The Association of Dietary Polyamines with Mortality and the Risk of Cardiovascular Disease: A Prospective Study in UK Biobank
Abstract
1. Introduction
2. Methods
2.1. Patients and Study Design
2.2. Exposure Assessment
2.3. Definitions of Covariates
2.4. Polygenic Risk Score
2.5. Definition of Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
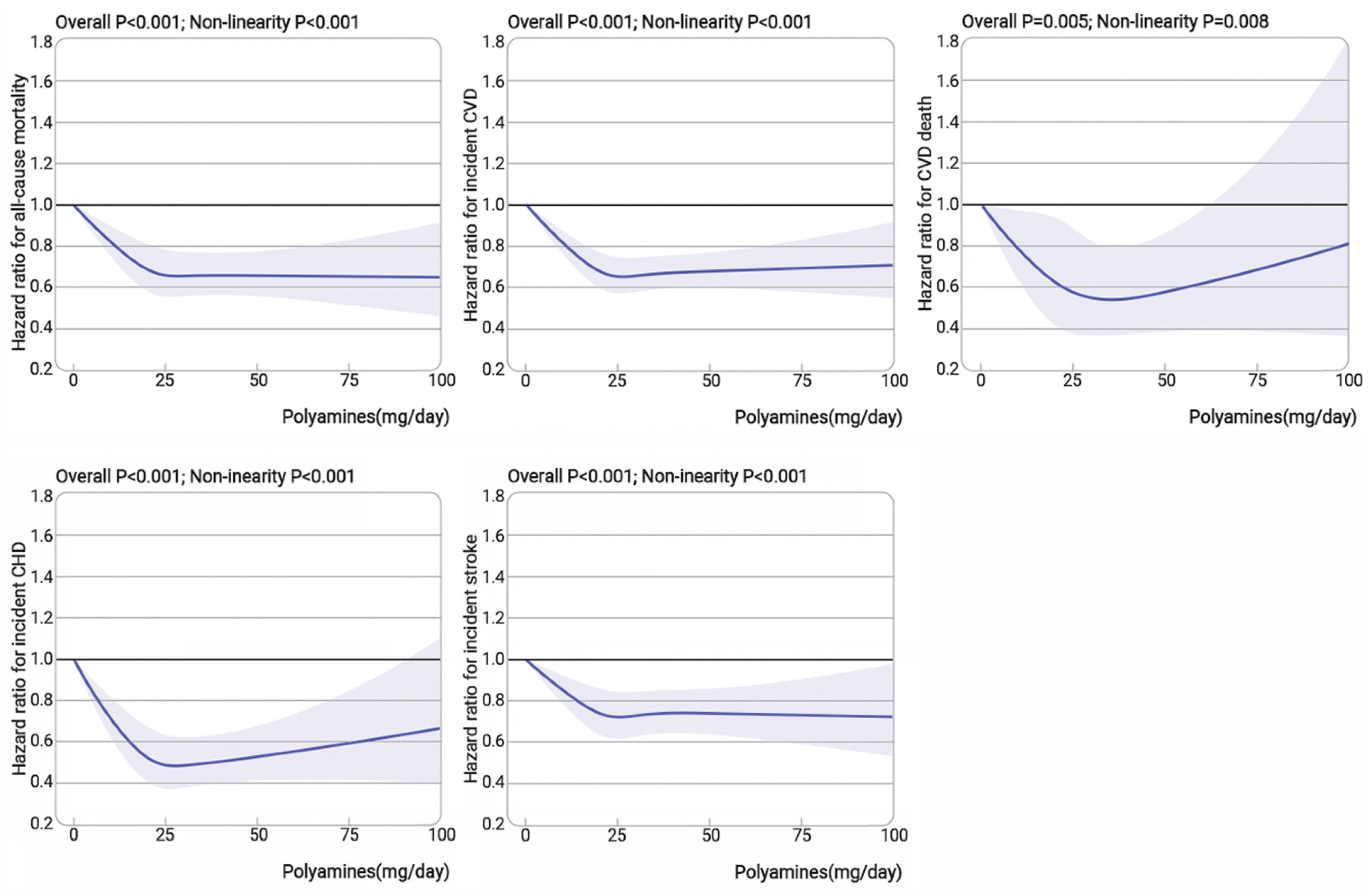
3.2. Association of Dietary Polyamines with All-Cause Mortality and Incident CVD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef]
- Zhao, B.; Gan, L.; Graubard, B.I.; Männistö, S.; Albanes, D.; Huang, J. Associations of Dietary Cholesterol, Serum Cholesterol, and Egg Consumption with Overall and Cause-Specific Mortality: Systematic Review and Updated Meta-Analysis. Circulation 2022, 145, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Casero, R.A.; Murray Stewart, T.; Pegg, A.E. Polyamine Metabolism and Cancer: Treatments, Challenges and Opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Arruabarrena-Aristorena, A.; Zabala-Letona, A.; Carracedo, A. Oil for the Cancer Engine: The Cross-Talk between Oncogenic Signaling and Polyamine Metabolism. Sci. Adv. 2018, 4, eaar2606. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, Y.; Bewley, M.C.; Pegg, A.E. Aminopropyltransferases: Function, Structure and Genetics. J. Biochem. 2006, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Kurihara, S.; Sawaki, E.; Koga, Y.; Benno, Y. Impact of Intestinal Microbiota on Intestinal Luminal Metabolome. Sci. Rep. 2012, 2, 233. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of Autophagy by Spermidine Promotes Longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Madeo, F.; Zimmermann, A.; Maiuri, M.C.; Kroemer, G. Essential Role for Autophagy in Life Span Extension. J. Clin. Investig. 2015, 125, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Mariño, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Bénit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and Resveratrol Induce Autophagy by Distinct Pathways Converging on the Acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and Lifespan Extension by the Natural Polyamine Spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Zimmermann, A.; Schroeder, S.; Pendl, T.; Harger, A.; Stekovic, S.; Schipke, J.; Magnes, C.; Schmidt, A.; et al. Dietary Spermidine for Lowering High Blood Pressure. Autophagy 2017, 13, 767–769. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher Spermidine Intake Is Linked to Lower Mortality: A Prospective Population-Based Study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Jiang, H.; Liu, X.; Sun, X.; Chen, Y.; Hu, C.; Wang, Z.; Han, T.; Sun, C.; et al. The Association of Dietary Spermidine with All-Cause Mortality and CVD Mortality: The U.S. National Health and Nutrition Examination Survey, 2003 to 2014. Front. Public Health 2022, 10, 949170. [Google Scholar] [CrossRef]
- Nagata, C.; Wada, K.; Yamakawa, M.; Nakashima, Y.; Sugino, M.; Mori, T. Dietary Polyamine Intake and All-Cause and Cause-Specific Mortality in Japanese Adults in the Takayama Study. Br. J. Nutr. 2024, 131, 343–350. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef]
- Perez-Cornago, A.; Pollard, Z.; Young, H.; van Uden, M.; Andrews, C.; Piernas, C.; Key, T.J.; Mulligan, A.; Lentjes, M. Description of the Updated Nutrition Calculation of the Oxford WebQ Questionnaire and Comparison with the Previous Version among 207,144 Participants in UK Biobank. Eur. J. Nutr. 2021, 60, 4019–4030. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and Evaluation of the Oxford WebQ, a Low-Cost, Web-Based Method for Assessment of Previous 24 h Dietary Intakes in Large-Scale Prospective Studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S.; Duguid, T.J.; Brown, D.S.; Grant, G.; Pusztai, A.; White, A.; Ralph, A. The Importance of Dietary Polyamines in Cell Regeneration and Growth. Br. J. Nutr. 1995, 73, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Esparza, N.C.; Costa-Catala, J.; Comas-Basté, O.; Toro-Funes, N.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Occurrence of Polyamines in Foods and the Influence of Cooking Processes. Foods 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Krausová, P.; Kalač, P.; Křížek, M.; Pelikánová, T. Content of Biologically Active Polyamines in Livers of Cattle, Pigs and Chickens after Animal Slaughter. Meat Sci. 2006, 73, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Atiya Ali, M.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in Foods: Development of a Food Database. Food Nutr. Res. 2011, 55, 5572. [Google Scholar] [CrossRef]
- Bardocz, S.; Grant, G.; Brown, D.; Ralph, A.; Pusztai, A. Polyamines in Food—Implications for Growth and Health. J. Nutr. Biochem. 1993, 4, 66–71. [Google Scholar] [CrossRef]
- Kalač, P.; Krausová, P. A Review of Dietary Polyamines: Formation, Implications for Growth and Health and Occurrence in Foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine Contents in Current Foods: A Basis for Polyamine Reduced Diet and a Study of Its Long Term Observance and Tolerance in Prostate Carcinoma Patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef]
- Kalač, P. Health Effects and Occurrence of Dietary Polyamines: A Review for the Period 2005–Mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in Polyamines with Aging and Their Ingestion from Food and Drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Romero, R.; Sánchez-Viñas, M.; Gázquez, D.; Bagur, M.G. Characterization of Selected Spanish Table Wine Samples According to Their Biogenic Amine Content from Liquid Chromatographic Determination. J. Agric. Food Chem. 2002, 50, 4713–4717. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Blane, D.; Townsend, P.; Phillimore, P.; Beattie, A. Health and Deprivation: Inequality and the North. Br. J. Sociol. 1989, 40, 344. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. Genome-Wide Genetic Data on ~500,000 UK Biobank Participants. bioRxiv 2017, 166298. [Google Scholar] [CrossRef]
- Nikpay, M.; Goel, A.; Won, H.-H.; Hall, L.M.; Willenborg, C.; Kanoni, S.; Saleheen, D.; Kyriakou, T.; Nelson, C.P.; Hopewell, J.C.; et al. A Comprehensive 1,000 Genomes-Based Genome-Wide Association Meta-Analysis of Coronary Artery Disease. Nat. Genet. 2015, 47, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.-K.; Van Der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry Genome-Wide Association Study of 520,000 Subjects Identifies 32 Loci Associated with Stroke and Stroke Subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The Role of Autophagy in Cardiomyocytes in the Basal State and in Response to Hemodynamic Stress. Nat. Med. 2007, 13, 619–624. [Google Scholar] [CrossRef]
- Taneike, M.; Yamaguchi, O.; Nakai, A.; Hikoso, S.; Takeda, T.; Mizote, I.; Oka, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; et al. Inhibition of Autophagy in the Heart Induces Age-Related Cardiomyopathy. Autophagy 2010, 6, 600–606. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Gioscia-Ryan, R.A.; Hearon, C.M.; Seals, D.R. The Autophagy Enhancer Spermidine Reverses Arterial Aging. Mech. Ageing Dev. 2013, 134, 314–320. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S.; Grant, G.; Brown, D.S.; Pusztai, A. Putrescine as a Source of Instant Energy in the Small Intestine of the Rat. Gut 1998, 42, 24–28. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-Term Oral Polyamine Intake Increases Blood Polyamine Concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef]
- Vargas, A.J.; Ashbeck, E.L.; Thomson, C.A.; Gerner, E.W.; Thompson, P.A. Dietary Polyamine Intake and Polyamines Measured in Urine. Nutr. Cancer 2014, 66, 1144–1153. [Google Scholar] [CrossRef]


| Characteristics | Total n = 187,432 | Quintile Groups of Polyamines (mg/d) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 (≤17.4) n = 37,487 | Quintile 2 (>17.4–22.3) n = 37,486 | Quintile 3 (>22.3–27.1) n = 37,487 | Quintile 4 (>27.1–33.5) n = 37,486 | Quintile 5 (>33.5–98.9) n = 37,487 | |||
| Age, mean (SD), y | 55.9 (7.92) | 54.64 (8.03) | 55.43 (7.96) | 56.04 (7.87) | 56.57 (7.78) | 56.93 (7.77) | <0.001 |
| Sex, female, n (%) | 105,924 (56.5) | 22,373 (59.7) | 21,872 (58.3) | 21,419 (57.1) | 20,763 (55.4) | 19,497 (52.0) | <0.001 |
| Ethnicity, n (%) | <0.001 | ||||||
| White European | 179,239 (95.6) | 35,132 (93.7) | 35,941 (95.9) | 36,148 (96.4) | 36,178 (96.5) | 35,840 (95.6) | |
| Mixed | 1120 (0.6) | 299 (0.8) | 207 (0.6) | 219 (0.6) | 194 (0.5) | 201 (0.5) | |
| Asian | 2958 (1.6) | 871 (2.3) | 580 (1.5) | 488 (1.3) | 460 (1.2) | 559 (1.5) | |
| Black | 2155 (1.1) | 697 (1.9) | 376 (1.0) | 305 (0.8) | 326 (0.9) | 451 (1.2) | |
| Others | 1960 (1.0) | 488 (1.3) | 382 (1.0) | 326 (0.9) | 328 (0.9) | 436 (1.2) | |
| Townsend deprivation index a | <0.001 | ||||||
| Least deprived | 37,547 (20.0) | 6690 (17.8) | 7350 (19.6) | 7943 (21.2) | 7950 (21.2) | 7614 (20.3) | |
| Moderate deprived | 112,399 (60.0) | 21,769 (58.1) | 22,542 (60.1) | 22,549 (60.1) | 22,756 (60.7) | 22,783 (60.8) | |
| Most deprived | 37,486 (20.0) | 9028 (24.1) | 7594 (20.3) | 6994 (18.7) | 6780 (18.1) | 7090 (18.9) | |
| Education, n (%) | <0.001 | ||||||
| College or university degree | 80,846 (43.1) | 14,156 (37.8) | 16,299 (43.5) | 16,830 (44.9) | 17,099 (45.6) | 16,462 (43.9) | |
| Others | 90,537 (48.3) | 19,243 (51.3) | 18,162 (48.4) | 17,783 (47.4) | 17,626 (47.0) | 17,723 (47.3) | |
| Unknown | 16,049 (8.6) | 4088 (10.9) | 3025 (8.1) | 2873 (7.7) | 2761 (7.4) | 3302 (8.8) | |
| Systolic Blood Pressure, mmHg | 135.08 (18.11) | 135.95 (18.23) | 136.61 (18.20) | 137.37 (18.24) | 138.11 (18.31) | <0.001 | |
| Body mass index, n (%) | <0.001 | ||||||
| Underweight (<18.5) | 954 (0.5) | 187 (0.5) | 183 (0.5) | 197 (0.5) | 201 (0.5) | 186 (0.5) | |
| Normal weight (18.5 to <25) | 68,801 (36.7) | 13,199 (35.2) | 14,041 (37.5) | 14,134 (37.7) | 14,122 (37.7) | 13,305 (35.5) | |
| Overweight (25 to <30) | 79,994 (42.7) | 15,967 (42.6) | 16,096 (42.9) | 15,947 (42.6) | 15,945 (42.5) | 16,039 (42.8) | |
| Obese ≥ 30 | 37,683 (20.1) | 8134 (21.7) | 7166 (19.1) | 7208 (19.2) | 7218 (19.3) | 7957 (21.2) | |
| Physical activity, n (%) b | <0.001 | ||||||
| Low | 164,138 (87.6) | 32,967 (87.9) | 33,321 (88.9) | 33,072 (88.2) | 32,878 (87.7) | 31,900 (85.1) | |
| High | 23,294 (12.4) | 4520 (12.1) | 4165 (11.1) | 4414 (11.8) | 4608 (12.3) | 5587 (14.9) | |
| Smoking, n (%) | <0.001 | ||||||
| Never | 107,387 (57.3) | 21,057 (56.2) | 21,488 (57.3) | 21,791 (58.1) | 21,846 (58.3) | 21,205 (56.6) | |
| Former | 65,177 (34.8) | 12,264 (32.7) | 12,963 (34.6) | 12,979 (34.6) | 13,255 (35.4) | 13,716 (36.6) | |
| Current | 14,393 (7.7) | 4048 (10.8) | 2950 (7.9) | 2628 (7.1) | 2295 (6.1) | 2472 (6.6) | |
| Unknown | 475 (0.2) | 118 (0.3) | 85 (0.2) | 88 (0.2) | 90 (0.2) | 94 (0.2) | |
| Alcohol, n (%) | <0.001 | ||||||
| Never | 5840 (3.1) | 1423 (3.8) | 1120 (3.0) | 1087 (2.9) | 1017 (2.7) | 1193 (3.2) | |
| Former | 5323 (2.8) | 1349 (3.6) | 1058 (2.8) | 886 (2.4) | 965 (2.6) | 1065 (2.8) | |
| Current | 176,096 (94.0) | 34,667 (92.5) | 35,265 (94.1) | 35,487 (94.6) | 35,478 (94.6) | 35,199 (93.9) | |
| Unknown | 173 (0.1) | 48 (0.1) | 43 (0.1) | 26 (0.1) | 26 (0.1) | 30 (0.1) | |
| Sleep duration, n (%) | <0.001 | ||||||
| <7 h | 41,887 (22.3) | 9097 (24.3) | 8218 (21.9) | 8005 (21.4) | 8005 (21.4) | 8562 (22.8) | |
| 7–8 h | 133,303 (71.1) | 25,686 (68.5) | 26,774 (71.4) | 27,180 (72.5) | 27,113 (72.3) | 26,550 (70.8) | |
| >8 h | 11,670 (6.3) | 2528 (6.7) | 2388 (6.4) | 2215 (5.9) | 2265 (6.0) | 2274 (6.1) | |
| Unknown | 572 (0.3) | 176 (0.5) | 106 (0.3) | 86 (0.2) | 103 (0.3) | 101 (0.3) | |
| Polyamines (SD), mg/day | |||||||
| Spermidine | 9.35 (4.78) | 5.05 (1.72) | 7.45 (1.62) | 8.98 (1.85) | 10.54 (2.23) | 14.71 (6.91) | <0.001 |
| Spermine | 4.18 (1.93) | 2.59 (1.16) | 3.58 (1.31) | 4.18 (1.50) | 4.78 (1.74) | 5.75 (2.14) | <0.001 |
| Putrescine | 12.3 (6.36) | 5.44 (2.24) | 8.90 (2.22) | 11.49 (2.59) | 14.68 (3.23) | 21.10 (5.72) | <0.001 |
| Energy, mean (SD), KJ | 8590 (2240) | 7311.85 (2024.45) | 8194.87 (1948.86) | 8660.61 (1990.94) | 9060.59 (2089.97) | 9742.72 (2326.27) | <0.001 |
| Hypertension, n (%) | 41,894 (22.4) | 7965 (21.2) | 8078 (21.5) | 8139 (21.7) | 8459 (22.6) | 9253 (24.7) | <0.001 |
| Diabetes, n (%) | 6593 (3.5) | 1233 (3.3) | 1218 (3.2) | 1192 (3.2) | 1311 (3.5) | 1639 (4.4) | <0.001 |
| Hypercholesteremia, n (%) | 18,787 (10.0) | 3616 (9.6) | 3605 (9.6) | 3586 (9.6) | 3842 (10.2) | 4138 (11.0) | <0.001 |
| Drug use, n (%) | |||||||
| Antihypertensive | 30,384 (16.2) | 5583 (14.9) | 5753 (15.3) | 5979 (15.9) | 6264 (16.7) | 6805 (18.2) | <0.001 |
| Lipid treatment | 24,005 (12.8) | 4526 (12.1) | 4573 (12.2) | 4618 (12.3) | 4941 (13.2) | 5347 (14.3) | <0.001 |
| Insulin treatment | 1342 (0.7) | 246 (0.7) | 254 (0.7) | 227 (0.6) | 266 (0.7) | 349 (0.9) | <0.001 |
| Genetic predisposition score † | |||||||
| CHD predisposition score | 12.2 (0.483) | 12.2 (0.484) | 12.2 (0.480) | 12.2 (0.483) | 12.2 (0.485) | 12.2 (0.482) | 0.11 |
| Stroke predisposition score | 1.83 (0.359) | 1.83 (0.361) | 1.83 (0.359) | 1.82 (0.358) | 1.82 (0.357) | 1.83 (0.359) | 0.074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Qian, M.; Zhang, N.; Zhang, R.; Liu, M.; Wang, J.; Li, F.; Zheng, L.; Sun, Z. The Association of Dietary Polyamines with Mortality and the Risk of Cardiovascular Disease: A Prospective Study in UK Biobank. Nutrients 2024, 16, 4335. https://doi.org/10.3390/nu16244335
Han S, Qian M, Zhang N, Zhang R, Liu M, Wang J, Li F, Zheng L, Sun Z. The Association of Dietary Polyamines with Mortality and the Risk of Cardiovascular Disease: A Prospective Study in UK Biobank. Nutrients. 2024; 16(24):4335. https://doi.org/10.3390/nu16244335
Chicago/Turabian StyleHan, Su, Mingxia Qian, Na Zhang, Rui Zhang, Min Liu, Jiangbo Wang, Furong Li, Liqiang Zheng, and Zhaoqing Sun. 2024. "The Association of Dietary Polyamines with Mortality and the Risk of Cardiovascular Disease: A Prospective Study in UK Biobank" Nutrients 16, no. 24: 4335. https://doi.org/10.3390/nu16244335
APA StyleHan, S., Qian, M., Zhang, N., Zhang, R., Liu, M., Wang, J., Li, F., Zheng, L., & Sun, Z. (2024). The Association of Dietary Polyamines with Mortality and the Risk of Cardiovascular Disease: A Prospective Study in UK Biobank. Nutrients, 16(24), 4335. https://doi.org/10.3390/nu16244335







