Mineral Intake and Cardiovascular Disease, Cancer, and All-Cause Mortality: Findings from the Golestan Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Intake
2.3. Outcome
2.4. Statistical Analysis
3. Results
3.1. All-Cause Mortality
3.2. CVD Mortality
3.3. Cancer Mortality
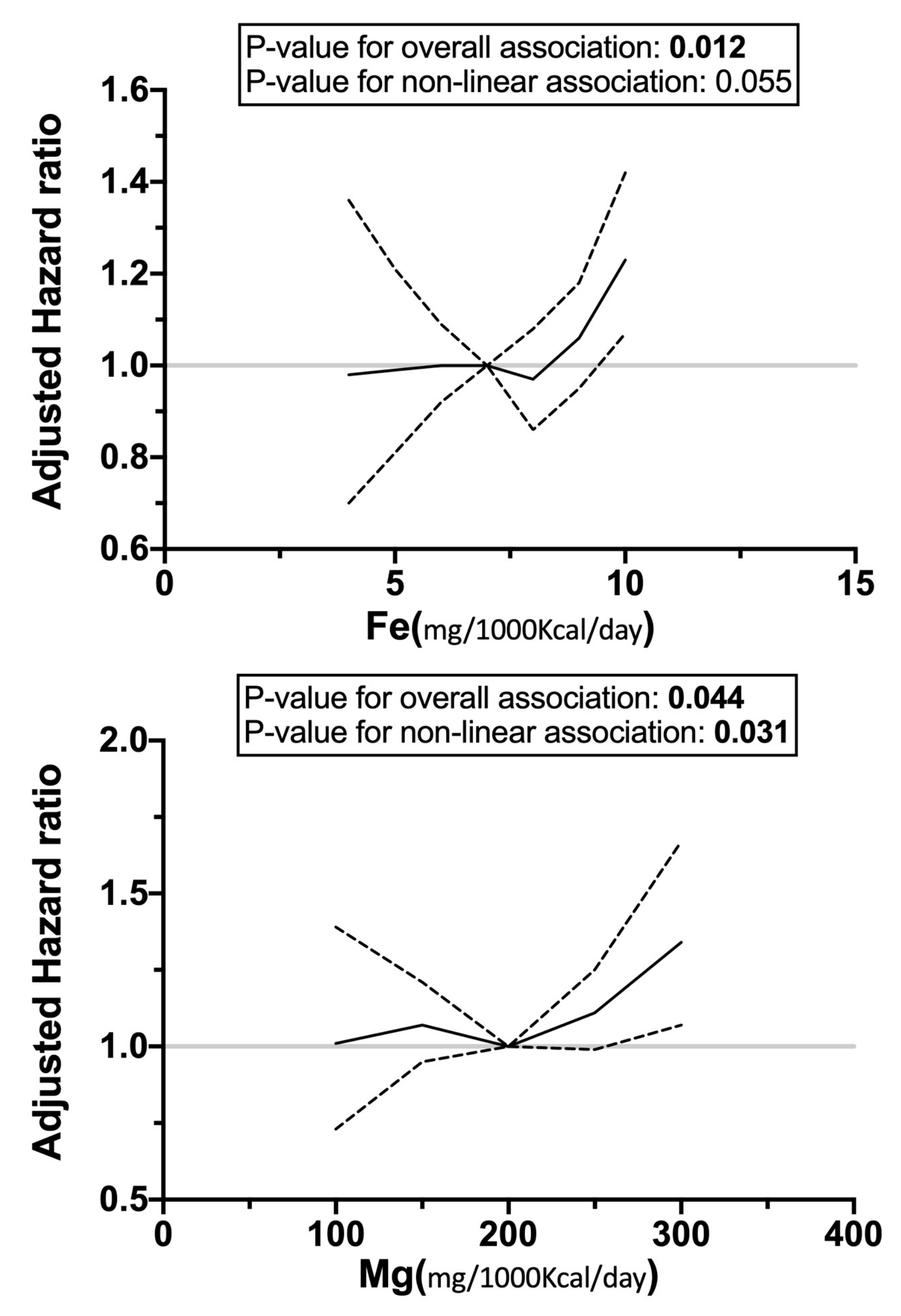
3.4. Restricted Cubic Splines
3.5. Sensitivity Analyses
4. Discussion
4.1. Calcium
4.2. Iron
4.3. Copper
4.4. Selenium
4.5. Phosphorus
4.6. Magnesium and Other Minerals
4.7. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Total NCD Deaths (in Thousands). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-ncd-deaths-in-thousands (accessed on 27 December 2022).
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 December 2022).
- World Health Organization, Regional Office for the Eastern Mediterranean. Noncommunicable Diseases in the Eastern Mediterranean Region; World Health Organization, Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2016. [Google Scholar]
- Bakhtiari, A.; Takian, A.; Majdzadeh, R.; Haghdoost, A.A. Assessment and prioritization of the WHO “best buys” and other recommended interventions for the prevention and control of non-communicable diseases in Iran. BMC Public Health 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Country Profiles 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Houston, M.C. The role of cellular micronutrient analysis, nutraceuticals, vitamins, antioxidants and minerals in the prevention and treatment of hypertension and cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 2010, 4, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Du, M.; Blumberg, J.B.; Chui, K.K.H.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association among Dietary Supplement Use, Nutrient Intake, and Mortality among U.S. Adults: A cohort study. Ann. Intern. Med. 2019, 170, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Hanley-Cook, G.T.; Huybrechts, I.; Biessy, C.; Remans, R.; Kennedy, G.; Deschasaux-Tanguy, M.; Murray, K.A.; Touvier, M.; Skeie, G.; Kesse-Guyot, E.; et al. Food biodiversity and total and cause-specific mortality in 9 European countries: An analysis of a prospective cohort study. PLoS Med. 2021, 18, e1003834. [Google Scholar] [CrossRef] [PubMed]
- Pourshams, A.; Khademi, H.; Malekshah, A.F.; Islami, F.; Nouraei, M.; Sadjadi, A.R.; Jafari, E.; Rakhshani, N.; Salahi, R.; Semnani, S.; et al. Cohort Profile: The Golestan Cohort Study—A prospective study of oesophageal cancer in northern Iran. Int. J. Behav. Nutr. Phys. Act. 2009, 39, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Sasani, N.; Mokhtari, Z.; Keshtkar, A.; Babajafari, S.; Poustchi, H.; Hashemian, M.; Malekzadeh, R. Comparing the risk of cardiovascular diseases and all-cause mortality in four lifestyles with a combination of high/low physical activity and healthy/unhealthy diet: A prospective cohort study. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, A.F.; Kimiagar, M.; Saadatian-Elahi, M.; Pourshams, A.; Nouraie, M.; Goglani, G.; Hoshiarrad, A.; Sadatsafavi, M.; Golestan, B.; Yoonesi, A.; et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: Pilot phase of Golestan cohort study of esophageal cancer. Eur. J. Clin. Nutr. 2006, 60, 971–977. [Google Scholar] [CrossRef]
- Haytowitz, D.; Lemar, L.; Pehrsson, P.; Exler, J.; Patterson, K.; Thomas, R.; Nickle, M.; Williams, J.; Showell, B.; Khan, M. USDA National Nutrient Database for Standard Reference, Release 24; US Department of Agriculture: Washington, DC, USA, 2011.
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1220S–1228S. [Google Scholar] [CrossRef]
- Khademi, H.; Etemadi, A.; Kamangar, F.; Nouraie, M.; Shakeri, R.; Abaie, B.; Pourshams, A.; Bagheri, M.; Hooshyar, A.; Islami, F.; et al. Verbal Autopsy: Reliability and Validity Estimates for Causes of Death in the Golestan Cohort Study in Iran. PLoS ONE 2010, 5, e11183. [Google Scholar] [CrossRef]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Kaluza, J.; Orsini, N.; Levitan, E.B.; Brzozowska, A.; Roszkowski, W.; Wolk, A. Dietary calcium and magnesium intake and mortality: A prospective study of men. Am. J. Epidemiol. 2010, 171, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Umesawa, M.; Iso, H.; Date, C.; Yamamoto, A.; Toyoshima, H.; Watanabe, Y.; Kikuchi, S.; Koizumi, A.; Kondo, T.; Inaba, Y.; et al. Dietary Intake of Calcium in Relation to Mortality from Cardiovascular Disease: The JACC Study. Stroke 2006, 37, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. A Prospective Cohort Study Examining the Associations of Dietary Calcium Intake with All-Cause and Cardiovascular Mortality in Older Chinese Community-Dwelling People. PLoS ONE 2013, 8, e80895. [Google Scholar] [CrossRef]
- van der Pols, J.C.; Gunnell, D.; Williams, G.M.; Holly, J.M.P.; Bain, C.; Martin, R.M. Childhood dairy and calcium intake and cardiovascular mortality in adulthood: 65-year follow-up of the Boyd Orr cohort. Heart 2009, 95, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, K.; Melhus, H.; Lemming, E.W.; Wolk, A.; Byberg, L. Long term calcium intake and rates of all cause and cardiovascular mortality: Community based prospective longitudinal cohort study. BMJ 2013, 346, f228. [Google Scholar] [CrossRef]
- Chung, M.; Tang, A.M.; Fu, Z.; Wang, D.D.; Newberry, S.J. Calcium Intake and Cardiovascular Disease Risk: An updated systematic review and meta-analysis. Ann. Intern. Med. 2016, 165, 856–866. [Google Scholar] [CrossRef]
- Xiao, Q.; Murphy, R.A.; Houston, D.K.; Harris, T.B.; Chow, W.-H.; Park, Y. Dietary and Supplemental Calcium Intake and Cardiovascular Disease Mortality: The National Institutes of Health–AARP diet and health study. JAMA Intern. Med. 2013, 173, 639–646. [Google Scholar] [CrossRef]
- Yang, B.; Campbell, P.T.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; McCullough, M.L. Calcium intake and mortality from all causes, cancer, and cardiovascular disease: The Cancer Prevention Study II Nutrition Cohort. Am. J. Clin. Nutr. 2016, 103, 886–894. [Google Scholar] [CrossRef]
- Pana, T.A.; Dehghani, M.; Baradaran, H.R.; Neal, S.R.; Wood, A.D.; Kwok, C.S.; Loke, Y.K.; Luben, R.N.; Mamas, M.A.; Khaw, K.-T.; et al. Calcium intake, calcium supplementation and cardiovascular disease and mortality in the British population: EPIC-norfolk prospective cohort study and meta-analysis. Eur. J. Epidemiol. 2021, 36, 669–683. [Google Scholar] [CrossRef]
- Asemi, Z.; Saneei, P.; Sabihi, S.-S.; Feizi, A.; Esmaillzadeh, A. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Sesso, H.D. Calcium Intake and Risk of Cardiovascular Disease. Am. J. Cardiovasc. Drugs 2012, 12, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.R.S.G.; Sanjuliani, A.F. Does calcium intake affect cardiovascular risk factors and/or events? Clinics 2012, 67, 839–844. [Google Scholar] [CrossRef]
- Renaud, S.; Morazain, R.; Godsey, F.; Dumont, E.; Symington, I.S.; Gillanders, E.M.; O’Brien, J.R. Platelet functions in relation to diet and serum lipids in British farmers. Heart 1981, 46, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; Morazain, R.; Godsey, F.; Dumont, E.; Thevenon, C.; Martin, J.; Mendy, F. Nutrients, platelet function and composition in nine groups of French and British farmers. Atherosclerosis 1986, 60, 37–48. [Google Scholar] [CrossRef]
- Abubakar, A.; Saito, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Structural Analysis of New Antihypertensive Peptides Derived from Cheese Whey Protein by Proteinase K Digestion. J. Dairy Sci. 1998, 81, 3131–3138. [Google Scholar] [CrossRef]
- Park, Y.; Leitzmann, M.F.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dairy Food, Calcium, and Risk of Cancer in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2009, 169, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G.; Wells, B.; Carek, P.J.; Gill, J.M.; Geesey, M.E. The Mortality Risk of Elevated Serum Transferrin Saturation and Consumption of Dietary Iron. Ann. Fam. Med. 2004, 2, 139–144. [Google Scholar] [CrossRef]
- Etemadi, A.; Sinha, R.; Ward, M.H.; Graubard, B.I.; Inoue-Choi, M.; Dawsey, S.M.; Abnet, C.C. Mortality from Different Causes Associated with Meat, Heme Iron, Nitrates, and Nitrites in the NIH-AARP Diet and Health Study: Population Based Cohort Study. BMJ 2017, 357, j1957. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Ohira, T.; Date, C.; Tanabe, N.; Kikuchi, S.; Tamakoshi, A. Associations of dietary iron intake with mortality from cardiovascular disease: The JACC study. J. Epidemiol. 2012, 22, 484–493. [Google Scholar] [CrossRef]
- Lee, D.-H.; Folsom, A.R.; Jacobs, D.R., Jr. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: The Iowa Women’s Health Study. Am. J. Clin. Nutr. 2005, 81, 787–791. [Google Scholar] [CrossRef]
- Casiglia, E.; Tikhonoff, V.; Bascelli, A.; Giordano, N.; Caffi, S.; Andreatta, E.; Mazza, A.; Boschetti, G.; Grasselli, C.; Saugo, M.; et al. Dietary Iron Intake and Cardiovascular Outcome in Italian Women: 10-Year Follow-Up. J. Women’s Health 2011, 20, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhen, S.; Zhou, Y.; Taylor, A.W. Hb level, iron intake and mortality in Chinese adults: A 10-year follow-up study. Br. J. Nutr. 2017, 117, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Updating the Mitochondrial Free Radical Theory of Aging: An Integrated View, Key Aspects, and Confounding Concepts. Antioxid. Redox Signal. 2013, 19, 1420–1445. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Ionizing and ultraviolet radiation generate ROI such as H2O2 and OH. Methods Enzym. 1990, 186, 1–85. [Google Scholar]
- Swapnil, R.; Joann, M.; Walter, C. Iron intake and the risk of type 2 diabetes in women. Diabetes Care 2006, 29, 1. [Google Scholar]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Chandar, S.S.; Bhaskara Rao, K. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS ONE 2014, 9, e90972. [Google Scholar] [CrossRef]
- Yang, W.; Li, B.; Dong, X.; Zhang, X.-Q.; Zeng, Y.; Zhou, J.-L.; Tang, Y.-H.; Xu, J.-J. Is heme iron intake associated with risk of coronary heart disease? A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 395–400. [Google Scholar] [CrossRef]
- Cross, A.J.; Leitzmann, M.F.; Gail, M.H.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R. A Prospective Study of Red and Processed Meat Intake in Relation to Cancer Risk. PLoS Med. 2007, 4, e325. [Google Scholar] [CrossRef]
- Romeu, M.; Aranda, N.; Giralt, M.; Ribot, B.; Nogues, M.R.; Arija, V. Diet, iron biomarkers and oxidative stress in a representative sample of Mediterranean population. Nutr. J. 2013, 12, 102. [Google Scholar] [CrossRef]
- Guéraud, F.; Taché, S.; Steghens, J.-P.; Milkovic, L.; Borovic-Sunjic, S.; Zarkovic, N.; Gaultier, E.; Naud, N.; Héliès-Toussaint, C.; Pierre, F.; et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic. Biol. Med. 2015, 83, 192–200. [Google Scholar] [CrossRef]
- Gan, X.; He, P.; Zhou, C.; Zu, C.; Meng, Q.; Liu, M.; Zhang, Y.; Yang, S.; Zhang, Y.; Ye, Z. J-shaped association between dietary copper intake and all-cause mortality: A prospective cohort study in Chinese adults. Br. J. Nutr. 2023, 129, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Eshak, E.S.; Iso, H.; Yamagishi, K.; Maruyama, K.; Umesawa, M.; Tamakoshi, A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J. Nutr. Biochem. 2018, 56, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of people aged 65 years and over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Rosen, H.; Chait, A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J. Clin. Investig. 1984, 74, 1890–1894. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S. Serum Copper Concentration and Coronary Heart Disease among US Adults. Am. J. Epidemiol. 2000, 151, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-W.; Shu, X.-O.; Li, H.-L.; Zhang, W.; Gao, J.; Zhao, L.-G.; Zheng, W.; Xiang, Y.-B. Dietary selenium intake and mortality in two population-based cohort studies of 133 957 Chinese men and women. Public Health Nutr. 2016, 19, 2991–2998. [Google Scholar] [CrossRef][Green Version]
- Hoque, B.; Shi, Z. Association between selenium intake, diabetes and mortality in adults: Findings from National Health and Nutrition Examination Survey (NHANES) 2003–2014. Br. J. Nutr. 2022, 127, 1098–1105. [Google Scholar] [CrossRef]
- Xie, B.; Wang, J.; Zhang, J.; Chen, M. Dietary and serum selenium in coronary heart disease and all-cause mortality: An international perspective. Asia Pac. J. Clin. Nutr. 2020, 29, 827–838. [Google Scholar] [CrossRef]
- Nouraie, M.; Pourshams, A.; Kamangar, F.; Sotoudeh, M.; Derakhshan, M.H.; Akbari, M.R.; Fakheri, H.; Zahedi, M.J.; Caldwell, K.; Abnet, C.C.; et al. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J. Gastroenterol. 2004, 10, 2544–2546. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Buss, C.; Marinho, C.; Maranhao, P.A.; Bouskela, E.; Kraemer-Aguiar, L.G. Long-term dietary intake of selenium, calcium, and dairy products is associated with improved capillary recruitment in healthy young men. Eur. J. Nutr. 2013, 52, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers: The selenium and vitamin E cancer prevention trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.J.; Shen, S.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F.; Glickman, L.T.; Oteham, C.; Schlittler, D.; Morris, J.S. Effects of Dietary Selenium Supplementation on DNA Damage and Apoptosis in Canine Prostate. J. Natl. Cancer Inst. 2003, 95, 237–241. [Google Scholar] [CrossRef]
- Waters, D.J.; Shen, S.; Glickman, L.T.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F.; Morris, J.S. Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis 2005, 26, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Schiar, V.P.P.; dos Santos, D.B.; Paixão, M.W.; Nogueira, C.W.; Rocha, J.B.T.; Zeni, G. Human erythrocyte hemolysis induced by selenium and tellurium compounds increased by GSH or glucose: A possible involvement of reactive oxygen species. Chem.-Biol. Interact. 2009, 177, 28–33. [Google Scholar] [CrossRef]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutierrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Ohira, T.; Date, C.; Tamakoshi, A.; Group, J.S. Associations of dietary magnesium intake with mortality from cardiovascular disease: The JACC study. Atherosclerosis 2012, 221, 587–595. [Google Scholar] [CrossRef]
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210. [Google Scholar] [CrossRef]
- Bagheri, A.; Naghshi, S.; Sadeghi, O.; Larijani, B.; Esmaillzadeh, A. Total, Dietary, and Supplemental Magnesium Intakes and Risk of All-Cause, Cardiovascular, and Cancer Mortality: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. Int. Rev. J. 2021, 12, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liang, C.; Li, M.; Montgomery, S.; Fall, K.; Aaseth, J.; Cao, Y. Dose-response relationship between dietary magnesium intake and cardiovascular mortality: A systematic review and dose-based meta-regression analysis of prospective studies. J. Trace Elem. Med. Biol. 2016, 38, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, M.; Poustchi, H.; Mohammadi-Nasrabadi, F.; Hekmatdoost, A. Systematic review of zinc biochemical indicators and risk of coronary heart disease. ARYA Atheroscler. 2015, 11, 357–365. [Google Scholar]
- Hashemian, M.; Hekmatdoost, A.; Poustchi, H.; Mohammadi Nasrabadi, F.; Abnet, C.C.; Malekzadeh, R. Systematic review of zinc biomarkers and esophageal cancer risk. Middle East J. Dig. Dis. 2014, 6, 177–185. [Google Scholar] [PubMed]
- Hashemian, M.; Poustchi, H.; Abnet, C.C.; Boffetta, P.; Dawsey, S.M.; Brennan, P.J.; Pharoah, P.; Etemadi, A.; Kamangar, F.; Sharafkhah, M.; et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: Results from the Golestan Cohort Study. Am. J. Clin. Nutr. 2015, 102, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.R.; Draper, H.H.; Tzeng, D.Y.M.; Shin, H.K.; Schmidt, G.R. Physiological Responses of Human Adults to Foods Containing Phosphate Additives. J. Nutr. 1977, 107, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Karp, H.J.; Vaihia, K.P.; Kärkkäinen, M.U.M.; Niemistö, M.J.; Lamberg-Allardt, C.J.E. Acute Effects of Different Phosphorus Sources on Calcium and Bone Metabolism in Young Women: A Whole-Foods Approach. Calcif. Tissue Int. 2007, 80, 251–258. [Google Scholar] [CrossRef]
- Sullivan, C.M.; Leon, J.B.; Sehgal, A.R. Phosphorus-Containing Food Additives and the Accuracy of Nutrient Databases: Implications for Renal Patients. J. Ren. Nutr. 2007, 17, 350–354. [Google Scholar] [CrossRef]
- Hashemian, M.; Farvid, M.S.; Poustchi, H.; Murphy, G.; Etemadi, A.; Hekmatdoost, A.; Kamangar, F.; Sheikh, M.; Pourshams, A.; Sepanlou, S.G. The application of six dietary scores to a Middle Eastern population: A comparative analysis of mortality in a prospective study. Eur. J. Epidemiol. 2019, 34, 371–382. [Google Scholar] [CrossRef]


| Total Population | Calcium Quintiles | p-Value | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Calcium, mg/1000 kcal/day | 321.68 ± 85.03 | 222.08 ± 26.90 | 272.99 ± 10.63 | 309.26 ± 10.59 | 352.46 ± 15.24 | 451.12 ± 69.61 | <0.001 |
| Energy Intake (Kcal/day) | 2160 ± 558 | 2065 ± 594 | 2159 ± 538 | 2206 ± 535 | 2220 ± 543 | 2151 ± 565 | <0.001 |
| Age, Years | 51.46 ± 8.73 | 51.62 ± 8.76 | 51.01 ± 8.58 | 51.03 ± 8.48 | 51.33 ± 8.71 | 52.3 3 ± 9.05 | <0.001 |
| Sex, Male | 17,870 (42.69) | 3135 (37.56) | 3544 (42.38) | 3733 (44.51) | 3788 (45.15) | 3670 (43.81) | <0.001 |
| Current smoker | 7136 (17.05) | 1457 (17.46) | 1479 (17.69) | 1467 (17.49) | 1445 (17.22) | 1288 (15.37) | <0.001 |
| Opium user | 6838 (16.33) | 1834 (21.97) | 1525 (18.24) | 1326 (15.81) | 1193 (14.22) | 960 (11.46) | <0.001 |
| BMI (Kg/m2) | 26.47 ± 5.40 | 25.40 ± 5.38 | 26.15 ± 5.31 | 26.53 ± 5.38 | 26.93 ± 5.40 | 27.32 ± 5.33 | <0.001 |
| Wealth score | <0.001 | ||||||
| Low | 16,041 (38.32) | 4800 (57.51) | 3759 (44.95) | 3025 (36.07) | 2448 (29.18) | 2009 (23.98) | |
| Medium | 12,092 (28.88) | 2222 (26.62) | 2586 (30.93) | 2548 (30.38) | 2502 (29.82) | 2234 (26.67) | |
| High | 13,730 (32.80) | 1325 (15.87) | 2017 (24.17) | 2813 (33.54) | 3440 (41.0) | 4135 (49.36) | |
| Physical Activity (MET) | <0.001 | ||||||
| Low | 13,044 (32.42) | 2739 (34.53) | 2539 (31.68) | 2463 (30.44) | 2555 (31.57) | 2748 (33.90) | |
| Intermediate | 13,250 (32.93) | 2363 (29.79) | 2550 (31.82) | 2661 (32.88) | 2790 (34.47) | 2886 (35.60) | |
| High | 13,945 (34.66) | 2831 (35.69) | 2926 (36.51) | 2968 (36.68) | 2748 (33.96) | 2472 (30.50) | |
| Rural place of residence | 33,730 (80.57) | 7677 (91.97) | 7328 (87.63) | 6885 (82.10) | 6363 (75.84) | 5447 (65.37) | <0.001 |
| Turkman Ethnicity | 31,486 (75.21) | 6154 (73.73) | 6308 (75.44) | 6352 (75.75) | 6465 (77.06) | 6207 (74.09) | <0.001 |
| Married Status | 37,045 (88.65) | 7014 (84.21) | 7389 (88.58) | 7543 (90.08) | 7574 (90.40) | 7525 (89.94) | <0.001 |
| No formal education | 29,022 (69.33) | 6815 (81.65) | 6188 (74.00) | 5710 (68.09) | 5375 (64.06) | 4934 (58.89) | <0.001 |
| History of hypertension | 6685 (15.97) | 1295 (15.51) | 1262 (15.09) | 1280 (15.26) | 1374 (16.38) | 1474 (17.59) | <0.001 |
| Quintile | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Elements | 1 | 2 | 3 | 4 | 5 | Trend | |
| Calcium | |||||||
| Intake (mg/1000 Kcal/day) | 222.08 ± 26.90 | 272.99 ± 10.63 | 309.26 ± 10.59 | 352.46 ± 15.24 | 451.12 ± 69.61 | 0.031 | |
| Person years | 113,424 | 114,506 | 116,035 | 116,885 | 117,841 | ||
| Cases, n | 1648 | 1437 | 1350 | 1350 | 1432 | ||
| Unadjusted HR | Ref | 0.86 (0.80–0.92) a | 0.79 (0.73–0.85) a | 0.78 (0.72–0.83) a | 0.80 (0.75–0.86) a | ||
| Age- and Sex-adjusted HR | Ref | 0.86 (0.83–0.96) b | 0.80 (0.75–0.86) a | 0.76 (0.70–0.81) a | 0.72 (0.67–0.77) a | ||
| Multi-variable-adjusted HR | Ref | 0.94 (0.88–1.02) | 0.93 (0.86–1.00) | 0.91 (0.84–0.98) c | 0.91 (0.85–0.99) c | ||
| Zinc | |||||||
| Intake (mg/1000 Kcal/day) | 3.86 ± 0.29 | 4.32 ± 0.08 | 4.57 ± 0.06 | 4.83 ± 0.08 | 5.33 ± 0.36 | 0.253 | |
| Person years | 116,557 | 116,502 | 116,105 | 115,380 | 114,148 | ||
| Cases, n | 1556 | 1356 | 1350 | 1392 | 1563 | ||
| Unadjusted HR | Ref | 0.88 (0.81–0.94) b | 0.88 (0.82–0.94) b | 0.91 (0.85–0.98) c | 1.04 (0.97–1.12) | ||
| Age- and Sex-adjusted HR | Ref | 0.92 (0.85–0.99) c | 0.91 (0.85–0.98) c | 0.93 (0.87–1.00) | 0.96 (0.90–1.03) | ||
| Multi-variable-adjusted HR | Ref | 0.97 (0.90–1.05) | 0.97 (0.90–1.05) | 1.00 (0.93–1.08) | 1.03 (0.96–1.11) | ||
| Iron | |||||||
| Intake (mg/1000 Kcal/day) | 6.23 ± 0.63 | 7.30 ± 0.18 | 7.86 ± 0.14 | 8.40 ± 0.17 | 9.33 ± 0.53 | 0.722 | |
| Person years | 116,246 | 116,471 | 116,155 | 115,389 | 114,431 | ||
| Cases, n | 1597 | 1417 | 1359 | 1357 | 1487 | ||
| Unadjusted HR | Ref | 0.89 (0.83–0.95) b | 0.86 (0.80–0.92) a | 0.86 (0.80–0.93) a | 0.96 (0.89–1.03) | ||
| Age- and Sex-adjusted HR | Ref | 0.96 (0.89–1.03) | 0.94 (0.87–1.01) | 0.99 (0.92–1.06) | 1.01 (0.94–1.08) | ||
| Multi-variable-adjusted HR | Ref | 0.98 (0.90–1.05) | 0.96 (0.89–1.04) | 0.97 (0.90–1.05) | 0.98 (0.91–1.06) | ||
| Magnesium | |||||||
| Intake (mg/1000 Kcal/day) | 163.20 ± 17.50 | 192.96 ± 5.03 | 208.32 ± 4.11 | 223.47 ± 4.86 | 249.63 ± 15.84 | 0.475 | |
| Person years | 115,220 | 116,729 | 116,688 | 115,944 | 114,111 | ||
| Cases, n | 1558 | 1357 | 1323 | 1381 | 1598 | ||
| Unadjusted HR | Ref | 0.85 (0.79–0.92) a | 0.83 (0.77–0.90) a | 0.88 (0.82–0.95) b | 1.04 (0.97–1.12) | ||
| Age- and Sex-adjusted HR | Ref | 0.91 (0.85–0.98) c | 0.89 (0.82–0.95) b | 0.95 (0.88–1.02) | 1.05 (0.97–1.12) | ||
| Multi-variable-adjusted HR | Ref | 0.92 (0.85–0.99) c | 0.90 (0.84–0.98) c | 0.93 (0.86–1.00) | 0.96 (0.89–1.04) | ||
| Phosphorus | |||||||
| Intake (mg/1000 Kcal/day) | 508.09 ± 41.18 | 572.14 ± 11.23 | 607.38 ± 9.52 | 642.78 ± 11.50 | 710.50 ± 46.25 | 0.647 | |
| Person years | 115,949 | 115,658 | 115,715 | 115,409 | 115,961 | ||
| Cases, n | 1517 | 1414 | 1390 | 1376 | 1520 | ||
| Unadjusted HR | Ref | 0.94 (0.87–1.01) | 0.92 (0.86–0.99) c | 0.91 (0.85–0.98) c | 1.00 (0.93–1.07) | ||
| Age- and Sex-adjusted HR | Ref | 0.94 (0.88–1.01) | 0.91 (0.84–0.97) c | 0.88 (0.81–0.94) b | 0.86 (0.80–0.93) a | ||
| Multi-variable-adjusted HR | Ref | 0.99 (0.92–1.07) | 0.99 (0.91–1.06) | 0.97 (0.89–1.05) | 0.98 (0.91–1.06) | ||
| Potassium | |||||||
| Intake (mg/1000 Kcal/day) | 1086.39 ± 71.36 | 1204.04 ± 22.82 | 1281.61 ± 22.92 | 1373.97 ± 32.33 | 1597.58 ± 179.11 | 0.405 | |
| Person years | 115,763 | 116,108 | 115,930 | 115,719 | 115,172 | ||
| Cases, n | 1491 | 1348 | 1352 | 1435 | 1591 | ||
| Unadjusted HR | Ref | 0.90 (0.84–0.97) b | 0.91 (0.84–0.97) c | 0.96 (0.89–1.03) | 1.07 (0.99–1.14) | ||
| Age- and Sex-adjusted HR | Ref | 0.92 (0.85–0.99) c | 0.92 (0.85–0.99) c | 0.92 (0.86–0.99) c | 0.99 (0.92–1.06) | ||
| Multi-variable-adjusted HR | Ref | 0.93 (0.86–1.00) | 0.93 (0.87–1.01) | 0.96 (0.89–1.04) | 1.00 (0.93–1.08) | ||
| Copper | |||||||
| Intake (mg/1000 Kcal/day) | 0.64 ± 0.04 | 0.71 ± 0.01 | 0.76 ± 0.1 | 0.80 ± 0.01 | 0.93 ± 0.16 | 0.297 | |
| Person years | 116,094 | 116,396 | 116,237 | 115,665 | 114,300 | ||
| Cases, n | 1497 | 1369 | 1367 | 1433 | 1551 | ||
| Unadjusted HR | Ref | 0.91 (0.85–0.98) c | 0.92 (0.85–0.99) c | 0.97 (0.90–1.04) | 1.06 (0.99–1.14) | ||
| Age- and Sex-adjusted HR | Ref | 0.98 (0.91–1.05) | 0.99 (0.92–1.06) | 1.01 (0.94–1.09) | 1.10 (1.02–1.18) b | ||
| Multi-variable-adjusted HR | Ref | 0.98 (0.90–1.05) | 0.98 (0.90–1.05) | 0.97 (0.90–1.04) | 1.06 (0.96–1.12) | ||
| Manganese | |||||||
| Intake (mg/1000 Kcal/day) | 2.96 ± 0.47 | 3.79 ± 0.14 | 4.26 ± 0.12 | 4.75 ± 0.16 | 5.75 ± 0.77 | 0.337 | |
| Person years | 116,690 | 116,977 | 116,353 | 115,909 | 112,763 | ||
| Cases, n | 1431 | 1306 | 1382 | 1399 | 1699 | ||
| Unadjusted HR | Ref | 0.91 (0.84–0.98) c | 0.97 (0.90–1.05) | 0.99 (0.92–1.07) | 1.25 (1.17–1.35) c | ||
| Age- and Sex-adjusted HR | Ref | 0.96 (0.89–1.04) | 1.02 (0.94–1.09) | 1.04 (0.96–1.12) | 1.23 (1.15–1.32) a | ||
| Multi-variable-adjusted HR | Ref | 0.94 (0.87–1.02) | 0.98 (0.91–1.06) | 0.93 (0.86–1.01) | 1.03 (0.96–1.12) | ||
| Selenium | |||||||
| Intake (mg/1000 Kcal/day) | 49.54 ± 7.19 | 62.28 ± 2.21 | 68.99 ± 1.75 | 75.30 ± 1.98 | 86.20 ± 6.30 | 0.366 | |
| Person years | 116,703 | 116,773 | 116,093 | 115,596 | 113,567 | ||
| Cases, n | 1463 | 1397 | 1396 | 1391 | 1570 | ||
| Unadjusted HR | Ref | 0.96 (0.89–1.03) | 0.97 (0.90–1.04) | 0.97 (0.90–1.05) | 1.13 (1.05–1.21) a | ||
| Age- and Sex-adjusted HR | Ref | 0.99 (0.92–1.07) | 1.02 (0.95–1.10) | 1.02 (0.95–1.10) | 1.11 (1.03–1.19) b | ||
| Multi-variable-adjusted HR | Ref | 0.99 (0.91–1.07) | 1.03 (0.96–1.12) | 1.02 (0.95–1.11) | 1.02 (0.94–1.10) | ||
| Quintile | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Trend | ||
| Elements | Calcium | ||||||
| Intake (mg/1000 Kcal/day) | 222.08 ± 26.90 | 272.99 ± 10.63 | 309.26 ± 10.59 | 352.46 ± 15.24 | 451.12 ± 69.61 | 0.003 | |
| Person years | 113,424 | 114,506 | 116,035 | 116,885 | 117,841 | ||
| Cases, n | 672 | 554 | 549 | 515 | 541 | ||
| Unadjusted HR | Ref | 0.81 (0.72–0.91) a | 0.79 (0.70–0.88) a | 0.73 (0.65–0.82) a | 0.75 (0.67–0.84) a | ||
| Age- and Sex-adjusted HR | Ref | 0.85 (0.76–0.95) b | 0.81 (0.72–0.91) a | 0.71 (0.64–0.80) a | 0.67 (0.59–0.75) a | ||
| Multi-variable-adjusted HR | Ref | 0.88 (0.78–0.99) c | 0.92 (0.82–1.04) | 0.84 (0.74–0.95) b | 0.82 (0.73–0.93) b | ||
| Zinc | |||||||
| Intake (mg/1000 Kcal/day) | 3.86 ± 0.29 | 4.32 ± 0.08 | 4.57 ± 0.06 | 4.83 ± 0.08 | 5.33 ± 0.36 | 0.073 | |
| Person years | 116,557 | 116,502 | 116,105 | 115,380 | 114,148 | ||
| Cases, n | 583 | 518 | 519 | 553 | 658 | ||
| Unadjusted HR | Ref | 0.81 (0.72–0.92) b | 0.82 (0.73–0.92) b | 0.84 (0.75–0.95) b | 1.12 (1.00–1.25) c | ||
| Age- and Sex-adjusted HR | Ref | 0.94 (0.84–1.06) | 0.94 (0.84–1.06) | 1.00 (0.89–1.12) | 1.08 (0.96–1.20) | ||
| Multi-variable-adjusted HR | Ref | 1.00 (0.88–1.13) | 0.98 (0.86–1.11) | 1.04 (0.92–1.18) | 1.10 (0.98–1.24) | ||
| Iron | |||||||
| Intake (mg/1000 Kcal/day) | 6.23 ± 0.63 | 7.30 ± 0.18 | 7.86 ± 0.14 | 8.40 ± 0.17 | 9.33 ± 0.53 | 0.191 | |
| Person years | 116,246 | 116,471 | 116,155 | 115,389 | 114,431 | ||
| Cases, n | 609 | 532 | 510 | 531 | 649 | ||
| Unadjusted HR | Ref | 0.87 (0.78–0.98) c | 0.84 (0.75–0.95) c | 0.89 (0.79–1.00) | 1.10 (0.98–1.23) | ||
| Age- and Sex-adjusted HR | Ref | 0.95 (0.85–1.07) | 0.94 (0.83–1.05) | 1.03 (0.91–1.16) | 1.17 (1.05–1.31) b | ||
| Multi-variable-adjusted HR | Ref | 0.95 (0.84–1.07) | 0.94 (0.83–1.06) | 0.98 (0.86–1.10) | 1.08 (0.96–1.22) | ||
| Magnesium | |||||||
| Intake (mg/1000 Kcal/day) | 163.20 ± 17.50 | 192.96 ± 5.03 | 208.32 ± 4.11 | 223.47 ± 4.86 | 249.63 ± 15.84 | 0.837 | |
| Person years | 115,220 | 116,729 | 116,688 | 115,944 | 114,111 | ||
| Cases, n | 613 | 510 | 513 | 520 | 675 | ||
| Unadjusted HR | Ref | 0.81 (0.72–0.92) b | 0.82 (0.73–0.92) b | 0.84 (0.75–0.95) b | 1.12 (1.00–1.25) c | ||
| Age- and Sex-adjusted HR | Ref | 0.88 (0.79–1.00) | 0.89 (0.79–1.00) | 0.92 (0.82–1.04) | 1.14 (1.02–1.27) b | ||
| Multi-variable-adjusted HR | Ref | 0.87 (0.77–0.99) c | 0.92 (0.81–1.04) | 0.88 (0.78–1.00) | 1.01 (0.90–1.14) | ||
| Phosphorus | |||||||
| Intake (mg/1000 Kcal/day) | 508.09 ± 41.18 | 572.14 ± 11.23 | 607.38 ± 9.52 | 642.78 ± 11.50 | 710.50 ± 46.25 | 0.626 | |
| Person years | 115,949 | 115,658 | 115,715 | 115,409 | 115,961 | ||
| Cases, n | 581 | 560 | 540 | 537 | 613 | ||
| Unadjusted HR | Ref | 0.97 (0.86–1.09) | 0.93 (0.83–1.05) | 0.93 (0.83–1.05) | 1.05 (0.94–1.18) | ||
| Age- and Sex-adjusted HR | Ref | 0.98 (0.87–1.10) | 0.92 (0.82–1.03) | 0.89 (0.79–1.00) | 0.90 (0.81–1.01) | ||
| Multi-variable-adjusted HR | Ref | 0.99 (0.88–1.12) | 0.97 (0.86–1.10) | 0.95 (0.84–1.07) | 0.98 (0.87–1.11) | ||
| Potassium | |||||||
| Intake (mg/1000 Kcal/day) | 1086.39 ± 71.36 | 1204.04 ± 22.82 | 1281.61 ± 22.92 | 1373.97 ± 32.33 | 1597.58 ± 179.11 | 0.772 | |
| Person years | 115,763 | 116,108 | 115,930 | 115,719 | 115,172 | ||
| Cases, n | 597 | 520 | 511 | 585 | 618 | ||
| Unadjusted HR | Ref | 0.87 (0.77–0.98) c | 0.85 (0.76–0.96) c | 0.98 (0.87–1.10) | 1.03 (0.92–1.16) | ||
| Age- and Sex-adjusted HR | Ref | 0.89 (0.79–1.00) | 0.87 (0.77–0.98) c | 0.93 (0.83–1.05) | 0.94 (0.84–1.06) | ||
| Multi-variable-adjusted HR | Ref | 0.89 (0.79–1.01) | 0.88 (0.78–1.00) | 0.96 (0.85–1.09) | 0.94 (0.83–1.06) | ||
| Copper | |||||||
| Intake (mg/1000 Kcal/day) | 0.64 ± 0.04 | 0.71 ± 0.01 | 0.76 ± 0.1 | 0.80 ± 0.01 | 0.93 ± 0.16 | 0.016 | |
| Person years | 116,094 | 116,396 | 116,237 | 115,665 | 114,300 | ||
| Cases, n | 582 | 500 | 497 | 586 | 666 | ||
| Unadjusted HR | Ref | 0.86 (0.76–0.97) a | 0.86 (0.76–0.96) a | 1.02 (0.91–1.14) | 1.17 (1.05–1.31) b | ||
| Age- and Sex-adjusted HR | Ref | 0.92 (0.82–1.04) | 0.93 (0.83–1.05) | 1.08 (0.96–1.21) | 1.23 (1.10–1.38) a | ||
| Multi-variable-adjusted HR | Ref | 0.91 (0.81–1.03) | 0.91 (0.81–1.04) | 0.99 (0.87–1.11) | 1.11 (0.99–1.26) | ||
| Manganese | |||||||
| Intake (mg/1000 Kcal/day) | 2.96 ± 0.47 | 3.79 ± 0.14 | 4.26 ± 0.12 | 4.75 ± 0.16 | 5.75 ± 0.77 | 0.293 | |
| Person years | 116,690 | 116,977 | 116,353 | 115,909 | 112,763 | ||
| Cases, n | 566 | 502 | 544 | 526 | 693 | ||
| Unadjusted HR | Ref | 0.88 (0.78–1.01) | 0.97 (0.86–1.09) | 0.94 (0.84–1.06) | 1.29 (1.16–1.44) a | ||
| Age- and Sex-adjusted HR | Ref | 0.94 (0.84–1.07) | 1.03 (0.91–1.16) | 1.00 (0.88–1.12) | 1.29 (1.15–1.44) a | ||
| Multi-variable-adjusted HR | Ref | 0.92 (0.81–1.04) | 0.99 (0.87–1.12) | 0.90 (0.79–1.02) | 1.07 (0.87–1.20) | ||
| Selenium | |||||||
| Intake (mg/1000 Kcal/day) | 49.54 ± 7.19 | 62.28 ± 2.21 | 68.99 ± 1.75 | 75.30 ± 1.98 | 86.20 ± 6.30 | 0.037 | |
| Person years | 116,703 | 116,773 | 116,093 | 115,596 | 113,567 | ||
| Cases, n | 554 | 539 | 534 | 523 | 681 | ||
| Unadjusted HR | Ref | 0.98 (0.87–1.10) | 0.98 (0.87–1.10) | 0.97 (0.86–1.09) | 1.29 (1.15–1.44) a | ||
| Age- and Sex-adjusted HR | Ref | 1.02 (0.90–1.15) | 1.05 (0.93–1.18) | 1.03 (0.91–1.16) | 1.28 (1.14–1.43) a | ||
| Multi-variable-adjusted HR | Ref | 1.01 (0.89–1.14) | 1.05 (0.93–1.19) | 1.00 (0.88–1.14) | 1.14 (1.01–1.29) c | ||
| Quintile | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Elements | 1 | 2 | 3 | 4 | 5 | Trend | |
| Calcium | |||||||
| Intake (mg/1000 Kcal/day) | 222.08 ± 26.90 | 272.99 ± 10.63 | 309.26 ± 10.59 | 352.46 ± 15.24 | 451.12 ± 69.61 | 0.132 | |
| Person years | 113,424 | 114,506 | 116,035 | 116,885 | 117,841 | ||
| Cases, n | 355 | 336 | 285 | 311 | 308 | ||
| Unadjusted HR | Ref | 0.93 (0.80–1.08) | 0.78 (0.66–0.91) b | 0.84 (0.72–0.98) c | 0.82 (0.70–0.95) c | ||
| Age- and Sex-adjusted HR | Ref | 0.95 (0.82–1.10) | 0.77 (0.66–0.90) b | 0.81 (0.69–0.94) b | 0.74 (0.63–0.86) a | ||
| Multi-variable-adjusted HR | Ref | 0.972 (0.83–1.14) | 0.84 (0.71–0.99) c | 0.91 (0.77–1.07) | 0.88 (0.75–1.04) | ||
| Zinc | |||||||
| Intake (mg/1000 Kcal/day) | 3.86 ± 0.29 | 4.32 ± 0.08 | 4.57 ± 0.06 | 4.83 ± 0.08 | 5.33 ± 0.36 | 0.085 | |
| Person years | 116,557 | 116,502 | 116,105 | 115,380 | 114,148 | ||
| Cases, n | 371 | 332 | 293 | 293 | 303 | ||
| Unadjusted HR | Ref | 0.89 (0.77–1.04) | 0.79 (0.68–0.92) b | 0.81 (0.69–0.94) b | 0.84 (0.72–0.97) c | ||
| Age- and Sex-adjusted HR | Ref | 0.92 (0.79–1.07) | 0.81 (0.69–0.94) b | 0.81 (0.69–0.94) b | 0.78 (0.67–0.91) b | ||
| Multi-variable-adjusted HR | Ref | 0.95 (0.81–1.11) | 0.86 (0.73–1.01) | 0.88 (0.75–1.03) | 0.88 (0.75–1.03) | ||
| Iron | |||||||
| Intake (mg/1000 Kcal/day) | 6.23 ± 0.63 | 7.30 ± 0.18 | 7.86 ± 0.14 | 8.40 ± 0.17 | 9.33 ± 0.53 | 0.109 | |
| Person years | 116,246 | 116,471 | 116,155 | 115,389 | 114,431 | ||
| Cases, n | 355 | 331 | 323 | 296 | 290 | ||
| Unadjusted HR | Ref | 0.93 (0.80–1.08) | 0.91 (0.78–1.06) | 0.84 (0.72–0.98) c | 0.83 (0.71–0.97) c | ||
| Age- and Sex-adjusted HR | Ref | 0.97 (0.84–1.13) | 0.96 (0.83–1.12) | 0.92 (0.78–1.07) | 0.85 (0.73–1.00) | ||
| Multi-variable-adjusted HR | Ref | 0.97 (0.83–1.14) | 0.95 (0.81–1.11) | 0.92 (0.78–1.08) | 0.87 (0.74–1.03) | ||
| Magnesium | |||||||
| Intake (mg/1000 Kcal/day) | 163.20 ± 17.50 | 192.96 ± 5.03 | 208.32 ± 4.11 | 223.47 ± 4.86 | 249.63 ± 15.84 | 0.263 | |
| Person years | 115,220 | 116,729 | 116,688 | 115,944 | 114,111 | ||
| Cases, n | 332 | 334 | 300 | 304 | 325 | ||
| Unadjusted HR | Ref | 0.99 (0.85–1.15) | 0.89 (0.76–1.04) | 0.90 (0.77–1.06) | 0.99 (0.85–1.15) | ||
| Age- and Sex-adjusted HR | Ref | 1.02 (0.88–1.19) | 0.91 (0.78–1.06) | 0.93 (0.80–1.09) | 0.96 (0.82–1.12) | ||
| Multi-variable-adjusted HR | Ref | 1.01 (0.86–1.18) | 0.86 (0.73–1.01) | 0.93 (0.79–1.09) | 0.93 (0.79–1.10) | ||
| Phosphorus | |||||||
| Intake (mg/1000 Kcal/day) | 508.09 ± 41.18 | 572.14 ± 11.23 | 607.38 ± 9.52 | 642.78 ± 11.50 | 710.50 ± 46.25 | 0.022 | |
| Person years | 115,949 | 115,658 | 115,715 | 115,409 | 115,961 | ||
| Cases, n | 348 | 342 | 294 | 330 | 281 | ||
| Unadjusted HR | Ref | 0.98 (0.85–1.14) | 0.84 (0.72–0.99) c | 0.95 (0.82–1.11) | 0.80 (0.69–0.94) b | ||
| Age- and Sex-adjusted HR | Ref | 0.98 (0.85–1.14) | 0.83 (0.71–0.97) c | 0.91 (0.78–1.06) | 0.71 (0.60–0.83) a | ||
| Multi-variable-adjusted HR | Ref | 1.01 (0.87–1.18) | 0.89 (0.76–1.05) | 1.00 (0.85–1.17) | 0.81 (0.69–0.96) c | ||
| Potassium | |||||||
| Intake (mg/1000 Kcal/day) | 1086.39 ± 71.36 | 1204.04 ± 22.82 | 1281.61 ± 22.92 | 1373.97 ± 32.33 | 1597.58 ± 179.11 | 0.648 | |
| Person years | 115,763 | 116,108 | 115,930 | 115,719 | 115,172 | ||
| Cases, n | 331 | 330 | 296 | 298 | 340 | ||
| Unadjusted HR | Ref | 0.99 (0.85–1.15) | 0.89 (0.76–1.04) | 0.90 (0.77–1.05) | 1.03 (0.88–1.20) | ||
| Age- and Sex-adjusted HR | Ref | 1.00 (0.86–1.17) | 0.90 (0.77–1.06) | 0.88 (0.75–1.03) | 0.99 (0.85–1.15) | ||
| Multi-variable-adjusted HR | Ref | 0.99 (0.85–1.16) | 0.92 (0.78–1.08) | 0.93 (0.79–1.09) | 1.05 (0.89–1.23) | ||
| Copper | |||||||
| Intake (mg/1000 Kcal/day) | 0.64 ± 0.04 | 0.71 ± 0.01 | 0.76 ± 0.011 | 0.80 ± 0.01 | 0.93 ± 0.16 | 0.028 | |
| Person years | 116,094 | 116,396 | 116,237 | 115,665 | 114,300 | ||
| Cases, n | 355 | 325 | 321 | 297 | 297 | ||
| Unadjusted HR | Ref | 0.91 (0.78–1.06) | 0.90 (0.77–1.05) | 0.84 (0.72–0.98) c | 0.85 (0.73–0.99) c | ||
| Age- and Sex-adjusted HR | Ref | 0.95 (0.82–1.11) | 0.94 (0.81–1.09) | 0.85 (0.73–0.99) c | 0.84 (0.72–0.98) c | ||
| Multi-variable-adjusted HR | Ref | 0.92 (0.79–1.08) | 0.93 (0.79–1.08) | 0.85 (0.72–1.00) | 0.84 (0.71–0.99) c | ||
| Manganese | |||||||
| Intake (mg/1000 Kcal/day) | 2.96 ± 0.47 | 3.79 ± 0.14 | 4.26 ± 0.12 | 4.75 ± 0.16 | 5.75 ± 0.77 | 0.628 | |
| Person years | 116,690 | 116,977 | 116,353 | 115,909 | 112,763 | ||
| Cases, n | 322 | 276 | 318 | 317 | 362 | ||
| Unadjusted HR | Ref | 0.85 (0.72–1.00) | 0.99 (0.85–1.15) | 0.99 (0.85–1.16) | 1.17 (1.01–1.36) c | ||
| Age- and Sex-adjusted HR | Ref | 0.87 (0.74–1.03) | 1.00 (0.85–1.17) | 1.00 (0.85–1.17) | 1.12 (0.96–1.31) | ||
| Multi-variable-adjusted HR | Ref | 0.83 (0.70–0.98) c | 0.95 (0.81–1.12) | 0.89 (0.76–1.05) | 1.00 (0.85–1.18) | ||
| Selenium | |||||||
| Intake (mg/1000 Kcal/day) | 49.54 ± 7.19 | 62.28 ± 2.21 | 68.99 ± 1.75 | 75.30 ± 1.98 | 86.20 ± 6.30 | 0.307 | |
| Person years | 116,703 | 116,773 | 116,093 | 115,596 | 113,567 | ||
| Cases, n | 319 | 330 | 328 | 313 | 305 | ||
| Unadjusted HR | Ref | 1.03 (0.88–1.20) | 1.03 (0.89–1.21) | 0.99 (0.85–1.16) | 0.99 (0.84–1.16) | ||
| Age- and Sex-adjusted HR | Ref | 1.04 (0.89–1.22) | 1.05 (0.90–1.23) | 1.01 (0.86–1.18) | 0.95 (0.81–1.11) | ||
| Multi-variable-adjusted HR | Ref | 0.98 (0.84–1.15) | 1.02 (0.87–1.20) | 0.99 (0.84–1.16) | 0.90 (0.76–1.06) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdanpanah, M.H.; Sharafkhah, M.; Poustchi, H.; Etemadi, A.; Sheikh, M.; Kamangar, F.; Pourshams, A.; Boffetta, P.; Dawsey, S.M.; Abnet, C.C.; et al. Mineral Intake and Cardiovascular Disease, Cancer, and All-Cause Mortality: Findings from the Golestan Cohort Study. Nutrients 2024, 16, 344. https://doi.org/10.3390/nu16030344
Yazdanpanah MH, Sharafkhah M, Poustchi H, Etemadi A, Sheikh M, Kamangar F, Pourshams A, Boffetta P, Dawsey SM, Abnet CC, et al. Mineral Intake and Cardiovascular Disease, Cancer, and All-Cause Mortality: Findings from the Golestan Cohort Study. Nutrients. 2024; 16(3):344. https://doi.org/10.3390/nu16030344
Chicago/Turabian StyleYazdanpanah, Mohammad Hosein, Maryam Sharafkhah, Hossein Poustchi, Arash Etemadi, Mahdi Sheikh, Farin Kamangar, Akram Pourshams, Paolo Boffetta, Sanford M. Dawsey, Christian C. Abnet, and et al. 2024. "Mineral Intake and Cardiovascular Disease, Cancer, and All-Cause Mortality: Findings from the Golestan Cohort Study" Nutrients 16, no. 3: 344. https://doi.org/10.3390/nu16030344
APA StyleYazdanpanah, M. H., Sharafkhah, M., Poustchi, H., Etemadi, A., Sheikh, M., Kamangar, F., Pourshams, A., Boffetta, P., Dawsey, S. M., Abnet, C. C., Malekzadeh, R., & Hashemian, M. (2024). Mineral Intake and Cardiovascular Disease, Cancer, and All-Cause Mortality: Findings from the Golestan Cohort Study. Nutrients, 16(3), 344. https://doi.org/10.3390/nu16030344





