health 2018, 10, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Binnie, M.J.; Goods, P.S.; Sim, M.; Burke, L.M. Evidence-based supplements for the enhancement of athletic performance. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 178–187. [Google Scholar] [CrossRef] [PubMed]
- DellaValle, D.M. Iron supplementation for female athletes: Effects on iron status and performance outcomes. Curr. Sports Med. Rep. 2013, 12, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Cowell, B.S.; Rosenbloom, C.A.; Skinner, R.; Summers, S.H. Policies on Screening Female Athletes for Iron Deficiency in NCAA Division I-A Institutions. Int. J. Sport. Nutr. Exerc. Metab. 2003, 13, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral iron supplementation in iron-deficient women: How much and how often? Mol. Aspects Med. 2020, 75, 100865. [Google Scholar] [CrossRef]
- Powers, J.M.; Buchanan, G.R.; Adix, L.; Zhang, S.; Gao, A.; McCavit, T.L. Effect of Low-Dose Ferrous Sulfate vs Iron Polysaccharide Complex on Hemoglobin Concentration in Young Children With Nutritional Iron-Deficiency Anemia: A Randomized Clinical Trial. JAMA 2017, 317, 2297–2304. [Google Scholar] [CrossRef]
- Gowin, E.; Horst-Sikorska, W. Iron stores—Who is at risk of iron deficiency in 21st century? Contemp. Pharm. 2010, 3, 139–146. [Google Scholar]
- Brune, M.; Rossander-Hultén, L.; Hallberg, L.; Gleerup, A.; Sandberg, A.S. Iron absorption from bread in humans: Inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J. Nutr. 1992, 122, 442–449. [Google Scholar] [CrossRef]
- Cook, J.D.; Hurrell, R.F.; Reddy, M. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Rosado, J.L.; Allen, L.H.; Abrams, S.; García, O.P. The efficacy of a local ascorbic acid-rich food in improving iron absorption from Mexican diets: A field study using stable isotopes. Am. J. Clin. Nutr. 2003, 78, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Shrestha, J.; LeClerq, S.C.; Khatry, S.K.; Jiang, T.; Wagner, T.; Katz, J.; West, K.P. Supplementation with micronutrients in addition to iron and folic acid does not further improve the hematologic status of pregnant women in rural Nepal. J. Nutr. 2003, 133, 3492–3498. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Results | Sports Discipline |
|---|---|---|
| EEE (kcal/day) | 272 ± 78 ExAnov 480 ± 53 ExOvul 494 ± 64 ExLPD | running [42] |
| 591 ± 95 | running [11] | |
| 600 ± 237 | running [45] | |
| 800 ± 132 EU 1300 ± 293 AM | endurance sports [14] | |
| 921 ± 256 | running [48] | |
| 940 ± 450 | endurance sports [43] | |
| EA (kcal/kg FFM per day) | 18.8 ± 3.2 ExAnov 23.3 ± 1.6 ExOvul 26.5 ± 1.8 ExLPD | running [42] |
| 18 ± 6.6 AM 29 ± 4.8 EU | endurance sports [14] | |
| 23 ± 3 ArtGym 33 ± 10 Swim | artistic gymnastics, swimming [46] | |
| 26 ± 13 | dancing [47] | |
| 29.6 ± 17.4 | running [45] | |
| 30.7 | running [49] | |
| 31.6 (21.2–37.6) | climbing [50] | |
| 33 ± 7 | middle- and long-distance running, race walking [51] | |
| 36.5 ± 4.5 | running [11] | |
| 37 ± 21 | running [48] | |
| 39.6 (35.3–43.9) | endurance sports [52] | |
| 42.5 ± 12.1 | endurance sports [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabia, M.; Perkowski, J.; Socha, K.; Markiewicz-Żukowska, R. Female Athlete Triad and Relative Energy Deficiency in Sport (REDs): Nutritional Management. Nutrients 2024, 16, 359. https://doi.org/10.3390/nu16030359
Grabia M, Perkowski J, Socha K, Markiewicz-Żukowska R. Female Athlete Triad and Relative Energy Deficiency in Sport (REDs): Nutritional Management. Nutrients. 2024; 16(3):359. https://doi.org/10.3390/nu16030359
Chicago/Turabian StyleGrabia, Monika, Jakub Perkowski, Katarzyna Socha, and Renata Markiewicz-Żukowska. 2024. "Female Athlete Triad and Relative Energy Deficiency in Sport (REDs): Nutritional Management" Nutrients 16, no. 3: 359. https://doi.org/10.3390/nu16030359
APA StyleGrabia, M., Perkowski, J., Socha, K., & Markiewicz-Żukowska, R. (2024). Female Athlete Triad and Relative Energy Deficiency in Sport (REDs): Nutritional Management. Nutrients, 16(3), 359. https://doi.org/10.3390/nu16030359









