Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice
Abstract
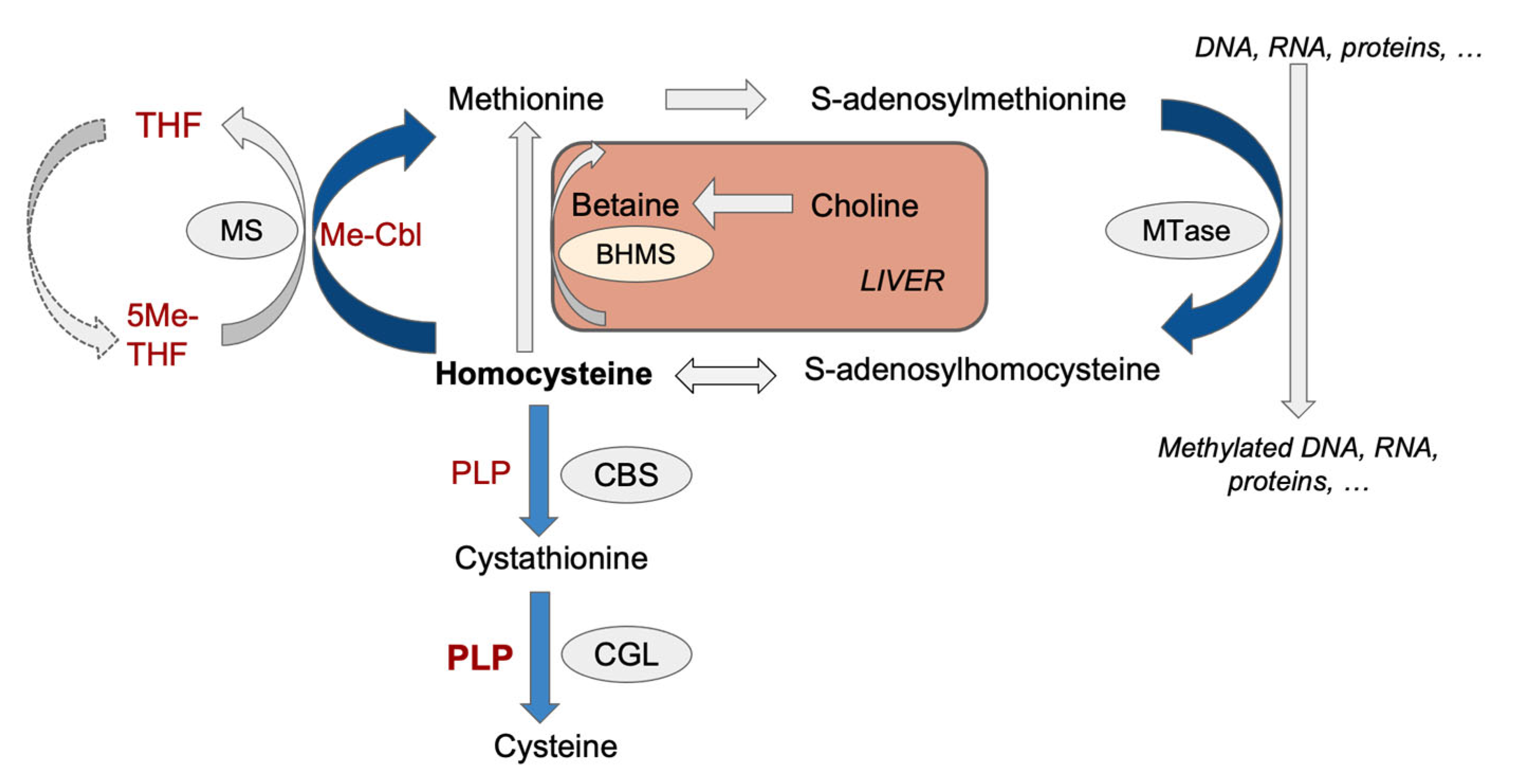
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Biochemical Analyses: Blood Glucose and ALT Levels
2.3. Blood Collection
2.4. Tissue Collection
2.5. Liver Histologic Analysis
2.6. Aortic Atheroma Quantification
2.7. Quantification of Homocysteine and Methylation Metabolites
2.8. Targeted Metabolomic Analysis
2.9. Statistical Analysis and Bioinformatics
3. Results and Discussion
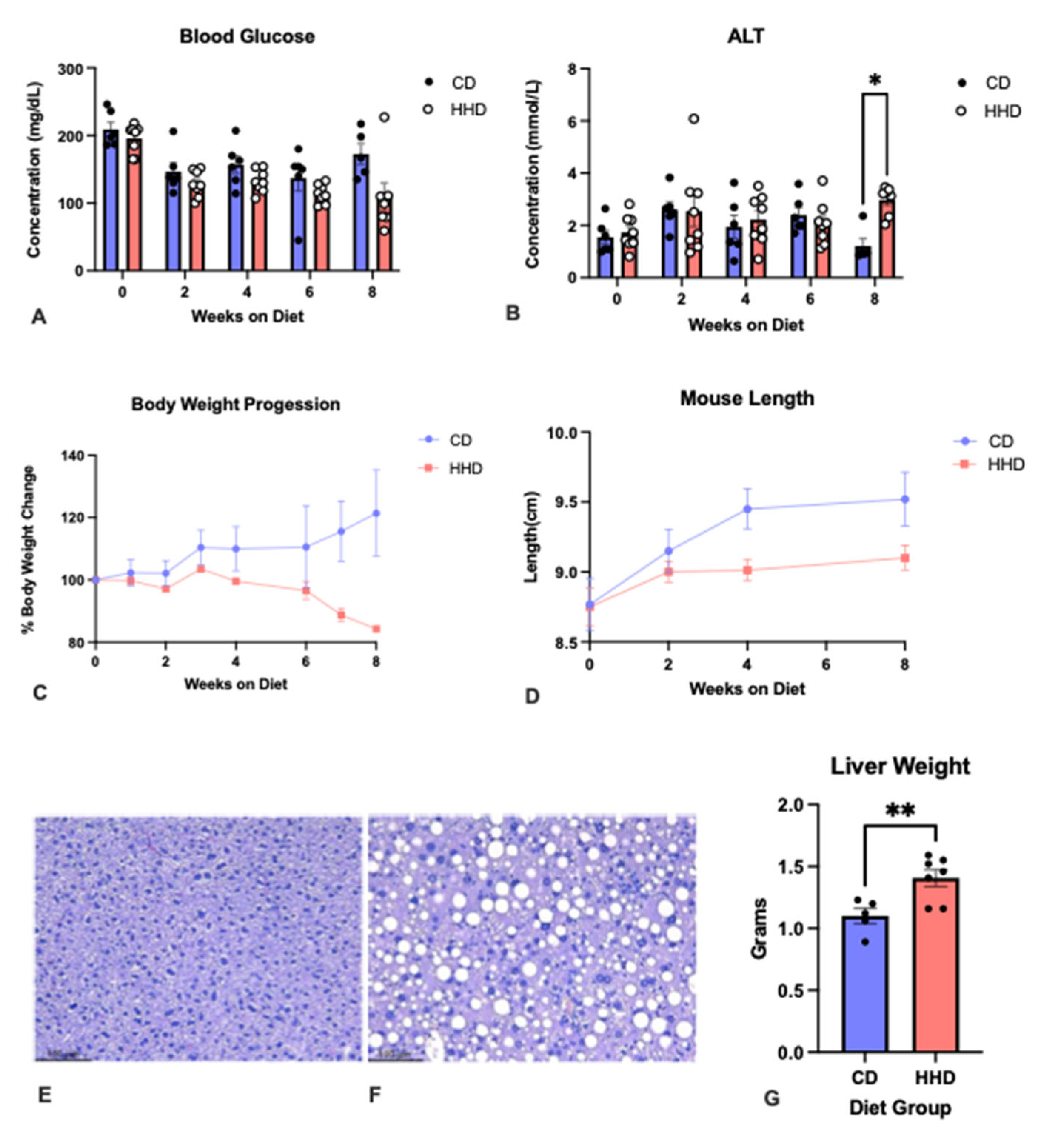
3.1. General Characteristics
3.2. Homocysteine Levels and Atherosclerotic Plaque Burden
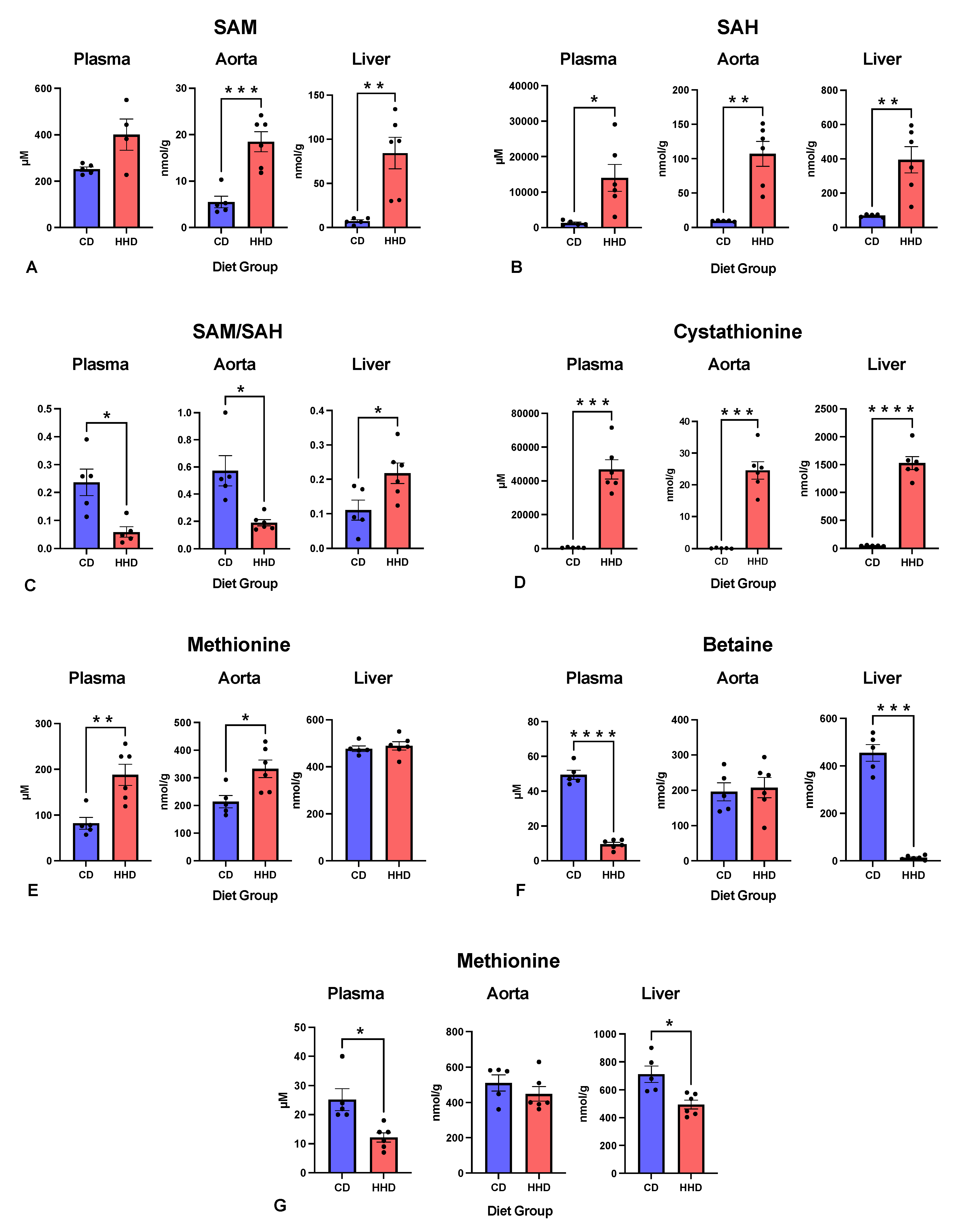
3.3. Methylation Indexes and the Concentrations of Relevant Metabolites in Different Tissues
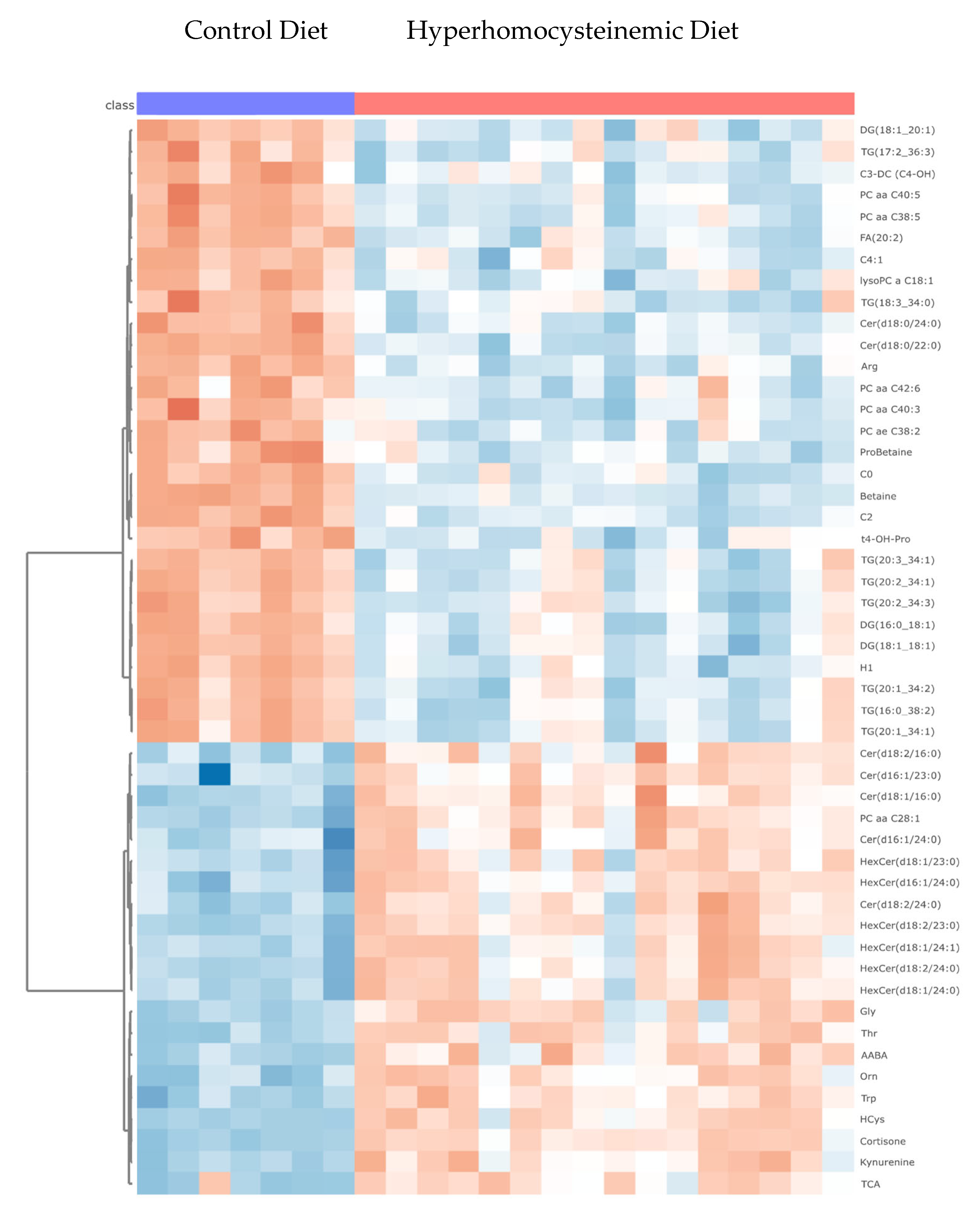
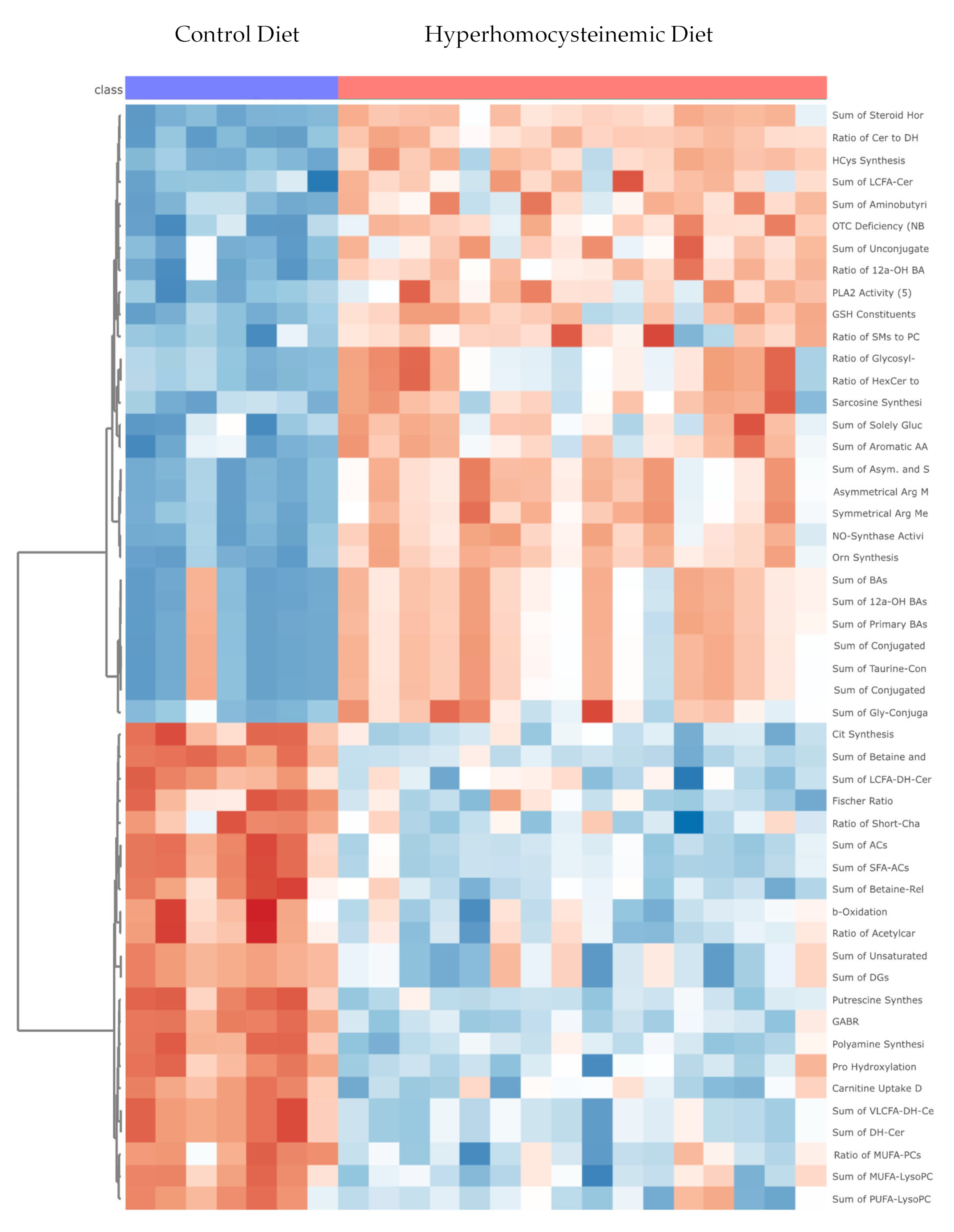
3.4. Targeted Metabolomic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Joseph, J.; Handy, D.E.; Loscalzo, J. Quo vadis: Whither homocysteine research? Cardiovasc. Toxicol. 2009, 9, 53–63. [Google Scholar] [CrossRef]
- Lai, W.K.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Gueant, J.L.; Gueant-Rodriguez, R.M.; Oussalah, A.; Zuily, S.; Rosenberg, I. Hyperhomocysteinemia in Cardiovascular Diseases: Revisiting Observational Studies and Clinical Trials. Thromb. Haemost. 2023, 123, 270–282. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Loscalzo, J.; Handy, D.E. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease (2013 Grover Conference series). Pulm. Circ. 2014, 4, 169–174. [Google Scholar] [CrossRef]
- Barroso, M.; Handy, D.E.; Castro, R. The link between hyperhomocysteinemia and hypomethylation: Implications for cardiovascular disease. J. Inborn Errors Metab. Screen. 2019, 5, e160024. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Caldeira-Araujo, H.; Ramos, R.; Florindo, C.; Rivera, I.; Castro, R.; Tavares de Almeida, I. Homocysteine Metabolism in Children and Adolescents: Influence of Age on Plasma Biomarkers and Correspondent Genotype Interactions. Nutrients 2019, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Barroso, M.; Rocha, M.; Esse, R.; Ramos, R.; Ravasco, P.; Rivera, I.; de Almeida, I.T. The TCN2 776CNG polymorphism correlates with vitamin B(12) cellular delivery in healthy adult populations. Clin. Biochem. 2010, 43, 645–649. [Google Scholar] [CrossRef]
- Troen, A.M.; Lutgens, E.; Smith, D.E.; Rosenberg, I.H.; Selhub, J. The atherogenic effect of excess methionine intake. Proc. Natl. Acad. Sci. USA 2003, 100, 15089–15094. [Google Scholar] [CrossRef]
- Esse, R.; Florindo, C.; Imbard, A.; Rocha, M.S.; de Vriese, A.S.; Smulders, Y.M.; Teerlink, T.; Tavares de Almeida, I.; Castro, R.; Blom, H.J. Global protein and histone arginine methylation are affected in a tissue-specific manner in a rat model of diet-induced hyperhomocysteinemia. Biochim. Biophys. Acta 2013, 1832, 1708–1714. [Google Scholar] [CrossRef]
- Dayal, S.; Lentz, S.R. Murine models of hyperhomocysteinemia and their vascular phenotypes. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1596–1605. [Google Scholar] [CrossRef]
- Saulnier-Blache, J.S.; Wilson, R.; Klavins, K.; Graham, D.; Alesutan, I.; Kastenmuller, G.; Wang-Sattler, R.; Adamski, J.; Roden, M.; Rathmann, W.; et al. Ldlr(-)(/)(-) and ApoE(-)(/)(-) mice better mimic the human metabolite signature of increased carotid intima media thickness compared to other animal models of cardiovascular disease. Atherosclerosis 2018, 276, 140–147. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Do the Apoe-/- and Ldlr-/- Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. ApoE knockout and knockin mice: The history of their contribution to the understanding of atherogenesis. J. Lipid. Res. 2016, 57, 758–766. [Google Scholar] [CrossRef]
- Caligiuri, G.; Nicoletti, A.; Zhou, X.; Tornberg, I.; Hansson, G.K. Effects of sex and age on atherosclerosis and autoimmunity in apoE-deficient mice. Atherosclerosis 1999, 145, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Whalen, C.A.; Gullette, S.; Mattie, F.J.; Florindo, C.; Heil, S.G.; Huang, N.K.; Neuberger, T.; Ross, A.C. A Hypomethylating Ketogenic Diet in Apolipoprotein E-Deficient Mice: A Pilot Study on Vascular Effects and Specific Epigenetic Changes. Nutrients 2021, 13, 3576. [Google Scholar] [CrossRef] [PubMed]
- Whalen, C.A.; Mattie, F.J.; Florindo, C.; van Zelst, B.; Huang, N.K.; Tavares de Almeida, I.; Heil, S.G.; Neuberger, T.; Ross, A.C.; Castro, R. No Effect of Diet-Induced Mild Hyperhomocysteinemia on Vascular Methylating Capacity, Atherosclerosis Progression, and Specific Histone Methylation. Nutrients 2020, 12, 2182. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Whalen, C.A.; Mattie, F.J.; Florindo, C.; Huang, N.K.; Heil, S.G.; Neuberger, T.; Ross, A.C.; Soveral, G.; Castro, R. An Atherogenic Diet Disturbs Aquaporin 5 Expression in Liver and Adipocyte Tissues of Apolipoprotein E-Deficient Mice: New Insights into an Old Model of Experimental Atherosclerosis. Biomedicines 2021, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Gullette, S.; Florindo, C.; Huang, N.K.; Neuberger, T.; Ross, A.C.; Soveral, G.; Castro, R. The Effect of Nutritional Ketosis on Aquaporin Expression in Apolipoprotein E-Deficient Mice: Potential Implications for Energy Homeostasis. Biomedicines 2022, 10, 1159. [Google Scholar] [CrossRef]
- Castro, R.; Gullette, S.; Whalen, C.; Mattie, F.J.; Ge, X.; Ross, A.C.; Neuberger, T. High-field magnetic resonance microscopy of aortic plaques in a mouse model of atherosclerosis. MAGMA 2023, 36, 887–896. [Google Scholar] [CrossRef]
- Ducros, V.; Belva-Besnet, H.; Casetta, B.; Favier, A. A robust liquid chromatography tandem mass spectrometry method for total plasma homocysteine determination in clinical practice. Clin. Chem. Lab. Med. 2006, 44, 987–990. [Google Scholar] [CrossRef]
- Lai, S.C.; Nakayama, Y.; Sequeira, J.M.; Wlodarczyk, B.J.; Cabrera, R.M.; Finnell, R.H.; Bottiglieri, T.; Quadros, E.V. The transcobalamin receptor knockout mouse: A model for vitamin B12 deficiency in the central nervous system. FASEB J. 2013, 27, 2468–2475. [Google Scholar] [CrossRef]
- Kalecky, K.; Ashcraft, P.; Bottiglieri, T. One-Carbon Metabolism in Alzheimer's Disease and Parkinson’s Disease Brain Tissue. Nutrients 2022, 14, 599. [Google Scholar] [CrossRef]
- Rooney, M.; Bottiglieri, T.; Wasek-Patterson, B.; McMahon, A.; Hughes, C.F.; McCann, A.; Horigan, G.; Strain, J.J.; McNulty, H.; Ward, M. Impact of the MTHFR C677T polymorphism on one-carbon metabolites: Evidence from a randomised trial of riboflavin supplementation. Biochimie 2020, 173, 91–99. [Google Scholar] [CrossRef]
- Moretti, R.; Peinkhofer, C. B Vitamins and Fatty Acids: What Do They Share with Small Vessel Disease-Related Dementia? Int. J. Mol. Sci. 2019, 20, 5797. [Google Scholar] [CrossRef]
- Kulinski, A.; Vance, D.E.; Vance, J.E. A choline-deficient diet in mice inhibits neither the CDP-choline pathway for phosphatidylcholine synthesis in hepatocytes nor apolipoprotein B secretion. J. Biol. Chem. 2004, 279, 23916–23924. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Shinohara, M.; Vance, D.; Than, T.A.; Ookhtens, M.; Chan, C.; Kaplowitz, N. Effect of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver. Alcohol Clin. Exp. Res. 2008, 32, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Law, S.H.; Chan, H.C.; Ke, G.M.; Kamatam, S.; Marathe, G.K.; Ponnusamy, V.K.; Ke, L.Y. Untargeted Lipidomic Profiling Reveals Lysophosphatidylcholine and Ceramide as Atherosclerotic Risk Factors in apolipoprotein E Knockout Mice. Int. J. Mol. Sci. 2023, 24, 6956. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Q.; Guo, H.; Xia, M.; Yuan, Q.; Hu, Y.; Zhu, H.; Hou, M.; Ma, J.; Tang, Z.; et al. Plasma S-adenosylhomocysteine is a better biomarker of atherosclerosis than homocysteine in apolipoprotein E-deficient mice fed high dietary methionine. J. Nutr. 2008, 138, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Perla-Kajan, J.; Jakubowski, H. Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. Int. J. Mol. Sci. 2019, 20, 3140. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Rivera, I.; Martins, C.; Struys, E.A.; Jansen, E.E.; Clode, N.; Graca, L.M.; Blom, H.J.; Jakobs, C.; de Almeida, I.T. Intracellular S-adenosylhomocysteine increased levels are associated with DNA hypomethylation in HUVEC. J. Mol. Med. 2005, 83, 831–836. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; Tavares de Almeida, I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef]
- Barroso, M.; Florindo, C.; Kalwa, H.; Silva, Z.; Turanov, A.A.; Carlson, B.A.; de Almeida, I.T.; Blom, H.J.; Gladyshev, V.N.; Hatfield, D.L.; et al. Inhibition of cellular methyltransferases promotes endothelial cell activation by suppressing glutathione peroxidase 1 protein expression. J. Biol. Chem. 2014, 289, 15350–15362. [Google Scholar] [CrossRef]
- Barroso, M.; Rocha, M.S.; Esse, R.; Goncalves, I., Jr.; Gomes, A.Q.; Teerlink, T.; Jakobs, C.; Blom, H.J.; Loscalzo, J.; Rivera, I.; et al. Cellular hypomethylation is associated with impaired nitric oxide production by cultured human endothelial cells. Amino Acids 2012, 42, 1903–1911. [Google Scholar] [CrossRef]
- Esse, R.; Imbard, A.; Florindo, C.; Gupta, S.; Quinlivan, E.P.; Davids, M.; Teerlink, T.; Tavares de Almeida, I.; Kruger, W.D.; Blom, H.J.; et al. Protein arginine hypomethylation in a mouse model of cystathionine beta-synthase deficiency. FASEB J. 2014, 28, 2686–2695. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Y.; Wang, M.; Li, X.; Su, D.; Qiu, J.; Li, D.; Yang, Y.; Xia, M.; Ling, W. Plasma S-adenosylhomocysteine is associated with the risk of cardiovascular events in patients undergoing coronary angiography: A cohort study. Am. J. Clin. Nutr. 2013, 98, 1162–1169. [Google Scholar] [CrossRef]
- Zawada, A.M.; Rogacev, K.S.; Hummel, B.; Berg, J.T.; Friedrich, A.; Roth, H.J.; Obeid, R.; Geisel, J.; Fliser, D.; Heine, G.H. S-adenosylhomocysteine is associated with subclinical atherosclerosis and renal function in a cardiovascular low-risk population. Atherosclerosis 2014, 234, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Cheng, J.; Huang, H.; Zhou, Y.; Yang, X.; Su, X.; Ke, Y.; Ling, W. Inhibition of S-Adenosylhomocysteine Hydrolase Induces Endothelial Dysfunction via Epigenetic Regulation of p66shc-Mediated Oxidative Stress Pathway. Circulation 2019, 139, 2260–2277. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Martinez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2013, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ramani, K.; Lu, S.C. Methionine adenosyltransferases in liver health and diseases. Liver Res. 2017, 1, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.M.; Lodha, P.H.; Morneau, D.J. The enzymes of the transsulfuration pathways: Active-site characterizations. Biochim. Biophys. Acta 2011, 1814, 1511–1517. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Iwamoto, J.; Hirayama, T.; Morishita, Y.; Monma, T.; Ueda, H.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid. Res. 2020, 61, 54–69. [Google Scholar] [CrossRef]
- Gregory, J.F.; DeRatt, B.N.; Rios-Avila, L.; Ralat, M.; Stacpoole, P.W. Vitamin B6 nutritional status and cellular availability of pyridoxal 5′-phosphate govern the function of the transsulfuration pathway's canonical reactions and hydrogen sulfide production via side reactions. Biochimie 2016, 126, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, T.; Pini, M.; Zhou, Z.; Fantuzzi, G.; Song, Z. Betaine improved adipose tissue function in mice fed a high-fat diet: A mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G634–G642. [Google Scholar] [CrossRef]
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, O.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Safo, M.K.; Contestabile, R. Biomedical aspects of pyridoxal 5′-phosphate availability. Front. Biosci. 2012, 4, 897–913. [Google Scholar]
- Kannan, K.; Jain, S.K. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic. Biol. Med. 2004, 36, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Sepulveda, J.; Papachristou, D.J. Nature and nurture in atherosclerosis: The roles of acylcarnitine and cell membrane-fatty acid intermediates. Vasc. Pharmacol. 2016, 78, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Boachie, J.; Adaikalakoteswari, A.; Samavat, J.; Saravanan, P. Low Vitamin B12 and Lipid Metabolism: Evidence from Pre-Clinical and Clinical Studies. Nutrients 2020, 12, 1925. [Google Scholar] [CrossRef]
- Bjorndal, B.; Alteras, E.K.; Lindquist, C.; Svardal, A.; Skorve, J.; Berge, R.K. Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutr. Metab. 2018, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, F.; Cheng, Y.; Xiao, X.R.; Hu, D.D.; Tang, Y.M.; Bao, W.M.; Yang, J.H.; Jiang, T.; Hu, J.P.; et al. Celastrol Protects From Cholestatic Liver Injury Through Modulation of SIRT1-FXR Signaling. Mol. Cell Proteom. 2019, 18, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef]
- Wei, S.; Ma, X.; Zhao, Y. Mechanism of Hydrophobic Bile Acid-Induced Hepatocyte Injury and Drug Discovery. Front. Pharmacol. 2020, 11, 1084. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Summers, S.A. Could Ceramides Become the New Cholesterol? Cell Metab. 2018, 27, 276–280. [Google Scholar] [CrossRef]
- Nelson, J.C.; Jiang, X.C.; Tabas, I.; Tall, A.; Shea, S. Plasma sphingomyelin and subclinical atherosclerosis: Findings from the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2006, 163, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, W.; Yancey, P.G.; Koury, M.J.; Zhang, Y.; Fazio, S.; Linton, M.F. Macrophage apolipoprotein E reduces atherosclerosis and prevents premature death in apolipoprotein E and scavenger receptor-class BI double-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 150–156. [Google Scholar] [CrossRef]
- Park, T.S.; Panek, R.L.; Mueller, S.B.; Hanselman, J.C.; Rosebury, W.S.; Robertson, A.W.; Kindt, E.K.; Homan, R.; Karathanasis, S.K.; Rekhter, M.D. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 2004, 110, 3465–3471. [Google Scholar] [CrossRef] [PubMed]
- Ronn, T.; Perfilyev, A.; Jonsson, J.; Eriksson, K.F.; Jorgensen, S.W.; Brons, C.; Gillberg, L.; Vaag, A.; Stener-Victorin, E.; Ling, C. Circulating triglycerides are associated with human adipose tissue DNA methylation of genes linked to metabolic disease. Hum. Mol. Genet. 2023, 32, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in inflammation in the blood and the vessel. Front. Pharmacol. 2022, 13, 997403. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, A.; Mannucci, L.; Tamburi, F.; Cardillo, C.; Schinzari, F.; Rovella, V.; Nistico, S.; Bennardo, L.; Di Daniele, N.; Tesauro, M. Lp-PLA(2), a new biomarker of vascular disorders in metabolic diseases. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419827154. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Li, G.; Chen, J.; Li, X.; Li, L.; Zhou, Y. Increased serum level of Lp-PLA2 is independently associated with the severity of coronary artery diseases: A cross-sectional study of Chinese population. BMC Cardiovasc. Disord. 2015, 15, 14. [Google Scholar] [CrossRef][Green Version]
- Ma, J.; Shen, L.; Bao, L.; Yuan, H.; Wang, Y.; Liu, H.; Wang, Q. A novel prognosis prediction model, including cytotoxic T lymphocyte-associated antigen-4, ischemia-modified albumin, lipoprotein-associated phospholipase A2, glial fibrillary acidic protein, and homocysteine, for ischemic stroke in the Chinese hypertensive population. J. Clin. Lab. Anal. 2021, 35, e23756. [Google Scholar]
- Li, B.; Gao, G.; Zhang, W.; Li, B.; Yang, C.; Jiang, X.; Tian, Y.; Liang, H. Metabolomics analysis reveals an effect of homocysteine on arachidonic acid and linoleic acid metabolism pathway. Mol. Med. Rep. 2018, 17, 6261–6268. [Google Scholar] [CrossRef]

| CD | HHD | |||
|---|---|---|---|---|
| gm | Kcal | gm | Kcal | |
| Casein | 180 | 720 | 180 | 720 |
| Corn Starch | 431 | 1725 | 431 | 1725 |
| Maltodextrin 10 | 155 | 620 | 155 | 620 |
| Sucrose | 100 | 400 | 100 | 400 |
| Cellulose | 35 | 0 | 35 | 0 |
| Primex 1 | 25 | 225 | 25 | 225 |
| Corn Oil | 25 | 225 | 25 | 225 |
| Mineral Mix S10001 | 35 | 0 | 35 | 0 |
| Vitamin Mix V10001 2 | 10 | 40 | 0 | 0 |
| Vitamin Mix V14904 3 | 0 | 0 | 10 | 40 |
| L-Cystine | 3 | 12 | 3 | 12 |
| L-Methionine | 0 | 0 | 3.1 | 12 |
| Choline bitartrate | 2.5 | 0 | 0 | 0 |
| Pyridoxine HCl (×103) | 0 | 0 | 0.2 | 0 |
| Folic acid (×103) | 0 | 0 | 0.1 | 0 |
| Cyanocobalamin, 0.1% (×10−6) | 0 | 0 | 2 | 0 |
| Succinylsulfathiazole | 8.6 | 0 | 8.6 | 0 |
| Metabolite Class | Potential | Detected | FDR (<0.05) (CD vs. HHD) | ||
|---|---|---|---|---|---|
| Metabolites | Metabolites | Increased | Decreased | ||
| LC-MS/MS Metabolites | Alkaloids | 1 | 0 | 0 | 0 |
| Amine Oxides | 1 | 0 | 0 | 0 | |
| Amino Acids | 20 | 20 | 13 | 4 | |
| Amino Acids Related | 30 | 24 | 7 | 8 | |
| Bile Acids | 14 | 12 | 12 | 0 | |
| Biogenic Amines | 9 | 7 | 0 | 0 | |
| Carboxylic Acids | 7 | 4 | 0 | 0 | |
| Cresols | 1 | 1 | 0 | 0 | |
| Fatty Acids | 12 | 9 | 0 | 3 | |
| Hormones | 4 | 1 | 1 | 0 | |
| Indoles and Derivatives | 4 | 3 | 1 | 0 | |
| Nucleobases Related | 2 | 0 | 0 | 0 | |
| Sugars | 1 | 1 | 0 | 1 | |
| Vitamins and Cofactors | 1 | 1 | 0 | 1 | |
| FIA-MS/MS metabolites | Acylcarnitines | 40 | 13 | 0 | 8 |
| Glycerophospholipids | 90 | 87 | 3 | 26 | |
| (Lysophosphatidylcholines & Phosphatidylcholines) | |||||
| Sphingolipids | 15 | 10 | 10 | 0 | |
| Cholesterol Esters | 22 | 21 | 0 | 4 | |
| Ceramides | 28 | 28 | 19 | 4 | |
| Dihydroceramides | 8 | 4 | 0 | 0 | |
| Glyceroceramides | 34 | 34 | 17 | 0 | |
| (Mono-, Di-, and Trihexosylceramindes) | |||||
| Diacylglycerols | 44 | 20 | 0 | 16 | |
| Triacylglycerols | 242 | 237 | 0 | 214 | |
| Total | 630 | 537 | 83 | 289 | |
| FDR (<0.05) CD vs. HHD | Relevant to Inflammation and Atherosclerosis | |||
|---|---|---|---|---|
| Metabolic Indicator Class | Significant | Increased | Decreased | |
| Amino Acids | 13 | 9 | 4 | 1 |
| Amino Acid Related | 18 | 11 | 7 | 4 |
| Acylcarnitines | 13 | 2 | 11 | 1 |
| Bile Acids | 15 | 13 | 2 | 0 |
| Biogenic Amines | 4 | 1 | 3 | 1 |
| Carboxylic Acids | 1 | 1 | 0 | 0 |
| Ceramides | 14 | 9 | 5 | 1 |
| Cholesteryl Esters | 1 | 0 | 1 | 1 |
| Diglycerides | 4 | 1 | 4 | 1 |
| Fatty Acids | 5 | 2 | 3 | 1 |
| Hormones | 1 | 1 | 0 | 0 |
| Indole and Derivatives | 2 | 1 | 1 | 0 |
| Lysophosphatidylcholines | 5 | 1 | 4 | 0 |
| Phosphatidylcholines | 5 | 2 | 3 | 3 |
| Sphingomyelins | 4 | 4 | 0 | 0 |
| Triacylglycerols | 4 | 0 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrews, S.G.; Koehle, A.M.; Paudel, D.; Neuberger, T.; Ross, A.C.; Singh, V.; Bottiglieri, T.; Castro, R. Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice. Nutrients 2024, 16, 330. https://doi.org/10.3390/nu16030330
Andrews SG, Koehle AM, Paudel D, Neuberger T, Ross AC, Singh V, Bottiglieri T, Castro R. Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice. Nutrients. 2024; 16(3):330. https://doi.org/10.3390/nu16030330
Chicago/Turabian StyleAndrews, Stephen G., Anthony M. Koehle, Devendra Paudel, Thomas Neuberger, A. Catharine Ross, Vishal Singh, Teodoro Bottiglieri, and Rita Castro. 2024. "Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice" Nutrients 16, no. 3: 330. https://doi.org/10.3390/nu16030330
APA StyleAndrews, S. G., Koehle, A. M., Paudel, D., Neuberger, T., Ross, A. C., Singh, V., Bottiglieri, T., & Castro, R. (2024). Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice. Nutrients, 16(3), 330. https://doi.org/10.3390/nu16030330








