Urinary Metabolite Profiles of Participants with Overweight and Obesity Prescribed a Weight Loss High Fruit and Vegetable Diet: A Single Arm Intervention Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment
2.3. Dietary Intake
2.4. Urine Collection
2.5. Urine Analysis
2.5.1. Chemicals
2.5.2. H NMR Sample Preparation and Metabolomics Data Collection
2.5.3. Metabolite Identification and Quantification Using 1H NMR Spectroscopy
2.6. Biostatistical and Bioinformatic Analysis
2.6.1. Univariate Analysis
2.6.2. Statistics
2.6.3. Machine Learning Models to Identify Significant Change Between the Weeks
2.6.4. Model Development
2.6.5. Metabolite Set Enrichment Analysis
3. Results
3.1. Participant Characteristics, Summary of Urinary Metabolites and Dietary Intake
3.2. Training Model Accuracy
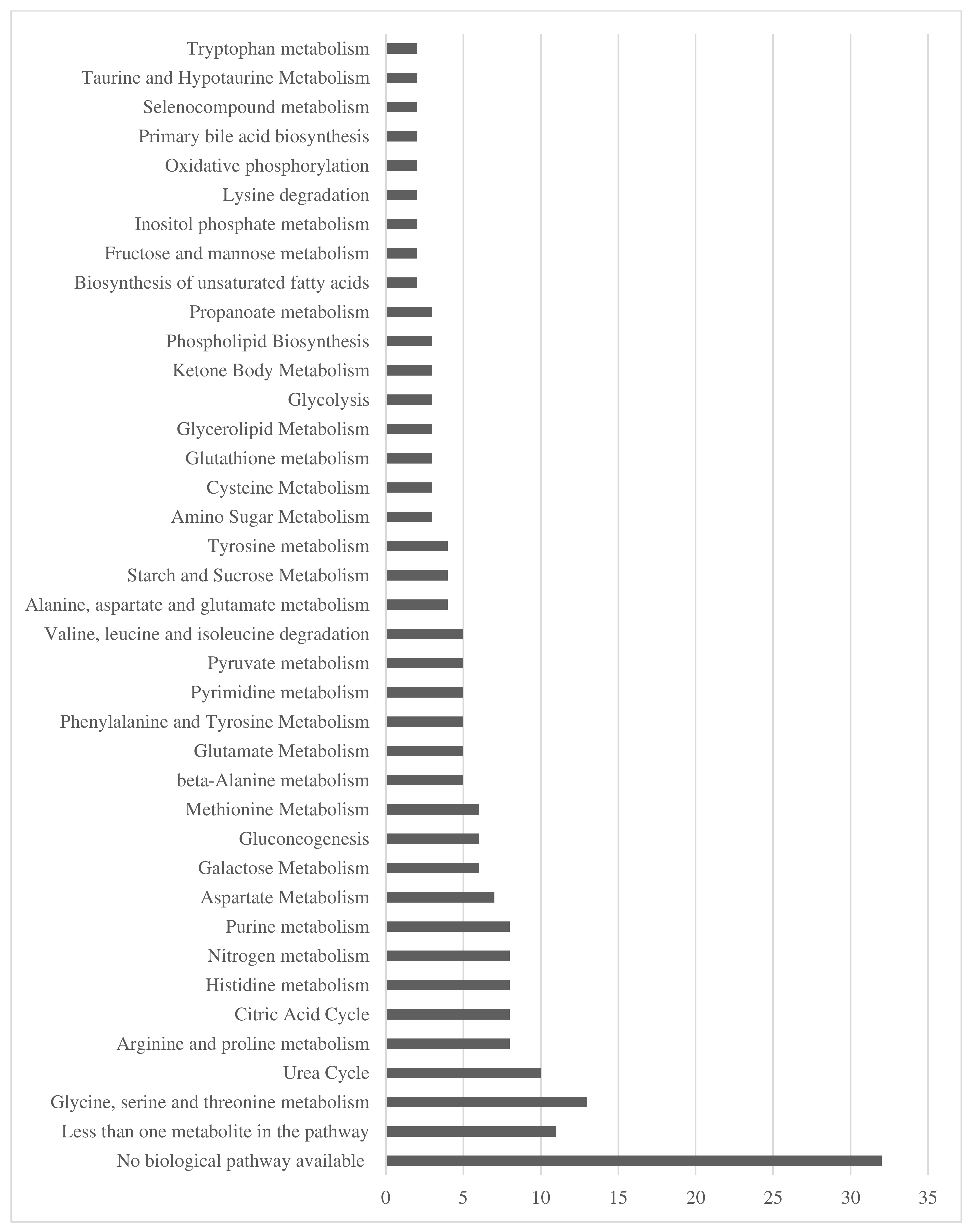
3.3. Urinary Metabolites
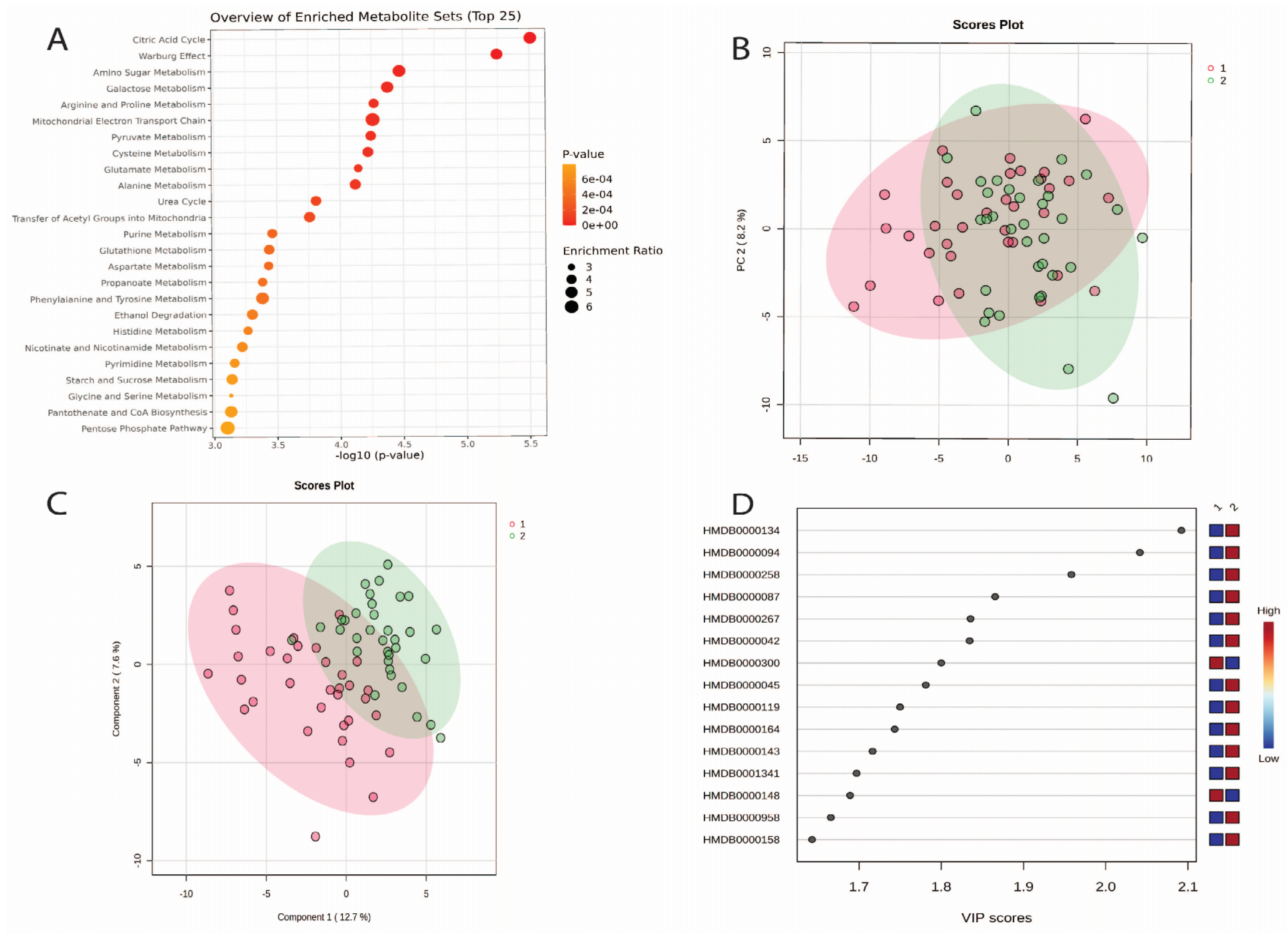
3.4. Changes in Urine Metabolites Baseline and Week 2
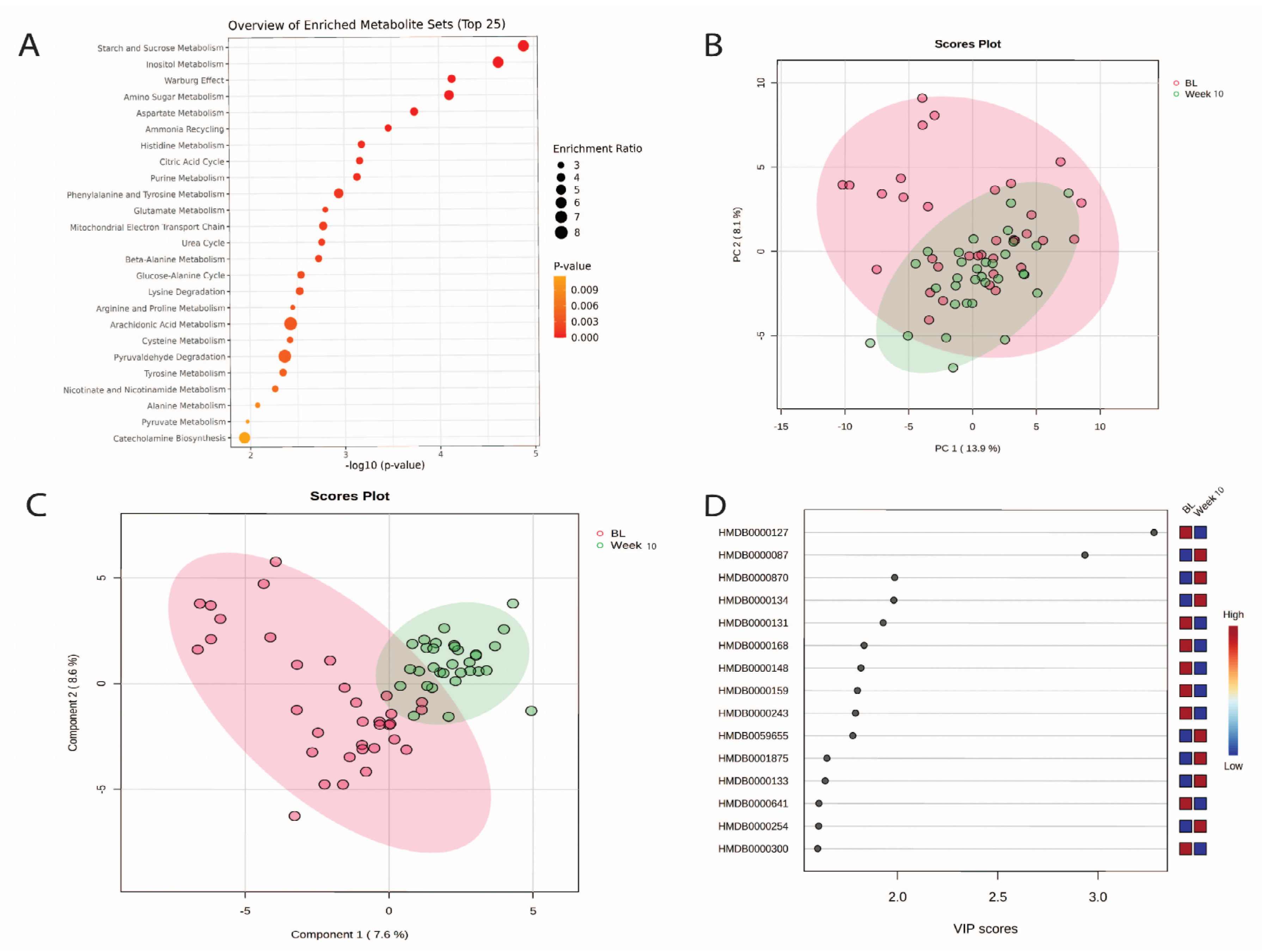
3.5. Changes in Urinary Metabolites from Baseline to Week 10
3.6. Changes in Urinary Metabolites from Week 2 to Week 10
3.7. Machine Learning Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yárnoz-Esquiroz, P.; Olazarán, L.; Aguas-Ayesa, M.; Perdomo, C.M.; García-Goñi, M.; Silva, C.; Fernández-Formoso, J.A.; Escalada, J.; Montecucco, F.; Portincasa, P.; et al. ‘Obesities’: Position statement on a complex disease entity with multifaceted drivers. Eur. J. Clin. Investig. 2022, 52, e13811. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 October 2024).
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO European Regional Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022; pp. 132–141. [Google Scholar]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Trius-Soler, M.; Lamuela-Raventós, R.M.; Zamora-Ros, R. Vegetable and Fruit Consumption and Prognosis Among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2020, 11, 1569–1582. [Google Scholar] [CrossRef]
- Kalmpourtzidou, A.; Eilander, A.; Talsma, E.F. Global Vegetable Intake and Supply Compared to Recommendations: A Systematic Review. Nutrients 2020, 12, 1558. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 28 September 2022).
- Australian Bureau of Statistics. Dietary Behaviour. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/dietary-behaviour/latest-release (accessed on 2 April 2024).
- National Health and Medical Research Council. Eat for Health: Australian Dietary Guidelines; Department of Health and Ageing: Canberra, Australia, 2013. [Google Scholar]
- Nouri, M.; Shateri, Z.; Faghih, S. The relationship between intake of fruits, vegetables and dairy products with overweight and obesity in a large sample in Iran: Findings of STEPS 2016. Front. Nutr. 2023, 9, 1082976. [Google Scholar] [CrossRef]
- Nour, M.; Lutze, S.A.; Grech, A.; Allman-Farinelli, M. The Relationship between Vegetable Intake and Weight Outcomes: A Systematic Review of Cohort Studies. Nutrients 2018, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Hefner, M.; Kudchadkar, G.; Hia, R.A.; Sultan, M.A.; Booe, H.; Dhurandhar, N.V. Confronting the challenge of promoting fruit and vegetable intake for obesity management: An alternative approach. Int. J. Obes. 2024, 48, 1043–1044. [Google Scholar] [CrossRef] [PubMed]
- Mytton, O.T.; Nnoaham, K.; Eyles, H.; Scarborough, P.; Ni Mhurchu, C. Erratum to: Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health 2017, 17, 662. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.A.; Brown, A.W.; Bohan Brown, M.M.; Shikany, J.M.; Mattes, R.D.; Allison, D.B. Increased fruit and vegetable intake has no discernible effect on weight loss: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 567–576. [Google Scholar] [CrossRef]
- Ojo, O. Nutrition and Chronic Conditions. Nutrients 2019, 11, 459. [Google Scholar] [CrossRef]
- Bailey, R.L. Overview of dietary assessment methods for measuring intakes of foods, beverages, and dietary supplements in research studies. Curr. Opin. Biotechnol. 2021, 70, 91–96. [Google Scholar] [CrossRef]
- Kirkpatrick, S.I.; Baranowski, T.; Subar, A.F.; Tooze, J.A.; Frongillo, E.A. Best Practices for Conducting and Interpreting Studies to Validate Self-Report Dietary Assessment Methods. J. Acad. Nutr. Diet. 2019, 119, 1801–1816. [Google Scholar] [CrossRef]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Djoumbou-Feunang, Y.; Dorrestein, P.C.; Dragsted, L.O.; Draper, J.; et al. Perspective: Dietary Biomarkers of Intake and Exposure-Exploration with Omics Approaches. Adv. Nutr. 2020, 11, 200–215. [Google Scholar] [CrossRef]
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef]
- Kaaks, R.; Ferrari, P.; Ciampi, A.; Plummer, M.; Riboli, E. Uses and limitations of statistical accounting for random error correlations, in the validation of dietary questionnaire assessments. Public. Health Nutr. 2002, 5, 969–976. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Serra, F.; Rodríguez, A.M.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O.; Gao, Q.; Scalbert, A.; Vergères, G.; Kolehmainen, M.; Manach, C.; Brennan, L.; Afman, L.A.; Wishart, D.S.; Andres Lacueva, C.; et al. Validation of biomarkers of food intake-critical assessment of candidate biomarkers. Genes. Nutr. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.D.; Rollo, M.E.; Pezdirc, K.; Collins, C.E.; Haslam, R.L. Urinary biomarkers of dietary intake: A review. Nutr. Rev. 2020, 78, 364–381. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.D.; Ferguson, J.J.; Stanford, J.; Collins, C.E. Dietary Assessment and Metabolomic Methodologies in Human Feeding Studies: A Scoping Review. Adv. Nutr. 2023, 14, 1453–1465. [Google Scholar] [CrossRef]
- Macias, S.; Kirma, J.; Yilmaz, A.; Moore, S.E.; McKinley, M.C.; McKeown, P.P.; Woodside, J.V.; Graham, S.F.; Green, B.D. Application of (1)H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet. Metabolites 2019, 9, 201. [Google Scholar] [CrossRef]
- Macias, S.; Yilmaz, A.; Kirma, J.; Moore, S.E.; Woodside, J.V.; Graham, S.F.; Green, B.D. Non-targeted LC-MS/MS metabolomic profiling of human plasma uncovers a novel Mediterranean diet biomarker panel. Metabolomics 2023, 20, 3. [Google Scholar] [CrossRef]
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Schumacher, T.; Haslam, R.L. Changes in vegetable and fruit intakes and effects on anthropometric outcomes in males and females. Nutr. Diet. 2021, 78, 192–201. [Google Scholar] [CrossRef]
- Subar, A.; Thompson, F.; Potischman, N.; Forsyth, B.; Buday, R.; Richards, D.; McNutt, S.; Hull, S.; Guenther, P.; Schatzkin, A.; et al. Formative research of a quick list for an automated self-administered 24-hour dietary recall. J. Am. Diet. Assoc. 2007, 107, 1002–1007. [Google Scholar] [CrossRef]
- Yuan, C.; Spiegelman, D.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Barnett, J.B.; Chavarro, J.E.; Rood, J.C.; Harnack, L.J.; Sampson, L.K.; et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records as Compared with Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am. J. Epidemiol. 2017, 187, 1051–1063. [Google Scholar] [CrossRef]
- Food Standards Australia and New Zealand. AUSNUT 2011-13 Food Nutrient Database. Available online: https://www.foodstandards.gov.au/science-data/food-composition-databases/ausnut-2011-13/ausnutdatafiles/foodnutrient (accessed on 21 October 2019).
- Clarke, E.D.; Rollo, M.E.; Collins, C.E.; Wood, L.; Callister, R.; Philo, M.; Kroon, P.A.; Haslam, R.L. The Relationship between Dietary Polyphenol Intakes and Urinary Polyphenol Concentrations in Adults Prescribed a High Vegetable and Fruit Diet. Nutrients 2020, 12, 3431. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; MacDonald, P.E.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Ashton, L.; Williams, R.; Wood, L.; Schumacher, T.; Burrows, T.; Rollo, M.; Pezdirc, K.; Callister, R.; Collins, C.E. The comparative validity of a brief diet screening tool for adults: The Fruit and Vegetable VAriety index (FAVVA). Clin. Nutr. ESPEN 2019, 29, 189–197. [Google Scholar] [CrossRef]
- McNaughton, S.A.; Hughes, M.C.; Marks, G.C. Validation of a FFQ to estimate the intake of PUFA using plasma phospholipid fatty acids and weighed foods records. Br. J. Nutr. 2007, 97, 561–568. [Google Scholar] [CrossRef]
- Slavin, J.; Carlson, J. Carbohydrates. Adv. Nutr. 2014, 5, 760–761. [Google Scholar] [CrossRef]
- Shimi, G. Dietary approaches for controlling cancer by limiting the Warburg effect: A review. Nutr. Rev. 2024, 82, 1281–1291. [Google Scholar] [CrossRef]
- Tran, Q.; Lee, H.; Kim, C.; Kong, G.; Gong, N.; Kwon, S.H.; Park, J.; Kim, S.H.; Park, J. Revisiting the Warburg Effect: Diet-Based Strategies for Cancer Prevention. BioMed Res. Int. 2020, 2020, 8105735. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Bona, E.; Leone, I.; Samà, M.T.; Nuzzo, A.; Ferrero, A.; Aimaretti, G.; Marzullo, P.; Prodam, F. Inositols and metabolic disorders: From farm to bedside. J. Tradit. Complement. Med. 2020, 10, 252–259. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Dehghani, A.; Faghfouri, A.H.; Radkhah, N.; Naemi Kermanshahi, M.; Hamedi Kalajahi, F.; Mohammadzadeh Honarvar, N.; Ghoreishi, Z.; Ostadrahimi, A.; Ebrahimi Mamaghani, M. Inositol supplementation and body mass index: A systematic review and meta-analysis of randomized clinical trials. Obes. Sci. Pract. 2022, 8, 387–397. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Kohlmeier, M. Acetate. In Nutrient Metabolism; Kohlmeier, M., Ed.; Academic Press: London, UK, 2003; pp. 147–153. [Google Scholar] [CrossRef]
- Bose, S.; Ramesh, V.; Locasale, J.W. Acetate Metabolism in Physiology, Cancer, and Beyond. Trends Cell Biol. 2019, 29, 695–703. [Google Scholar] [CrossRef]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef]
- Yuan, L.; Muli, S.; Huybrechts, I.; Nöthlings, U.; Ahrens, W.; Scalbert, A.; Floegel, A. Assessment of Fruit and Vegetables Intake with Biomarkers in Children and Adolescents and Their Level of Validation: A Systematic Review. Metabolites 2022, 12, 126. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef]
- Madrid-Gambin, F.; Llorach, R.; Vázquez-Fresno, R.; Urpi-Sarda, M.; Almanza-Aguilera, E.; Garcia-Aloy, M.; Estruch, R.; Corella, D.; Andres-Lacueva, C. Urinary (1)H Nuclear Magnetic Resonance Metabolomic Fingerprinting Reveals Biomarkers of Pulse Consumption Related to Energy-Metabolism Modulation in a Subcohort from the PREDIMED study. J. Proteome Res. 2017, 16, 1483–1491. [Google Scholar] [CrossRef]
- Zheng, Y.; He, J.Q. Pathogenic Mechanisms of Trimethylamine N-Oxide-induced Atherosclerosis and Cardiomyopathy. Curr. Vasc. Pharmacol. 2022, 20, 29–36. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dimethylamine in human urine. Clin. Chim. Acta 1995, 233, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Gibbons, H.; Rundle, M.; Frost, G.; McNulty, B.A.; Nugent, A.P.; Walton, J.; Flynn, A.; Brennan, L. The Relationship between Fish Intake and Urinary Trimethylamine-N-Oxide. Mol. Nutr. Food Res. 2020, 64, e1900799. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C.; Zhang, A.Q.; Smith, R.L. Dimethylamine and diet. Food Chem. Toxicol. 2008, 46, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Spevacek, A.R.; Benson, K.H.; Bamforth, C.W.; Slupsky, C.M. Beer metabolomics: Molecular details of the brewing process and the differential effects of late and dry hopping on yeast purine metabolism. J. Inst. Brew. 2016, 122, 21–28. [Google Scholar] [CrossRef]
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Sánchez-Pérez, S.; Comas-Basté, O.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Low-Histamine Diets: Is the Exclusion of Foods Justified by Their Histamine Content? Nutrients 2021, 13, 1395. [Google Scholar] [CrossRef]
- Yamashita, H. Biological Function of Acetic Acid-Improvement in Obesity and Glucose Tolerance by Acetic Acid in Type 2 Diabetic Rats. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. 1), S171–S175. [Google Scholar] [CrossRef]
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Boo, S.Y.; Chua, J.Y.H.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Long, K.; et al. Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat-diet-induced obese mice. Sci. Rep. 2017, 7, 6664. [Google Scholar] [CrossRef]
- Ilyés, T.; Silaghi, C.N.; Crăciun, A.M. Diet-Related Changes of Short-Chain Fatty Acids in Blood and Feces in Obesity and Metabolic Syndrome. Biology 2022, 11, 1556. [Google Scholar] [CrossRef]
- Elliott, P.; Posma, J.M.; Chan, Q.; Garcia-Perez, I.; Wijeyesekera, A.; Bictash, M.; Ebbels, T.M.; Ueshima, H.; Zhao, L.; van Horn, L.; et al. Urinary metabolic signatures of human adiposity. Sci. Transl. Med. 2015, 7, ra262–ra285. [Google Scholar] [CrossRef] [PubMed]
- Provensi, G.; Blandina, P.; Passani, M.B. The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology 2016, 106, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kukucka, T.; Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Kovacova, V.; Macejova, A.; Mlyncekova, Z.; Tonhajzerova, I. Mechanisms Involved in the Link between Depression, Antidepressant Treatment, and Associated Weight Change. Int. J. Mol. Sci. 2024, 25, 4511. [Google Scholar] [CrossRef]
- Khouma, A.; Moeini, M.M.; Plamondon, J.; Richard, D.; Caron, A.; Michael, N.J. Histaminergic regulation of food intake. Front. Endocrinol. 2023, 14, 1202089. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.; McCrickerd, K.; Yeomans, M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015, 41, 149–160. [Google Scholar] [CrossRef]
- Wanders, A.J.; van den Borne, J.J.; de Graaf, C.; Hulshof, T.; Jonathan, M.C.; Kristensen, M.; Mars, M.; Schols, H.A.; Feskens, E.J. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obes. Rev. 2011, 12, 724–739. [Google Scholar] [CrossRef]
- Ribeiro, G.; Camacho, M.; Santos, O.; Pontes, C.; Torres, S.; Oliveira-Maia, A.J. Association between hedonic hunger and body-mass index versus obesity status. Sci. Rep. 2018, 8, 5857. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Young, S.N. L-tyrosine to alleviate the effects of stress? J. Psychiatry Neurosci. 2007, 32, 224. [Google Scholar]
- Kessler, T.A.; Raja, A. Biochemistry, Histidine; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]

| Characteristics | Baseline Mean ± SD | Week 2 Mean ± SD | Week 10 Mean ± SD |
|---|---|---|---|
| Age (years) | 33.9 ± 8.3 | - | - |
| Sex (n, % female) | 18 (52.3%) | - | - |
| Smoking status (n, % smokers) | 1 (2.9%) | - | - |
| Weight (kg) | 83.6 ± 13.0 | 82.9 ± 12.8 | 81.0 ± 11.5 |
| BMI (kg/m2) | 28.9 ± 1.9 | 28.6 ± 1.9 | 28.1 ± 1.7 |
| Energy (kJ/day) | 9961.5 ± 4269.4 | 9774.8 ± 9600.0 | 7792.1 ± 3236.7 * |
| Fruit (grammes/day) | 157.5 ± 133.5 | 224.9 ± 151.5 | 270.2 ± 157.3 * |
| Vegetables (grammes/day) | 333.0 ± 223.7 | 427.1 ± 318.2 | 437.2 ± 433.7 |
| Total Fruit and Vegetables (grammes/day) | 490.5 ± 267.7 | 651.9 ± 374.7 * | 707.4 ± 469.5 * |
| Metabolite Name | Baseline | Week 2 | Week 10 | Change Baseline vs. Week 2 | Change Baseline vs. Week 10 | Change Week 2 vs. Week 10 |
|---|---|---|---|---|---|---|
| Acetic acid | 76.89 | 130.61 | 106.23 | 53.73 | 26.92 | −19.74 |
| (58.05) | (137.34) | (66.86) | (129.03) * | (71.05) * | (132.60) | |
| Dimethylamine | 60.85 | 199.47 | 282.46 | 138.61 | 226.09 | 113.48 |
| (154.02) | (273.16) | (274.65) | (330.64) * | (300.78) * | (250.24) * | |
| Choline | 28.61 | 20.20 | 33.32 | −8.41 | 3.57 | 14.83 |
| (34.12) | (22.06) | (76.41) | (29.04) * | (73.82) * | (71.77) | |
| Fumaric acid | 171.13 | 279.58 | 283.48 | 108.45 | 104.76 | 20.08 |
| (176.57) | (215.40) | (220.37) | (229.01) * | (251.33) * | (193.11) | |
| Glutamic acid | 256.90 | 178.90 | 156.61 | −78.00 | −107.74 | −11.62 |
| (312.19) | (201.65) | (127.75) | (372.24) * | (349.37) * | (160.98) | |
| L-tyrosine | 314.26 | 533.01 | 471.12 | 218.75 | 142.33 | 2.79 |
| (309.82) | (651.49) | (490.67) | (642.76) * | (582.79) * | (712.35) | |
| Histidine | 1308.04 | 2215.59 | 2154.74 | 907.55 | 806.09 | 249.87 |
| (1363.49) | (2685.40) | (2133.23) | (2624.58) * | (1666.04) * | (2618.68) | |
| Succinic acid | 50.47 | 82.25 | 87.64 | 31.78 | 35.22 | 10.80 |
| (49.86) | (73.00) | (79.98) | (76.59) * | (66.33) * | (87.36) | |
| Uracil | 95.61 | 61.57 | 68.81 | −34.03 | −28.76 | 12.16 |
| (91.38) | (67.85) | (50.59) | (108.04) * | (94.48) * | (60.82) | |
| Histamine | 262.46 | 397.61 | 608.75 | 135.16 | 331.48 | 221.90 |
| (402.60) | (496.59) | (759.52) | (618.69) * | (806.95) * | (763.01) | |
| 2-hydroxyglutarate | 254.93 | 562.82 | 379.36 | 307.89 | 114.97 | −146.39 |
| (272.00) | (855.46) | (336.66) | (742.99) * | (316.03) * | (809.86) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, E.D.; Gómez-Martín, M.; Stanford, J.; Yilmaz, A.; Ustun, I.; Wood, L.; Green, B.; Graham, S.F.; Collins, C.E. Urinary Metabolite Profiles of Participants with Overweight and Obesity Prescribed a Weight Loss High Fruit and Vegetable Diet: A Single Arm Intervention Study. Nutrients 2024, 16, 4358. https://doi.org/10.3390/nu16244358
Clarke ED, Gómez-Martín M, Stanford J, Yilmaz A, Ustun I, Wood L, Green B, Graham SF, Collins CE. Urinary Metabolite Profiles of Participants with Overweight and Obesity Prescribed a Weight Loss High Fruit and Vegetable Diet: A Single Arm Intervention Study. Nutrients. 2024; 16(24):4358. https://doi.org/10.3390/nu16244358
Chicago/Turabian StyleClarke, Erin D., María Gómez-Martín, Jordan Stanford, Ali Yilmaz, Ilyas Ustun, Lisa Wood, Brian Green, Stewart F. Graham, and Clare E. Collins. 2024. "Urinary Metabolite Profiles of Participants with Overweight and Obesity Prescribed a Weight Loss High Fruit and Vegetable Diet: A Single Arm Intervention Study" Nutrients 16, no. 24: 4358. https://doi.org/10.3390/nu16244358
APA StyleClarke, E. D., Gómez-Martín, M., Stanford, J., Yilmaz, A., Ustun, I., Wood, L., Green, B., Graham, S. F., & Collins, C. E. (2024). Urinary Metabolite Profiles of Participants with Overweight and Obesity Prescribed a Weight Loss High Fruit and Vegetable Diet: A Single Arm Intervention Study. Nutrients, 16(24), 4358. https://doi.org/10.3390/nu16244358










