Short-Term Supplementation of Sauerkraut Induces Favorable Changes in the Gut Microbiota of Active Athletes: A Proof-of-Concept Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Supplementation Protocol
2.4. Standardization of Physical Activity, Sleep, and Diet
2.5. 16S rRNA NGS of the Gut Microbiota
2.6. Laboratory Analysis
- Blood count: erythrocytes, leukocytes, neutrophils, lymphocytes;
- Metabolism: serum low-dense lipoprotein cholesterol levels (LDL), uric acid levels;
- Hormone levels: thyroid (TSH, FT3), testosterone, blood glucose (insulin, HOMA-IR), cortisol;
- Vitamins: vitamin D, B12, folic acid.
2.7. Statistical Analysis
3. Results
3.1. Sauerkraut Microbiota
3.2. The Intervention
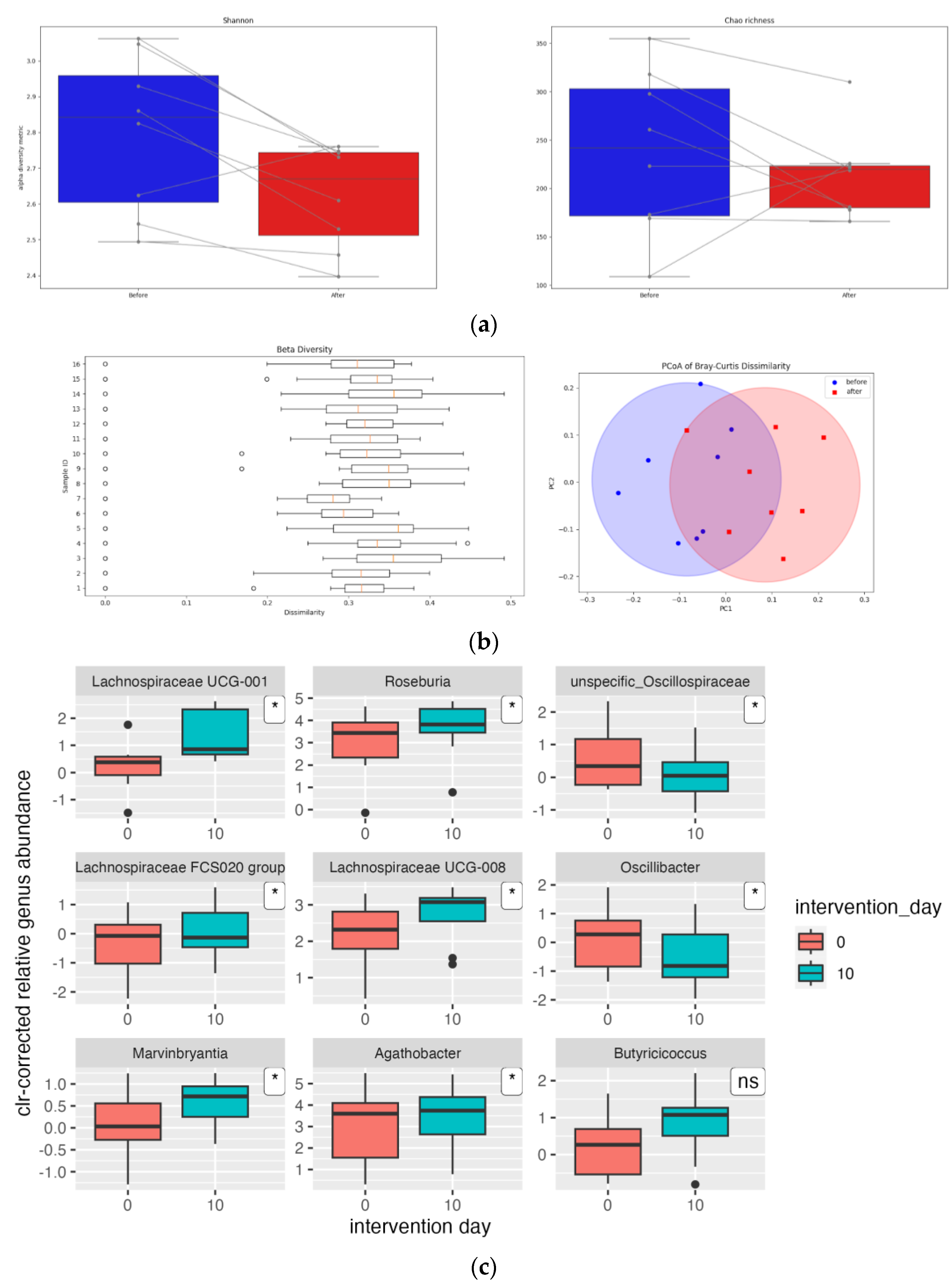
3.3. Gut Microbiota
3.4. Gut Microbiota Functionality
3.5. Laboratory Analyses
3.6. Bowel Movement and Adverse Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gagliardi, A.; Totino, V.; Cacciotti, F.; Iebba, V.; Neroni, B.; Bonfiglio, G.; Trancassini, M.; Passariello, C.; Pantanella, F.; Schippa, S. Rebuilding the Gut Microbiota Ecosystem. Int. J. Environ. Res. Public Health 2018, 15, 1679. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G. Update on the Gut Microbiome in Health and Diseases. World J. Methodol. 2024, 14, 89196. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The Role of the Microbiome for Human Health: From Basic Science to Clinical Applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Paravati, M.R.; Scarlata, G.G.M.; Boccuto, L.; Tilocca, B.; Roncada, P.; Luzza, F. Gut Microbiota and Critically Ill Patients: Immunity and Its Modulation via Probiotics and Immunonutrition. Nutrients 2023, 15, 3569. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Nolte, S.; Krüger, K.; Lenz, C.; Zentgraf, K. Optimizing the Gut Microbiota for Individualized Performance Development in Elite Athletes. Biology 2023, 12, 1491. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The Athlete Gut Microbiome and Its Relevance to Health and Performance: A Review. Sports Med. 2022, 52, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; O’Sullivan, O.; Barton, W.; Cotter, P.D.; Molloy, M.G.; Shanahan, F. Gut Microbiota: Implications for Sports and Exercise Medicine. Br. J. Sports Med. 2017, 51, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Turpin-Nolan, S.M.; Joyner, M.J.; Febbraio, M.A. Can Microbes Increase Exercise Performance in Athletes? Nat. Rev. Endocrinol. 2019, 15, 629–630. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-Omics Analysis of Elite Athletes Identifies a Performance-Enhancing Microbe That Functions via Lactate Metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef]
- Petersen, L.M.; Bautista, E.J.; Nguyen, H.; Hanson, B.M.; Chen, L.; Lek, S.H.; Sodergren, E.; Weinstock, G.M. Community Characteristics of the Gut Microbiomes of Competitive Cyclists. Microbiome 2017, 5, 98. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The Microbiome of Professional Athletes Differs from That of More Sedentary Subjects in Composition and Particularly at the Functional Metabolic Level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The Human Gut Microbiome of Athletes: Metagenomic and Metabolic Insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-Fermented Sauerkraut Improves Symptoms in IBS Patients Independent of Product Pasteurisation—A Pilot Study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Fijan, P.; Wei, L.; Marco, M.L. Health Benefits of Kimchi, Sauerkraut, and Other Fermented Foods of the Genus Brassica. Appl. Microbiol. 2024, 4, 1165–1176. [Google Scholar] [CrossRef]
- Gaudioso, G.; Weil, T.; Marzorati, G.; Solovyev, P.; Bontempo, L.; Franciosi, E.; Bertoldi, L.; Pedrolli, C.; Tuohy, K.M.; Fava, F. Microbial and Metabolic Characterization of Organic Artisanal Sauerkraut Fermentation and Study of Gut Health-Promoting Properties of Sauerkraut Brine. Front. Microbiol. 2022, 13, 929738. [Google Scholar] [CrossRef] [PubMed]
- Beganović, J.; Kos, B.; Leboš Pavunc, A.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally Produced Sauerkraut as Source of Autochthonous Functional Starter Cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Raak, C.; Ostermann, T.; Boehm, K.; Molsberger, F. Regular Consumption of Sauerkraut and Its Effect on Human Health: A Bibliometric Analysis. Glob. Adv. Health Med. 2014, 3, 12–18. [Google Scholar] [CrossRef]
- Han, K.; Bose, S.; Wang, J.; Kim, B.-S.; Kim, M.J.; Kim, E.-J.; Kim, H. Contrasting Effects of Fresh and Fermented Kimchi Consumption on Gut Microbiota Composition and Gene Expression Related to Metabolic Syndrome in Obese Korean Women. Mol. Nutr. Food Res. 2015, 59, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.H.; Noh, J.S.; Han, J.-S.; Kim, H.J.; Han, E.-S.; Song, Y.O. Kimchi, a Fermented Vegetable, Improves Serum Lipid Profiles in Healthy Young Adults: Randomized Clinical Trial. J. Med. Food 2013, 16, 223–229. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, K.-Y. Clinical Trials of Kimchi Intakes on the Regulation of Metabolic Parameters and Colon Health in Healthy Korean Young Adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lessard-Lord, J.; Roussel, C.; Lupien-Meilleur, J.; Généreux, P.; Richard, V.; Guay, V.; Roy, D.; Desjardins, Y. Short Term Supplementation with Cranberry Extract Modulates Gut Microbiota in Human and Displays a Bifidogenic Effect. NPJ Biofilms Microbiomes 2024, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of Short-Term Dietary Intervention and Probiotic Mix Supplementation on the Gut Microbiota of Elderly Obese Women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef]
- Capling, L.; Gifford, J.A.; Beck, K.L.; Flood, V.M.; Slater, G.J.; Denyer, G.S.; O’Connor, H.T. Development of an Athlete Diet Index for Rapid Dietary Assessment of Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 643–650. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Villanueva, R.A.M.; Chen, Z.J. Ggplot2: Elegant Graphics for Data Analysis. In Measurement: Interdisciplinary Research and Perspective, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2019; Volume 17, pp. 160–167. [Google Scholar] [CrossRef]
- Hiseni, P.; Rudi, K.; Wilson, R.C.; Hegge, F.T.; Snipen, L. HumGut: A Comprehensive Human Gut Prokaryotic Genomes Collection Filtered by Metagenome Data. Microbiome 2021, 9, 165. [Google Scholar] [CrossRef]
- Somerfield, P.J.; Clarke, K.R.; Gorley, R.N. A Generalised Analysis of Similarities (ANOSIM) Statistic for Designs with Ordered Factors. Austral Ecol. 2021, 46, 901–910. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wu, L.; Zhang, L.; Wang, S. Characterization of Microbiota of Naturally Fermented Sauerkraut by High-Throughput Sequencing. Food Sci. Biotechnol. 2023, 32, 855–862. [Google Scholar] [CrossRef]
- Palmnäs-Bédard, M.; de Santa Izabel, A.; Dicksved, J.; Landberg, R. Characterization of the Bacterial Composition of 47 Fermented Foods in Sweden. Foods 2023, 12, 3827. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Thriene, K.; Hansen, S.S.; Binder, N.; Michels, K.B.; Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; et al. Effects of Fermented Vegetable Consumption on Human Gut Microbiome Diversity—A Pilot Study. Fermentation 2022, 8, 118. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, A.; Baccouche, H.; Mahjoub, S.; Ben Romdhane, N. Assessment of Peripheral Blood Lymphocytosis in Adults: Determination of Thresholds for Differential Diagnosis between Clonal and Reactive Lymphocytosis. Med. Lab. J. 2021, 15, 23–30. [Google Scholar] [CrossRef]
- Chen, V.L.; Kasper, D.L. Interactions between the Intestinal Microbiota and Innate Lymphoid Cells. Gut Microbes 2014, 5, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, A.; Brosseau, C.; Bodinier, M. Immunomodulation of B Lymphocytes by Prebiotics, Probiotics and Synbiotics: Application in Pathologies. Nutrients 2023, 15, 269. [Google Scholar] [CrossRef]
- Tavakoly, R.; Hadi, A.; Rafie, N.; Talaei, B.; Marx, W.; Arab, A. Effect of Probiotic Consumption on Immune Response in Athletes: A Meta-Analysis. Int. J. Sports Med. 2021, 42, 769–781. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.-Y.; Lee, M.; Jang, J.-Y.; Choue, R. Immunomodulatory Effects of Kimchi in Chinese Healthy College Students: A Randomized Controlled Trial. Clin. Nutr. Res. 2014, 3, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Farquharson, J.; Adams, J.F. The Forms of Vitamin B12 in Foods. Br. J. Nutr. 1976, 36, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic Genome Assessment of B-Vitamin Biosynthesis Suggests Co-Operation among Gut Microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Arendt, J.F.B.; Pedersen, L.; Nexo, E.; Sørensen, H.T. Elevated Plasma Vitamin B12 Levels as a Marker for Cancer: A Population-Based Cohort Study. JNCI J. Natl. Cancer Inst. 2013, 105, 1799–1805. [Google Scholar] [CrossRef]
- Marteau, P.; Seksik, P. Tolerance of Probiotics and Prebiotics. J. Clin. Gastroenterol. 2004, 38, S67–S69. [Google Scholar] [CrossRef]
| Calories | 80 kJ/81 kcal |
|---|---|
| Protein | 0.8 g |
| Carbohydrates | 3.61 g |
| Sugar | 0.2 g |
| Fat | 0.1 g |
| Salt | 1.99 g |
| Fiber | 1.5 g |
| (a) Personal Information | ||||||
|---|---|---|---|---|---|---|
| Participant | Gender | Age (Years) | Sport | Years in Sport | Participant Classification Framework | |
| 1 | M | 27 | Karate | 20 | Tier 5 | |
| 2 | M | 37 | Table tennis | 31 | Tier 5 | |
| 3 | F | 28 | Bodybuilding | 20 | Tier 2 | |
| 4 | M | 38 | Triathlon | 30 | Tier 3 | |
| 5 | M | 30 | Kayaking/Kanu/ Rafting | 23 | Tier 5 | |
| 6 | M | 26 | Triathlon | 13 | Tier 2 | |
| 7 | M | 27 | Kayaking/Kanu/ Rafting | 19 | Tier 5 | |
| 8 | M | 23 | Soccer | 18 | Tier 3 | |
| 9 | M | 27 | Bodybuilding | 12 | Tier 4 | |
| 10 | M | 27 | Kayaking/Kanu/ Rafting | 21 | Tier 5 | |
| Average value, SD | 29 ± 4.81 | 20.7 ± 6.18 | ||||
| (b) Physical characteristics and body composition variables of participants | ||||||
| Participant | Height (cm) | Body mass (kg) | FFM (kg) | SMM (kg) | BF (%) | FM (kg) |
| 1 | 193.0 | 88.4 | 76.2 | 44.8 | 13.8 | 12.2 |
| 2 | 185.0 | 80.9 | 69.7 | 40.3 | 13.9 | 11.2 |
| 3 | 168.3 | 60.0 | - | - | - | - |
| 4 | 177.5 | 73.0 | 64.5 | 37.9 | 11.7 | 8.5 |
| 5 | 190.0 | 99.0 | 80.8 | 48.2 | 18.4 | 18.2 |
| 6 | 190.0 | 87.2 | 79.3 | 48.6 | 9.1 | 7.9 |
| 7 | 188.0 | 99.1 | 77.0 | 44.5 | 22.3 | 22.1 |
| 8 | 180.0 | 79.4 | 66.1 | 39.0 | 16.8 | 13.3 |
| 9 | 185.0 | 108.5 | 89.3 | 58.3 | 17.7 | 19.2 |
| 10 | 184.0 | 87.5 | 71.2 | 41.5 | 18.6 | 16.3 |
| (c) Data on physical activity and sleep/ | ||||||
| Before intervention | During intervention | Difference (p-value) | ||||
| Training frequency (per week) | 6.22 ± 2.28 | 6.22 ± 2.63 | 1.000 | |||
| Training duration (minutes per day) | 54.22 ± 36.94 | 61.61 ± 38.13 | 0.104 | |||
| Sleep time (hours) | 7.74 ± 0.77 | 8.06 ± 0.82 | 0.073 | |||
| (d) Daily dietary intake | ||||||
| Before intervention (average, SD) | During intervention (average, SD) | Difference (p-value) | ||||
| Energy intake (kcal) | 2741.59 ± 660.90 | 2747.72 ± 1017.84 | 0.983 | |||
| Protein intake (g) | 158.29 ± 42.08 | 160.49 ± 55.55 | 0.868 | |||
| Protein intake (g/kg) | 1.84 ± 0.29 | 1.84 ± 0.47 | 0.995 | |||
| Carbohydrate intake (g) | 293.39 ± 100.09 | 267.14 ± 76.32 | 0.288 | |||
| Carbohydrate intake (g/kg) | 3.48 ± 1.22 | 3.14 ± 0.95 | 0.208 | |||
| Fat intake (g) | 94.63 ± 19.10 | 97.25 ± 38.06 | 0.813 | |||
| Fat intake (% energy intake) | 31.39 ± 3.41 | 31.66 ± 2.14 | 0.854 | |||
| Fiber intake (g) | 21.91 ± 5.89 | 25.09 ± 5.02 | 0.111 | |||
| Fiber intake (g/1000 kcal) | 8.20 ± 2.28 | 9.98 ± 3.10 | 0.030 * | |||
| (e) ADI Results | ||||||
| Score (maximum points) | N | Before intervention (average, SD) | During intervention (average, SD) | Difference (p-value) | ||
| Special Nutrients subscale (35) | 7 | 18.4 ± 2.2 | 17.4 ± 3.2 | 0.448 | ||
| Core Nutrition subscale (80) | 7 | 47.6 ± 12.9 | 46.9 ± 8.7 | 0.860 | ||
| Dietary Habits (10) | 7 | 6.6 ± 1.6 | 6.1 ± 1.5 | 0.289 | ||
| Overall Score (125) | 10 | 72.2 ± 12.5 | 71.4 ± 10.6 | 0.937 | ||
| Overall Score (%) | 10 | 57.7 ± 9.8 | 57.1 ± 8.6 | 0.863 | ||
| (a) | |||
|---|---|---|---|
| Pathway | p-Value | Correlation Coefficient | FDR |
| pyrimidine deoxyribonucleotides de novo biosynthesis I | 0.000 | −0.928 | 0.026 |
| pyrimidine deoxyribonucleotide phosphorylation | 0.000 | −0.925 | 0.026 |
| superpathway of guanosine nucleotides de novo biosynthesis I | 0.001 | −0.916 | 0.026 |
| superpathway of pyrimidine ribonucleosides salvage | 0.001 | −0.911 | 0.026 |
| superpathway of guanosine nucleotides de novo biosynthesis II | 0.001 | −0.909 | 0.026 |
| pyrimidine deoxyribonucleotides de novo biosynthesis II | 0.001 | −0.898 | 0.026 |
| superpathway of pyrimidine ribonucleotides de novo biosynthesis | 0.001 | −0.896 | 0.026 |
| superpathway of purine nucleotides de novo biosynthesis I | 0.001 | −0.894 | 0.026 |
| superpathway of purine nucleotides de novo biosynthesis II | 0.001 | −0.889 | 0.026 |
| pyrimidine deoxyribonucleotides de novo biosynthesis III | 0.001 | −0.889 | 0.026 |
| superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis (E. coli) | 0.002 | −0.878 | 0.032 |
| superpathway of pyrimidine deoxyribonucleoside salvage | 0.002 | −0.874 | 0.033 |
| peptidoglycan maturation (meso-diaminopimelate containing) | 0.004 | 0.852 | 0.052 |
| superpathway of glycolysis and Entner-Doudoroff | 0.005 | −0.835 | 0.066 |
| superpathway of thiamin diphosphate biosynthesis I | 0.005 | −0.834 | 0.066 |
| glycogen degradation I (bacterial) | 0.009 | 0.806 | 0.104 |
| superpathway of L-alanine biosynthesis | 0.010 | 0.796 | 0.116 |
| superpathway of N-acetylneuraminate degradation | 0.011 | −0.791 | 0.118 |
| galactose degradation I (Leloir pathway) | 0.015 | 0.773 | 0.140 |
| thiamin salvage II | 0.015 | 0.772 | 0.140 |
| sucrose degradation III (sucrose invertase) | 0.016 | 0.767 | 0.142 |
| superpathway of histidine, purine, and pyrimidine biosynthesis | 0.016 | −0.764 | 0.142 |
| gluconeogenesis I | 0.022 | 0.744 | 0.163 |
| sucrose degradation IV (sucrose phosphorylase) | 0.022 | 0.741 | 0.163 |
| S-adenosyl-L-methionine cycle I | 0.022 | 0.741 | 0.163 |
| NAD salvage pathway I | 0.023 | 0.740 | 0.163 |
| CMP−3-deoxy-D-manno-octulosonate biosynthesis I | 0.024 | −0.737 | 0.163 |
| superpathway of hexuronide and hexuronate degradation | 0.024 | 0.735 | 0.163 |
| TCA cycle VI (obligate autotrophs) | 0.032 | −0.709 | 0.208 |
| superpathway of fucose and rhamnose degradation | 0.033 | 0.708 | 0.208 |
| L-glutamate and L-glutamine biosynthesis | 0.035 | 0.702 | 0.214 |
| superpathway of β-D-glucuronide and D-glucuronate degradation | 0.037 | 0.697 | 0.214 |
| L-rhamnose degradation I | 0.038 | 0.695 | 0.214 |
| starch degradation V | 0.038 | 0.694 | 0.214 |
| superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | 0.043 | −0.683 | 0.232 |
| (b) | |||
| Module | p-value | correlation coefficient | FDR |
| Polysaccharide degradation | 0.019 | 0.755 | 0.142 |
| Sugar degradation | 0.026 | 0.729 | 0.142 |
| Indicators of inflammation | 0.041 | −0.687 | 0.142 |
| Protein fermentation | 0.043 | 0.681 | 0.142 |
| Butyrate metabolism | 0.047 | −0.672 | 0.142 |
| Parameter | Unit | Before Intervention (Mean, SD) | Day after Intervention (Mean, SD) | p-Value |
|---|---|---|---|---|
| Testosterone | (mg/mL) | 19.14 ± 6.84 | 23.60 ± 10.04 | 0.363 |
| Cortisol | (mg/mL) | 500.8 ± 87.69 | 498.51 ± 144.44 | 0.974 |
| Folic acid | (mg/mL) | 25.67 ± 5.58 | 25.48 ± 5.49 | 0.882 |
| Vitamin B12 | (mg/mL) | 394.1 ± 101.1 | 356.9 ± 108.1 | 0.012 * |
| Vitamin D | (mg/mL) | 74.48 ± 12.82 | 80.2 ± 11.96 | 0.37 |
| beta | (%) | 114.84 ± 38.33 | 115.38 ± 45.36 | 0.974 |
| S | (%) | 145.19 ± 50.61 | 103.35 ± 46.2 | 0.054 |
| IR | (%) | 0.93 ± 0.46 | 0.94 ± 0.42 | 0.971 |
| Insulin | (pmol/L) | 55.51 ± 33.47 | 56.16 ± 30.09 | 0.846 |
| TSH | (mIU/L) | 2.94 ± 1.45 | 3.69 ± 2.13 | 0.146 |
| T3 | (pmol/L) | 5.21 ± 1.11 | 5.11 ± 1.07 | 0.233 |
| CRP | (mg/L) | 0.65 ± 0.24 | 0.57 ± 0.11 | 0.466 |
| LDL cholesterol | (mmol/L) | 3.39 ± 1.33 | 3.08 ± 1.13 | 0.239 |
| Urate | (μmol/L) | 285.4 ± 38.7 | 290.6 ± 44.32 | 0.824 |
| Lymphocytes | (%) | 42.04 ± 3.764 | 45.55 ± 5.38 | 0.022 * |
| Neutrophils | (%) | 47.39 ± 3.89 | 45.17 ± 6.99 | 0.221 |
| Leukocytes | (109/L) | 6.05 ± 1.31 | 6.02 ± 1.14 | 0.937 |
| Erythrocytes | (1012/L) | 4.96 ± 0.23 | 4.99 ± 0.18 | 0.681 |
| Participants (N) Indicating BTS | Probability for BTS 3 and 4 | Participants (N) Indicating Adverse Effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 4 | 5 | 6 | HR, CI, p-Value | Bloating | Diarrhea | Pain | Constipation |
| 1 | 0 | 4 | 3 | 2 | 0 | 1 | 50%, [18.7%, 81.3%], 1.000 | 1 | 1 | 1 | 0 |
| 2 | 0 | 3 | 2 | 2 | 3 | 0 | 40%, [12.2%, 73.8%], 0.527 | 1 | 0 | 0 | 0 |
| 3 | 0 | 2 | 0 | 5 | 3 | 0 | 50%, [18.8%, 81.3%], 1.000 | 2 | 0 | 0 | 0 |
| 4 | 0 | 2 | 2 | 4 | 2 | 0 | 60%, [26.2%, 87.8%], 0.527 | 1 | 0 | 0 | 0 |
| 5 | 0 | 1 | 2 | 4 | 2 | 1 | 60%, [26.2%, 87.8%], 0.527 | 3 | 1 | 1 | 0 |
| 6 | 0 | 2 | 1 | 4 | 1 | 2 | 50%, [18.7%, 81.3%], 1.000 | 3 | 2 | 1 | 0 |
| 7 | 0 | 2 | 2 | 2 | 4 | 0 | 40%, [12.2%, 73.8%], 0.527 | 2 | 0 | 0 | 0 |
| 8 | 0 | 0 | 2 | 8 | 0 | 0 | 100%, [69.2%, 100%], 0.002 * | 1 | 0 | 0 | 0 |
| 9 | 0 | 1 | 1 | 8 | 0 | 0 | 100%, [55.5%, 99.7%], 0.011 * | 1 | 0 | 0 | 0 |
| 10 | 0 | 0 | 2 | 8 | 0 | 0 | 100%, [69.2%, 100%], 0.002 * | 1 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karačić, A.; Zonjić, J.; Stefanov, E.; Radolović, K.; Starčević, A.; Renko, I.; Krznarić, Ž.; Ivančić, M.; Šatalić, Z.; Liberati Pršo, A.-M. Short-Term Supplementation of Sauerkraut Induces Favorable Changes in the Gut Microbiota of Active Athletes: A Proof-of-Concept Study. Nutrients 2024, 16, 4421. https://doi.org/10.3390/nu16244421
Karačić A, Zonjić J, Stefanov E, Radolović K, Starčević A, Renko I, Krznarić Ž, Ivančić M, Šatalić Z, Liberati Pršo A-M. Short-Term Supplementation of Sauerkraut Induces Favorable Changes in the Gut Microbiota of Active Athletes: A Proof-of-Concept Study. Nutrients. 2024; 16(24):4421. https://doi.org/10.3390/nu16244421
Chicago/Turabian StyleKaračić, Andrija, Jadran Zonjić, Ena Stefanov, Katja Radolović, Antonio Starčević, Ira Renko, Željko Krznarić, Matija Ivančić, Zvonimir Šatalić, and Ana-Marija Liberati Pršo. 2024. "Short-Term Supplementation of Sauerkraut Induces Favorable Changes in the Gut Microbiota of Active Athletes: A Proof-of-Concept Study" Nutrients 16, no. 24: 4421. https://doi.org/10.3390/nu16244421
APA StyleKaračić, A., Zonjić, J., Stefanov, E., Radolović, K., Starčević, A., Renko, I., Krznarić, Ž., Ivančić, M., Šatalić, Z., & Liberati Pršo, A.-M. (2024). Short-Term Supplementation of Sauerkraut Induces Favorable Changes in the Gut Microbiota of Active Athletes: A Proof-of-Concept Study. Nutrients, 16(24), 4421. https://doi.org/10.3390/nu16244421








