Efficiency of Skeletal Muscle Mass/Weight Measurement for Distinguishing Metabolic Dysfunction-Associated Steatotic Liver Disease: A Prospective Analysis Using InBody Bioimpedance Devices
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diagnosis of Hepatic Steatosis by Abdominal Ultrasonography
2.3. Measurement of Body Composition Components, Hand Grip Strength, and Waist Circumference
2.4. Statistical Analysis
3. Results
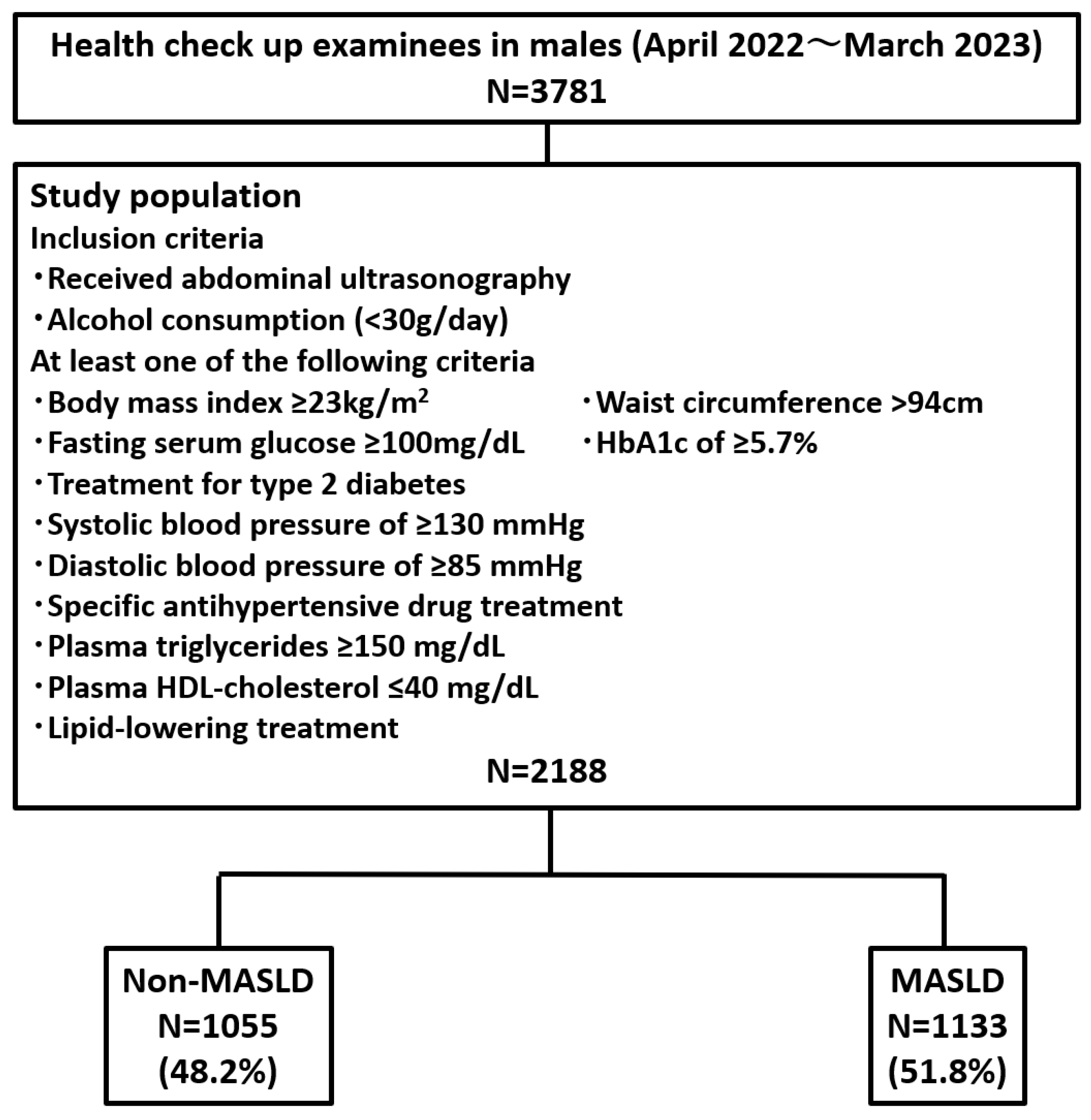
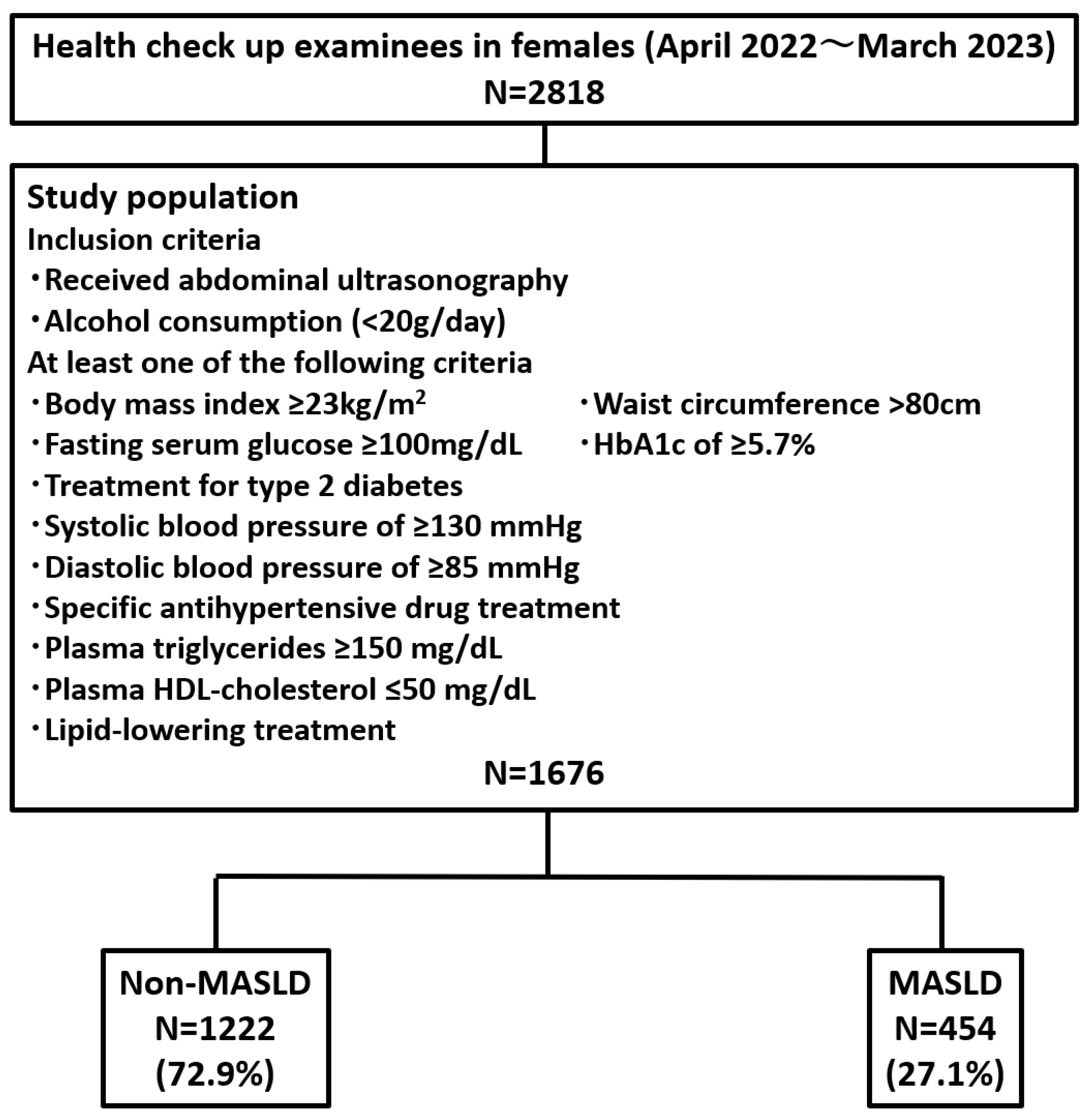
3.1. Prevalence of MASLD Among Health Checkup Examinees
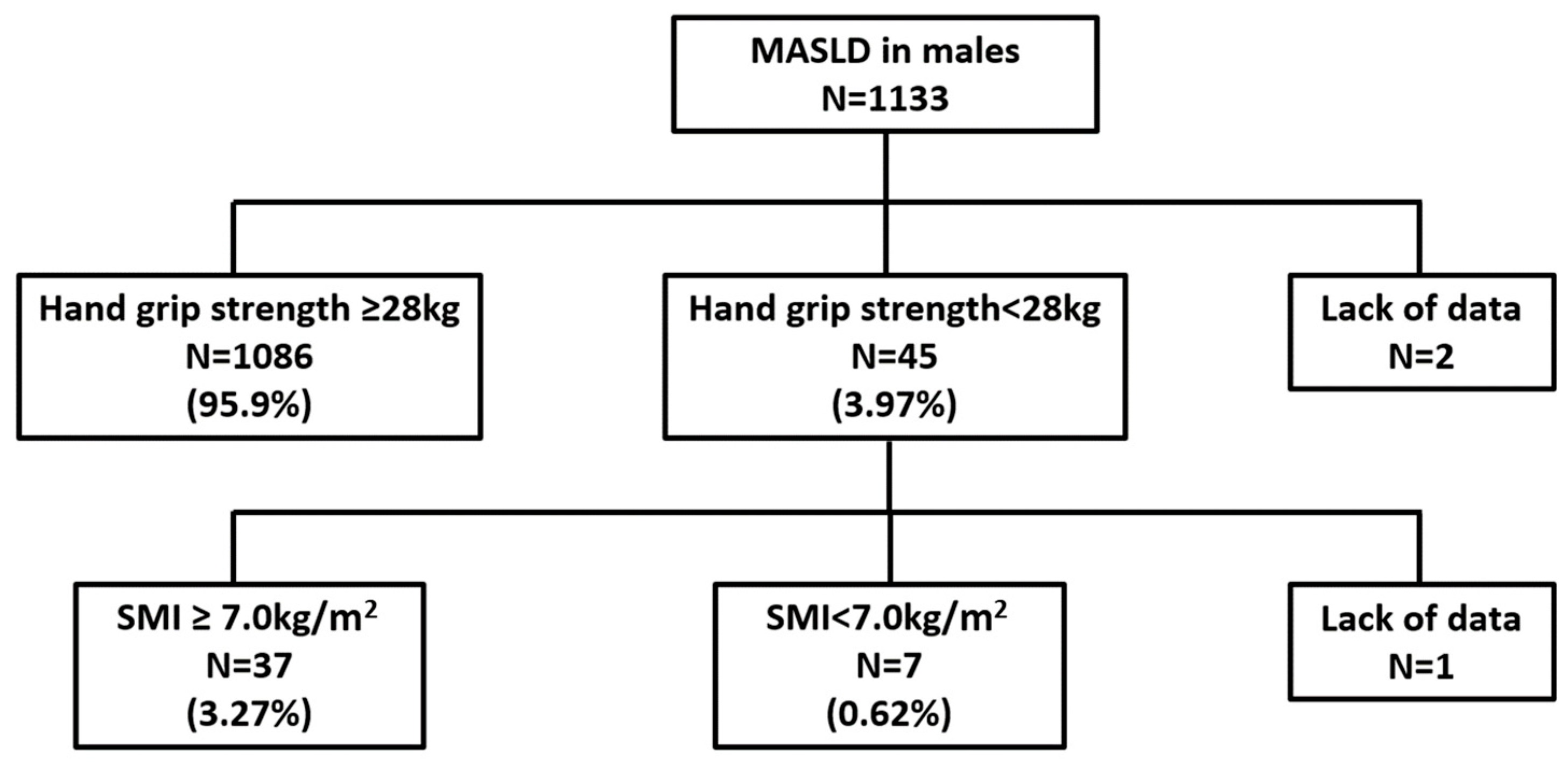
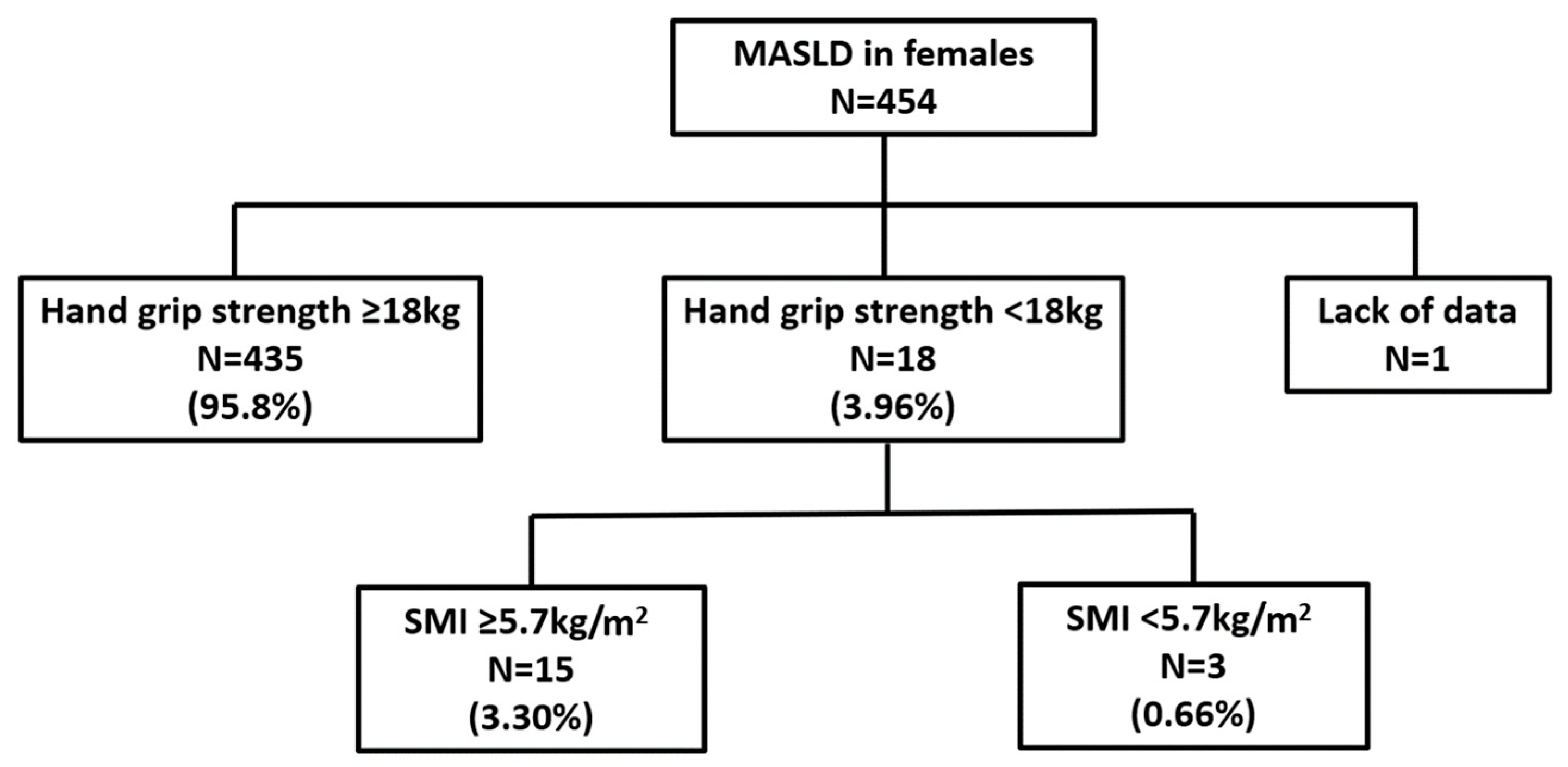
3.2. Prevalence of Sarcopenia Among Health Checkup Examinees
3.3. Characteristics of the Non-MASLD Group and the MASLD Group
3.4. Identification of Factors Strongly Associated with MASLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henry, L.; Paik, J.; Younossi, Z.M. Review article: The epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment. Pharmacol. Ther. 2022, 56, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Kawakami, M.; Murawaki, Y.; Senda, M. The impact of visceral fat in nonalcoholic fatty liver disease: Cross-sectional and longitudinal studies. J. Gastroenterol. 2007, 42, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef]
- Tokushige, K.; Ikejima, K.; Ono, M.; Eguchi, Y.; Kamada, Y.; Itoh, Y.; Akuta, N.; Yoneda, M.; Iwasa, M.; Yoneda, M.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. Hepatol. Res. 2021, 51, 1013–1025. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Takahashi, H.; Gerber, L.H. Clinics in liver disease: Update on nonalcoholic steatohepatitis: Sarcopenia and nonalcoholic fatty liver disease. Clin. Liver Dis. 2023, 27, 275–286. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Hara, N.; Moriya, K.; Hino, K.; Koike, K. Reduced handgrip strength predicts poorer survival in chronic liver diseases: A large multicenter study in Japan. Hepatol. Res. 2021, 51, 957–967. [Google Scholar] [CrossRef]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shinkai, S.; Murayama, H.; Mori, S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy x-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr. Gerontol. Int. 2015, 15, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Visser, M.; De Meersman, R.E.; Sepúlveda, D.; Baumgartner, R.N.; Pierson, R.N.; Harris, T.; Heymsfield, S.B. Appendicular skeletal muscle mass: Effects of age, gender, and ethnicity. J. Appl. Physiol. 1997, 83, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B.; et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef]
- Petta, S.; Ciminnisi, S.; Di Marco, V.; Cabibi, D.; Cammà, C.; Licata, A.; Marchesini, G.; Craxì, A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2017, 45, 510–518. [Google Scholar] [CrossRef]
- Cai, C.; Song, X.; Chen, Y.; Chen, X.; Yu, C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2020, 14, 115–126. [Google Scholar] [CrossRef]
- Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Zhu, K.F.; Zhou, W.J.; Zhang, Q.; Deng, D.F.; Yang, Y.C.; Lu, W.W.; Xu, J.; Yang, Y.M. Sarcopenia is associated with the presence of nonalcoholic fatty liver disease in Zhejiang Province, China: A cross-sectional observational study. BMC Geriatr. 2021, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Gray, S.R.; Forrest, E.; Welsh, P.; Sattar, N.; Celis-Morales, C.; Ho, F.K.; Pell, J.P. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 2022, 76, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Non–MASLD (n = 1055) | MASLD (n = 1133) | p–Value | non–MASLD (n = 1222) | MASLD (n = 454) | p–Value | ||
| Age (years) | 53.0 (45.0–60.0) | 54.0 (47.0–59.0) | n.s | Age (years) | 52.0 (46.0–58.0) | 54.0 (48.0–59.0) | 0.0012 |
| Height (cm) | 170.5 (166.4–174.4) | 170.4 (166.6–174.3) | n.s | Height (cm) | 157.7 (154.1–161.3) | 157.4 (153.6–160.8) | n.s |
| Weight (kg) | 65.9 (60.4–71.7) | 74.3 (67.9–82.8) | <0.0001 | Weight (kg) | 53.3 (48.1–59.1) | 65.6 (59.0–73.2) | <0.0001 |
| BMI (kg/m2) | 22.9 (21.1–24.3) | 25.7 (23.8–28.1) | <0.0001 | BMI (kg/m2) | 21.5 (19.6–23.5) | 26.3 (23.8–29.7) | <0.0001 |
| WC (cm) | 81.5 (76.8–86.1) | 89.8 (84.6–96.4) | <0.0001 | WC (cm) | 77.6 (71.5–83.5) | 89.0 (83.7–96.5) | <0.0001 |
| TBW (L) | 37.8 (35.3–40.8) | 40.0 (37.1–43.6) | <0.0001 | TBW (L) | 27.3 (25.6–29.4) | 29.7 (27.5–32.0) | <0.0001 |
| ICW (L) | 23.5 (21.8–25.3) | 24.9 (23.1–27.1) | <0.0001 | ICW (L) | 16.8 (15.7–18.1) | 18.3 (16.9–19.7) | <0.0001 |
| ECW (L) | 14.4 (13.4–15.5) | 15.1 (14.0–16.5) | <0.0001 | ECW (L) | 10.6 (9.9–11.3) | 11.4 (10.6–12.3) | <0.0001 |
| Protein (kg) | 10.2 (9.4–10.9) | 10.7 (10.0–11.7) | <0.0001 | Protein (kg) | 7.3 (6.8–7.8) | 7.9 (7.3–8.5) | <0.0001 |
| Minerals (kg) | 3.42 (3.17–3.70) | 3.64 (3.37–3.99) | <0.0001 | Minerals (kg) | 2.56 (2.42–2.78) | 2.79 (2.55–3.02) | <0.0001 |
| BFM (kg) | 14.0 (11.0–17.4) | 20.0 (16.2–25.0) | <0.0001 | BFM (kg) | 16.2 (12.6–20.0) | 24.8 (20.5–30.6) | <0.0001 |
| SLM (kg) | 48.6 (45.3–52.5) | 51.4 (47.7–56.0) | <0.0001 | SLM (kg) | 35.0 (32.8–37.7) | 38.0 (35.2–41.0) | <0.0001 |
| FFM (kg) | 51.4 (47.9–55.5) | 54.4 (50.5–59.2) | <0.0001 | FFM (kg) | 37.2 (34.8–40.0) | 40.4 (37.3–43.5) | <0.0001 |
| SMM (kg) | 28.6 (26.4–31.0) | 30.4 (28.1–33.4) | <0.0001 | SMM (kg) | 19.9 (18.5–21.6) | 21.8 (20.0–23.7) | <0.0001 |
| PBF (%) | 21.3 (17.7–25.0) | 27.0 (23.4–31.1) | <0.0001 | PBF (%) | 30.4 (25.4–34.5) | 38.0 (34.3–42.1) | <0.0001 |
| SMI (kg/m2) | 7.6 (7.1–8.0) | 7.9 (7.5–8.5) | <0.0001 | SMI (kg/m2) | 5.9 (5.6–6.4) | 6.6 (6.2–7.1) | <0.0001 |
| VFA (cm2) | 59.6 (46.6–75.5) | 86.9 (69.1–110.9) | <0.0001 | VFA (cm2) | 71.5 (54.7–98.1) | 125.6 (97.4–160.9) | <0.0001 |
| SMM/WT (%) | 43.8 (41.7–45.9) | 40.8 (38.3–42.9) | <0.0001 | SMM/WT (%) | 37.3 (34.9–39.8) | 33.5 (31.3–35.4) | <0.0001 |
| FFMI (kg/m2) | 17.7 (16.8–18.8) | 18.8 (17.8–19.9) | <0.0001 | FFMI (kg/m2) | 15.0 (14.2–15.8) | 16.3 (15.4–17.5) | <0.0001 |
| FMI (kg/m2) | 4.8 (3.8–6.0) | 6.9 (5.6–8.7) | <0.0001 | FMI (kg/m2) | 6.5 (5.1–8.0) | 9.9 (8.3–12.6) | <0.0001 |
| Hand grip strength (kg) | 36.8 (33.0–40.1) | 37.2 (33.6–41.4) | 0.0019 | Hand grip strength (kg) | 23.6 (21.2–25.9) | 24.1 (21.9–26.9) | 0.0007 |
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Multivariate Analysis | Multivariate Analysis | |||||
| OR | 95%CI | p–Value | OR | 95%CI | p–Value | |
| BMI | 1.05 | 0.89–1.24 | 0.5367 | 1.00 | 0.84–1.20 | 0.9875 |
| WC | 1.09 | 1.05–1.13 | <0.0001 | 1.05 | 1.02–1.08 | 0.0004 |
| TBW | 1.54 | 0.99–2.38 | 0.0529 | 0.70 | 0.38–1.26 | 0.2307 |
| Protein | 0.15 | 0.03–0.78 | 0.0242 | 1.97 | 0.19–20.35 | 0.5683 |
| PBF | 2.78 | 2.03–3.79 | <0.0001 | 2.24 | 1.51–3.33 | <0.0001 |
| SMI | 0.77 | 0.40–1.48 | 0.4382 | 2.34 | 0.97–5.65 | 0.0589 |
| SMM/WT | 5.11 | 2.99–8.73 | <0.0001 | 3.41 | 1.68–6.93 | 0.0006 |
| Hand grip strength | 1.01 | 0.99–1.03 | 0.3458 | 1.00 | 0.96–1.05 | 0.8293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsuta, M.; Masaki, T.; Kimura, S.; Sato, Y.; Tomida, A.; Ishikawa, I.; Nakamura, Y.; Takuma, K.; Nakahara, M.; Oura, K.; et al. Efficiency of Skeletal Muscle Mass/Weight Measurement for Distinguishing Metabolic Dysfunction-Associated Steatotic Liver Disease: A Prospective Analysis Using InBody Bioimpedance Devices. Nutrients 2024, 16, 4422. https://doi.org/10.3390/nu16244422
Tatsuta M, Masaki T, Kimura S, Sato Y, Tomida A, Ishikawa I, Nakamura Y, Takuma K, Nakahara M, Oura K, et al. Efficiency of Skeletal Muscle Mass/Weight Measurement for Distinguishing Metabolic Dysfunction-Associated Steatotic Liver Disease: A Prospective Analysis Using InBody Bioimpedance Devices. Nutrients. 2024; 16(24):4422. https://doi.org/10.3390/nu16244422
Chicago/Turabian StyleTatsuta, Miwa, Tsutomu Masaki, Shungo Kimura, Yudai Sato, Akemi Tomida, Ichiro Ishikawa, Yu Nakamura, Kei Takuma, Mai Nakahara, Kyoko Oura, and et al. 2024. "Efficiency of Skeletal Muscle Mass/Weight Measurement for Distinguishing Metabolic Dysfunction-Associated Steatotic Liver Disease: A Prospective Analysis Using InBody Bioimpedance Devices" Nutrients 16, no. 24: 4422. https://doi.org/10.3390/nu16244422
APA StyleTatsuta, M., Masaki, T., Kimura, S., Sato, Y., Tomida, A., Ishikawa, I., Nakamura, Y., Takuma, K., Nakahara, M., Oura, K., Tadokoro, T., Fujita, K., Tani, J., Morishita, A., Himoto, T., Miyazaki, S., Maeta, T., Mori, Y., Kohi, F., & Kobara, H. (2024). Efficiency of Skeletal Muscle Mass/Weight Measurement for Distinguishing Metabolic Dysfunction-Associated Steatotic Liver Disease: A Prospective Analysis Using InBody Bioimpedance Devices. Nutrients, 16(24), 4422. https://doi.org/10.3390/nu16244422





