Curcumin Modulates Platelet Activation and ROS Production Induced by Amyloid Peptides: New Perspectives in Attenuating Prothrombotic Risk in Alzheimer’s Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fibrillar Amyloid Peptides
2.3. Preparation of Human Platelets
2.4. Platelet Aggregation Assay
2.5. SDS-PAGE, Western Blotting, and Immunoblotting
2.6. Flow Cytometric Analysis of Platelet Surface Expression of CD62P
2.7. Measurement of ROS Production
2.8. Statistical Analysis
3. Results
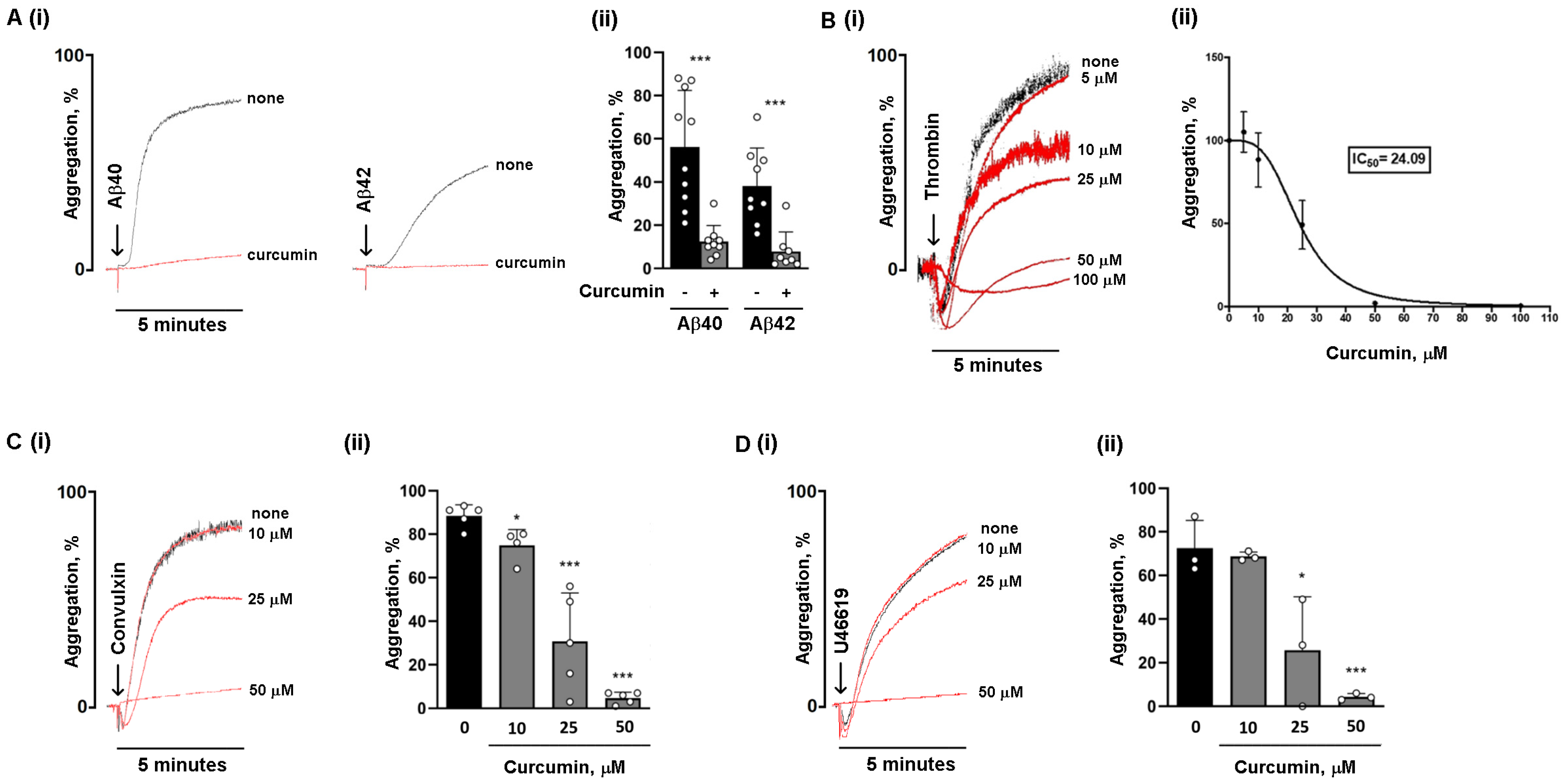
3.1. Curcumin Is More Potent in Reducing Platelet Aggregation Induced by Amyloid Peptides Compared to Classical Agonists
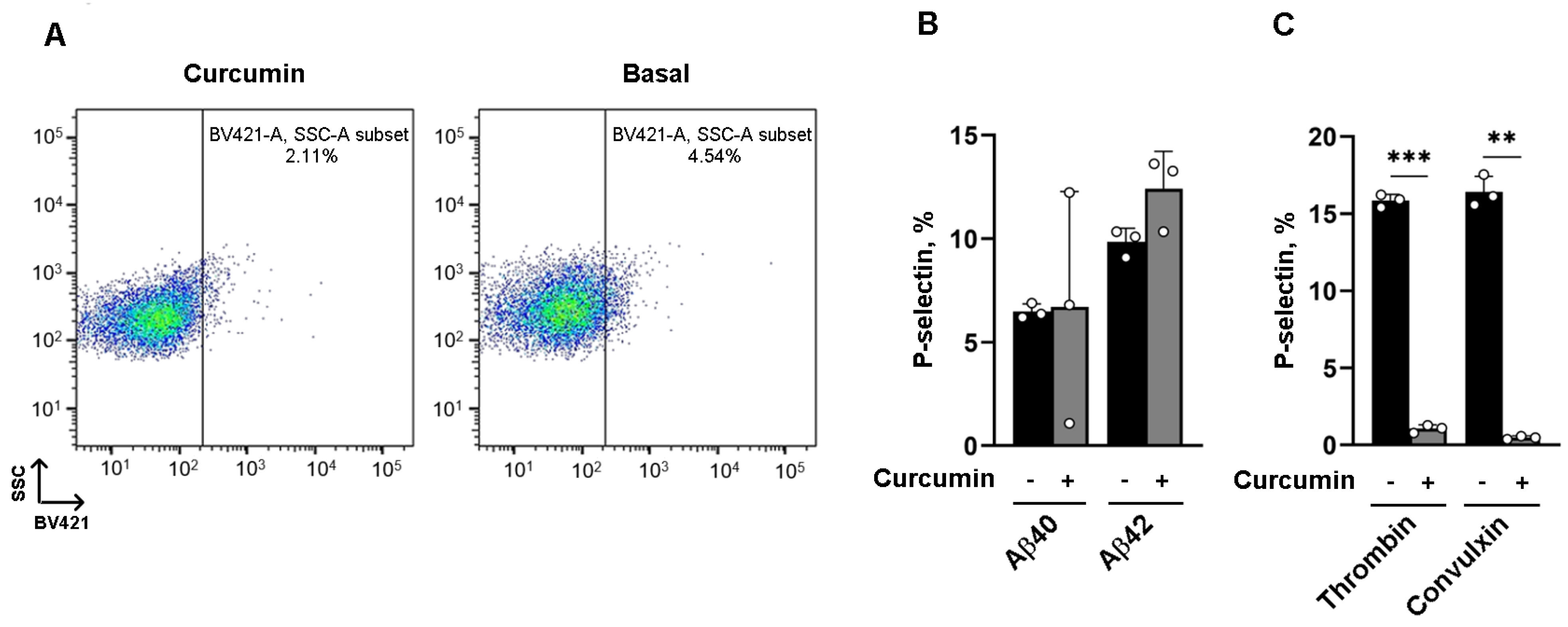
3.2. The Effect of Curcumin on α Granule Secretion Depends on the Platelet Agonist
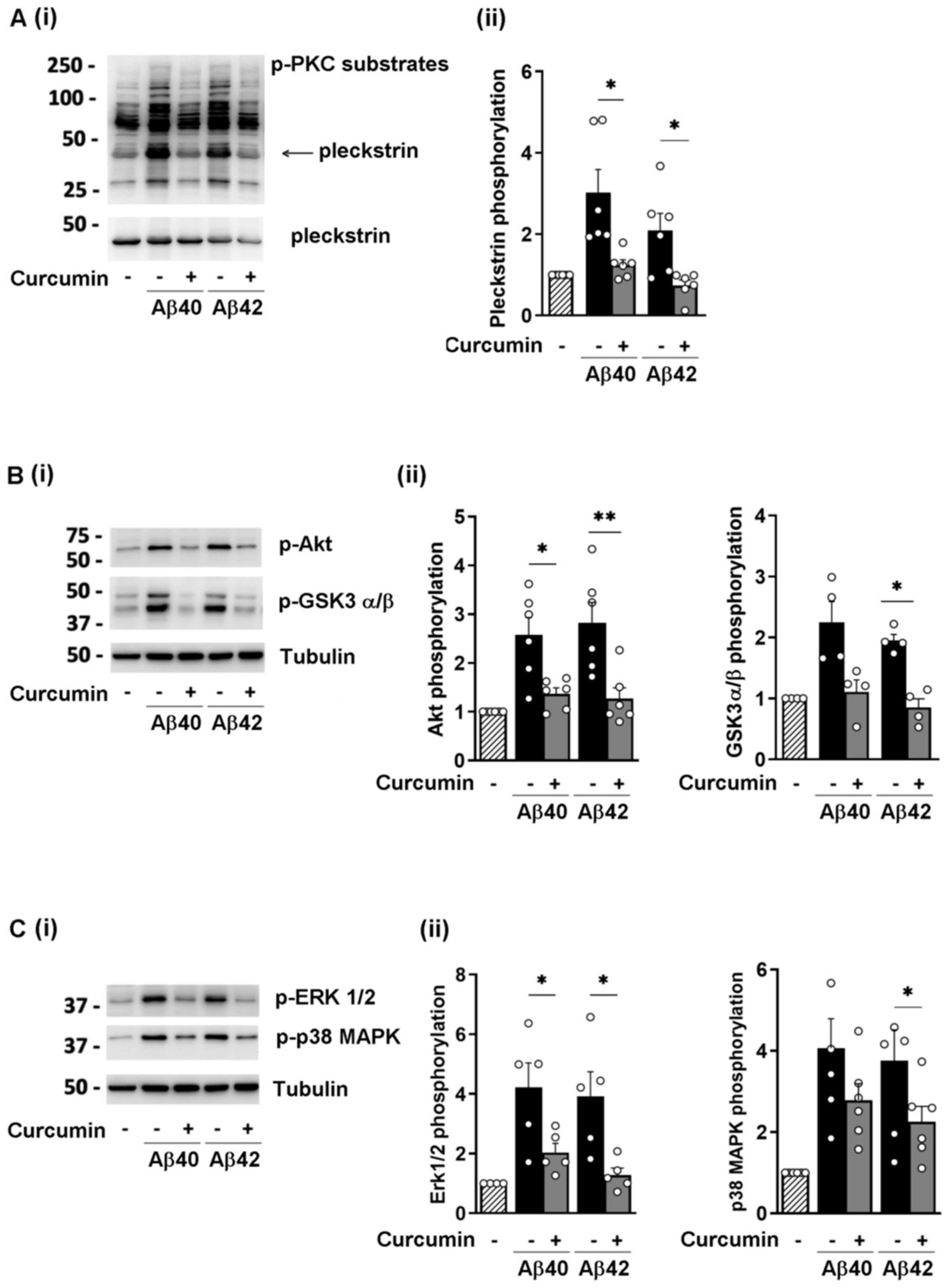
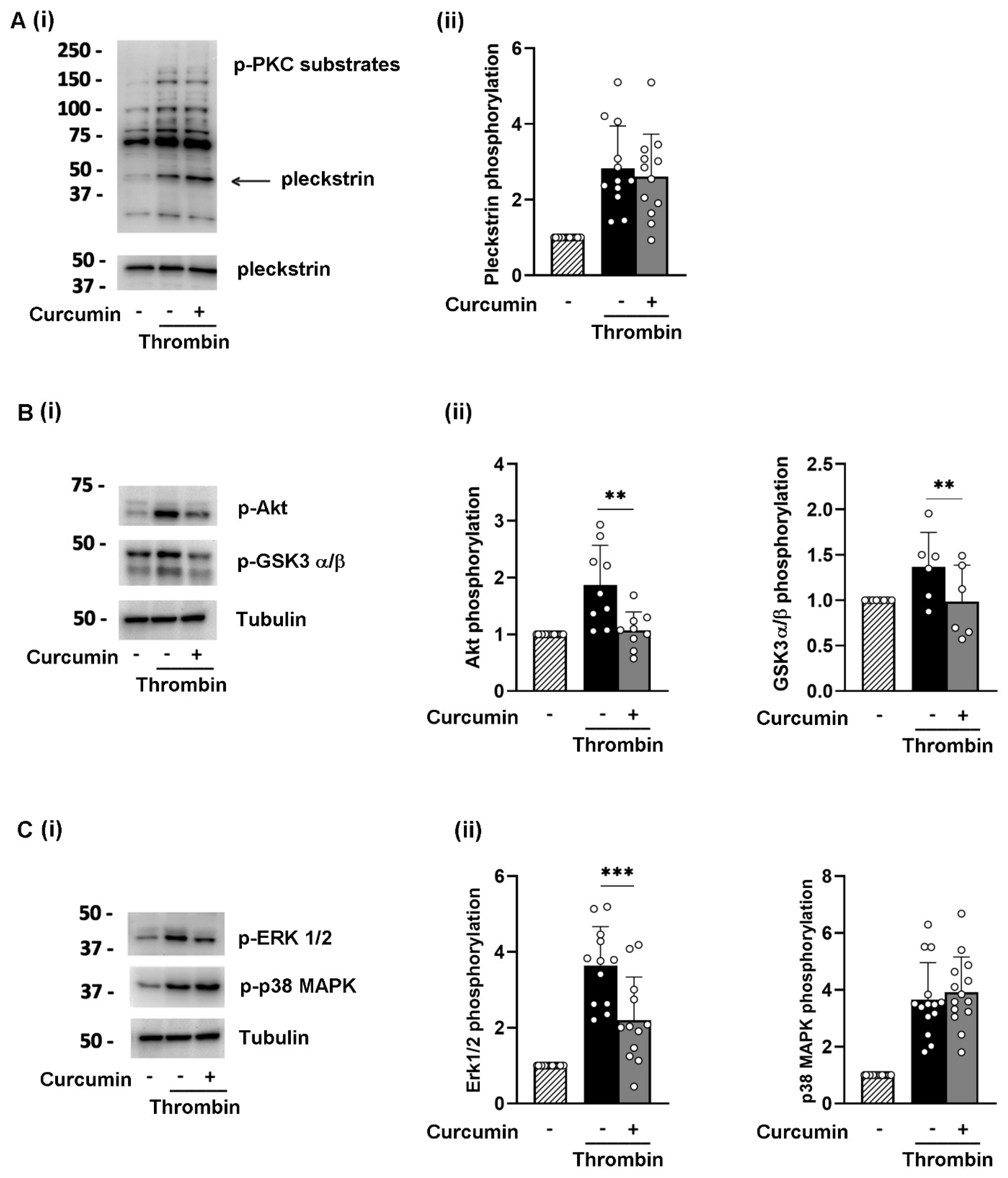
3.3. Curcumin Reduces the Phosphorylation of Selected Signaling Proteins
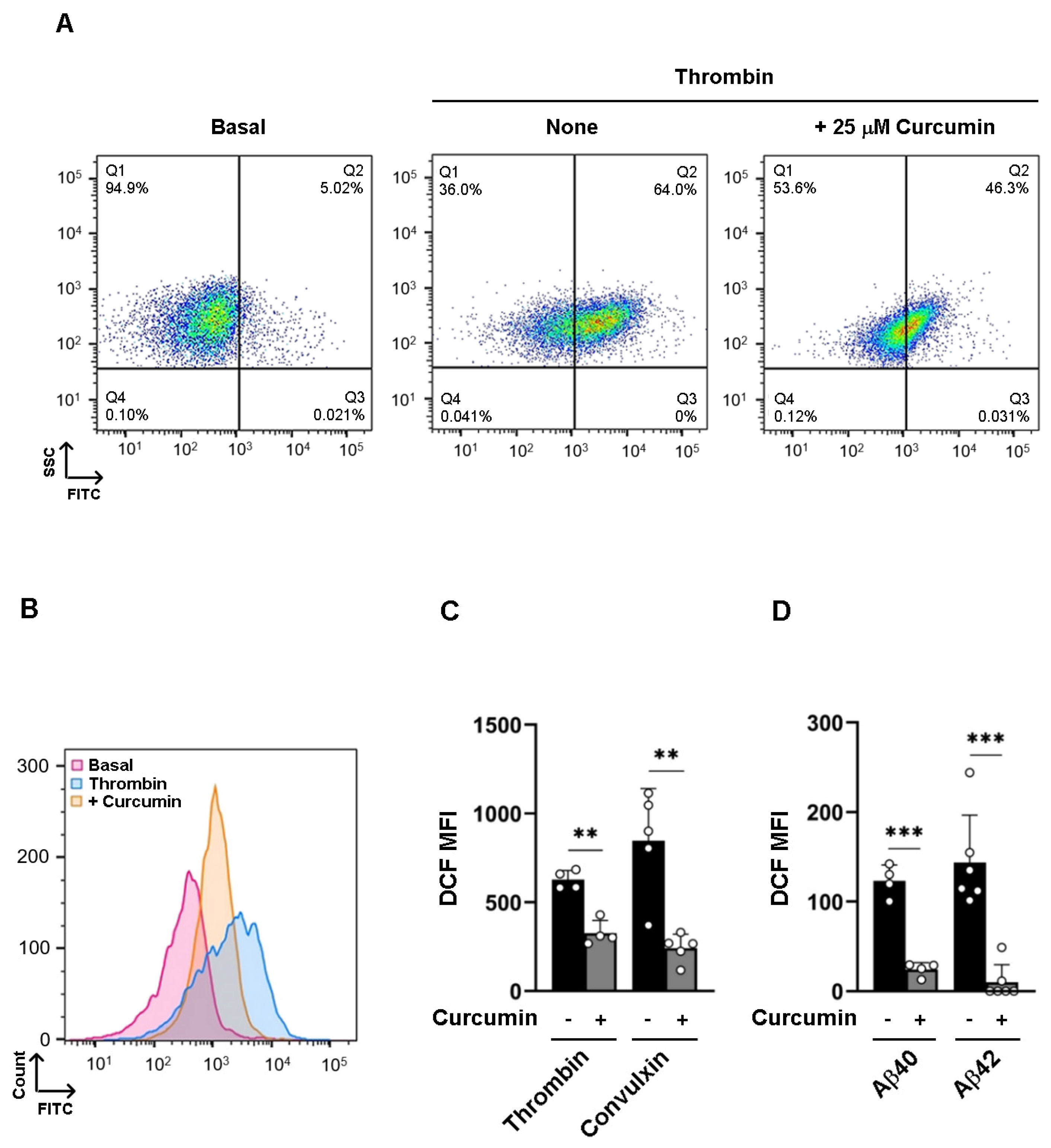
3.4. Curcumin Inhibits Intracellular ROS Formation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yubolphan, R.; Pratchayasakul, W.; Koonrungsesomboon, N.; Chattipakorn, N.; Chattipakorn, S.C. Potential links between platelets and amyloid-β in the pathogenesis of Alzheimer’s disease: Evidence from in vitro, in vivo, and clinical studies. Exp. Neurol. 2024, 374, 114683. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Martins, R.N.; Rumble, B.; Moir, R.; Fuller, S.; Milward, E.; Currie, J.; Ames, D.; Weidemann, A.; Fischer, P.; et al. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J. Biol. Chem. 1990, 265, 15977–15983. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, J.; Luo, X.; Zhu, W.; Chen, K.; Ma, J.; Jiang, H. Conformational transition of amyloid beta-peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 5403–5407. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Hsiao, G.; Fong, T.H.; Chen, H.M.; Chou, D.S.; Lin, C.H.; Sheu, J.R.; Hsu, C.Y. Amyloid beta peptide-activated signal pathways in human platelets. Eur. J. Pharmacol. 2008, 588, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Canobbio, I.; Guidetti, G.F.; Oliviero, B.; Manganaro, D.; Vara, D.; Torti, M.; Pula, G. Amyloid β-peptide-dependent activation of human platelets: Essential role for Ca2+ and ADP in aggregation and thrombus formation. Biochem. J. 2014, 462, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Donner, L.; Fälker, K.; Gremer, L.; Klinker, S.; Pagani, G.; Ljungberg, L.U.; Lothmann, K.; Rizzi, F.; Schaller, M.; Gohlke, H.; et al. Platelets contribute to amyloid-β aggregation in cerebral vessels through integrin αIIbβ3-induced outside-in signaling and clusterin release. Sci. Signal. 2016, 9, ra52. [Google Scholar] [CrossRef] [PubMed]
- Visconte, C.; Canino, J.; Guidetti, G.F.; Zarà, M.; Seppi, C.; Abubaker, A.A.; Pula, G.; Torti, M.; Canobbio, I. Amyloid precursor protein is required for in vitro platelet adhesion to amyloid peptides and potentiation of thrombus formation. Cell. Signal. 2018, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Visconte, C.; Canino, J.; Vismara, M.; Guidetti, G.F.; Raimondi, S.; Pula, G.; Torti, M.; Canobbio, I. Fibrillar amyloid peptides promote platelet aggregation through the coordinated action of ITAM- and ROS-dependent pathways. J. Thromb. Haemost. JTH 2020, 18, 3029–3042. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Washington, A.V.; Inyushin, M.Y. Amyloid Beta Peptide Is Released during Thrombosis in the Skin. Int. J. Mol. Sci. 2018, 19, 1705. [Google Scholar] [CrossRef]
- Hussain, Y.; Abdullah; Khan, F.; Alsharif, K.F.; Alzahrani, K.J.; Saso, L.; Khan, H. Regulatory Effects of Curcumin on Platelets: An Update and Future Directions. Biomedicines 2022, 10, 3180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, Z.L.; Qin, Z.H.; Liang, Z.Q. Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro. Acta Pharmacol. Sin. 2008, 29, 800–807. [Google Scholar] [CrossRef]
- Prakash, P.; Misra, A.; Surin, W.R.; Jain, M.; Bhatta, R.S.; Pal, R.; Raj, K.; Barthwal, M.K.; Dikshit, M. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb. Res. 2011, 127, 111–118. [Google Scholar] [CrossRef]
- Mayanglambam, A.; Dangelmaier, C.A.; Thomas, D.; Damodar Reddy, C.; Daniel, J.L.; Kunapuli, S.P. Curcumin inhibits GPVI-mediated platelet activation by interfering with the kinase activity of Syk and the subsequent activation of PLγ2. Platelets 2010, 21, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Rukoyatkina, N.; Shpakova, V.; Bogoutdinova, A.; Kharazova, A.; Mindukshev, I.; Gambaryan, S. Curcumin by activation of adenosine A(2A) receptor stimulates protein kinase a and potentiates inhibitory effect of cangrelor on platelets. Biochem. Biophys. Res. Commun. 2022, 586, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, Y.; Zhang, C.; Chen, B.; Zhang, X.; Yu, X.; Shuai, H.; He, Q.; Ya, F. Tetrahydrocurcumin Downregulates MAPKs/cPLA2 Signaling and Attenuates Platelet Thromboxane A2 Generation, Granule Secretion, and Thrombus Growth. Thromb. Haemost. 2022, 122, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Maheswaraiah, A.; Rao, L.J.; Naidu, K.A. Anti-platelet activity of water dispersible curcuminoids in rat platelets. Phytother. Res. PTR 2015, 29, 450–458. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Olas, B.; Saluk-Juszczak, J.; Wachowicz, B. Antioxidative properties of curcumin in the protection of blood platelets against oxidative stress in vitro. Platelets 2011, 22, 270–276. [Google Scholar] [CrossRef]
- Bisceglia, F.; Seghetti, F.; Serra, M.; Zusso, M.; Gervasoni, S.; Verga, L.; Vistoli, G.; Lanni, C.; Catanzaro, M.; De Lorenzi, E.; et al. Prenylated Curcumin Analogues as Multipotent Tools to Tackle Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Esh, C.L.; Kokjohn, T.A.; Castaño, E.M.; Van Vickle, G.D.; Kalback, W.M.; Patton, R.L.; Luehrs, D.C.; Daugs, I.D.; Kuo, Y.M.; et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2009, 5, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Gowert, N.S.; Donner, L.; Chatterjee, M.; Eisele, Y.S.; Towhid, S.T.; Münzer, P.; Walker, B.; Ogorek, I.; Borst, O.; Grandoch, M.; et al. Blood platelets in the progression of Alzheimer’s disease. PLoS ONE 2014, 9, e90523. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.C.; Bordia, A.; Verma, S.K. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot. Essent. Fat. Acids 1995, 52, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wolska, N.; Celikag, M.; Failla, A.V.; Tarafdar, A.; Renné, T.; Torti, M.; Canobbio, I.; Pula, G. Human platelets release amyloid peptides β(1-40) and β(1-42) in response to haemostatic, immune, and hypoxic stimuli. Res. Pract. Thromb. Haemost. 2023, 7, 100154. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Chen, Z.L.; Zamolodchikov, D.; Norris, E.H.; Strickland, S. Interactions of β-amyloid peptide with fibrinogen and coagulation factor XII may contribute to Alzheimer’s disease. Curr. Opin. Hematol. 2017, 24, 427–431. [Google Scholar] [CrossRef]
- Inyushin, M.; Zayas-Santiago, A.; Rojas, L.; Kucheryavykh, L. On the Role of Platelet-Generated Amyloid Beta Peptides in Certain Amyloidosis Health Complications. Front. Immunol. 2020, 11, 571083. [Google Scholar] [CrossRef] [PubMed]
- Gosztyla, M.L.; Brothers, H.M.; Robinson, S.R. Alzheimer’s Amyloid-β is an Antimicrobial Peptide: A Review of the Evidence. J. Alzheimer’s Dis. JAD 2018, 62, 1495–1506. [Google Scholar] [CrossRef]
- Doytchinova, I.; Atanasova, M.; Salamanova, E.; Ivanov, S.; Dimitrov, I. Curcumin Inhibits the Primary Nucleation of Amyloid-Beta Peptide: A Molecular Dynamics Study. Biomolecules 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef] [PubMed]
- Garodia, P.; Hegde, M.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin, inflammation, and neurological disorders: How are they linked? Integr. Med. Res. 2023, 12, 100968. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, R.; Lin, Q.; Liu, Y.; Ge, W.; Cao, H.; Li, J. Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-κB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J. Cell. Mol. Med. 2021, 25, 8947–8956. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, F.I.D.; Erinwusi, B.; Oyeyemi, O.T. Immunomodulatory activity of curcumin-entrapped poly d,l-lactic-co-glycolic acid nanoparticles in mice. Integr. Med. Res. 2018, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustichelli, S.; Lanni, C.; Zarà, M.; Guidetti, G.F.; Torti, M.; Canobbio, I. Curcumin Modulates Platelet Activation and ROS Production Induced by Amyloid Peptides: New Perspectives in Attenuating Prothrombotic Risk in Alzheimer’s Disease Patients. Nutrients 2024, 16, 4419. https://doi.org/10.3390/nu16244419
Rustichelli S, Lanni C, Zarà M, Guidetti GF, Torti M, Canobbio I. Curcumin Modulates Platelet Activation and ROS Production Induced by Amyloid Peptides: New Perspectives in Attenuating Prothrombotic Risk in Alzheimer’s Disease Patients. Nutrients. 2024; 16(24):4419. https://doi.org/10.3390/nu16244419
Chicago/Turabian StyleRustichelli, Serena, Cristina Lanni, Marta Zarà, Gianni Francesco Guidetti, Mauro Torti, and Ilaria Canobbio. 2024. "Curcumin Modulates Platelet Activation and ROS Production Induced by Amyloid Peptides: New Perspectives in Attenuating Prothrombotic Risk in Alzheimer’s Disease Patients" Nutrients 16, no. 24: 4419. https://doi.org/10.3390/nu16244419
APA StyleRustichelli, S., Lanni, C., Zarà, M., Guidetti, G. F., Torti, M., & Canobbio, I. (2024). Curcumin Modulates Platelet Activation and ROS Production Induced by Amyloid Peptides: New Perspectives in Attenuating Prothrombotic Risk in Alzheimer’s Disease Patients. Nutrients, 16(24), 4419. https://doi.org/10.3390/nu16244419






