Highlights
- This systematic review highlights the current impact of certain bioactive compounds and medicinal plants on the management and progression of CKD, as well as in dialysis patients.
- Bioactive compounds, such as turmeric, propolis, and green tea, exhibit significant anti-inflammatory and antioxidant properties that may improve outcomes in CKD and dialysis patients.
- Traditional Chinese medicine could play a role in a comprehensive approach to managing CKD and dialysis patients; however, there remains insufficient evidence to endorse its use in clinical practice.
Abstract
Background. The bioactive components of plant foods and medicinal plants have attracted interest due to their potential impact on the progression of chronic kidney disease (CKD) and outcomes. Objective. This study aimed to conduct a critical and quantitative systematic review of randomized clinical trials (RCTs) investigating the potential effects of selected phytochemicals from plant-based foods and medicinal plants in CKD and dialysis patients. Methods. The review included studies that related plant-based bioactive compounds (curcumin, propolis, sulforaphane, betalain, catechins, rhein, emodin, aloe-emodin, flavonoids, and triptolide) and medicinal plants (green tea, rhubarb, Astragalus membranaceus, and Tripterygium wilfordii Hook F) in CKD and dialysis patients. A literature search was conducted in PubMed, LILACS, Embase, Scopus, and WOS between December 2022 and October 2024. This review was performed according to the PRISMA flowchart and was registered in PROSPERO (595162). Results. In the eight RCTs conducted with curcumin, anti-inflammatory, antioxidant, and microbiota-modulating properties were reported. As for propolis, in three RCTs, anti-inflammatory, anti-proteinuric, and renal-protective properties were reported. Sulforaphane in one RCT showed antioxidant and cardiovascular benefits, and in another RCT no effects were observed. In one RCT, genistein was shown to be a potential anti-inflammatory agent and improved nutritional status. Allicin in two RCTs showed cardioprotective, antioxidant, anti-inflammatory, and lipid-lowering effects. Finally, beetroot showed a vasodilator effect in one RCT. As for the medicinal plants, green tea, rhubarb, Astragalus membranaceus, and Tripterygium Wilfordii Hook F, in six RCTs they showed antioxidant, anti-inflammatory, cardioprotective, antiproteinuric, and renoprotective properties. Conclusions. These results suggest that bioactive compounds of plant-based foods and medicinal plants have promising effects in terms of preventing or treating CKD progression and appear to improve inflammation and antioxidant capacity and support cardiovascular benefits and renoprotective effects; however, it is recommended that further studies be carried out.
1. Introduction
Chronic kidney disease (CKD) is a public health problem affecting 8.0–16.0% of the world’s adult population. It is expected to rise from the 16th leading cause of death in 2016 to the 5th in 2040 [1,2]. Aging, diabetes mellitus (DM), and cardiovascular disease (CVD) have been identified as the main causes of CKD, with an estimated prevalence of 27.9% to 44.0% in people aged 70 years and older [3]. Consequently, there is growing interest in the search for effective interventions to prevent or slow the progression of CKD and to delay the onset of dialysis. The effects that bioactive components of plant-based foods and medicinal plants may have on the treatment of CKD have attracted much interest because of their potential impact on disease progression and improving patient outcomes.
CKD involves a combination of different pathogenetic mechanisms, such as inflammation, oxidative stress, and endothelial dysfunction, which are closely linked to increased morbidity and mortality [4]. Some inflammatory biomarkers, such as C-reactive protein (CRP), and proinflammatory cytokines, e.g., interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), can induce oxidative stress through different signaling pathways. On the other hand, oxidative stress also promotes inflammation, establishing a potentially vicious circle through the activation of nuclear transcription factor κB (NF-κB), which is responsible for the recruitment of immune cells. In addition, proinflammatory cytokines associated with oxidative stress play an important role in the pathogenesis of CKD by inducing apoptosis, necrosis, and fibrosis. Uremic toxins also promote inflammation and oxidative stress by priming polymorphonuclear cells, activating interleukin-1 βeta (IL-1β) and IL-8 and the innate immune response [5].
Bioactive compounds, also termed phytochemicals, are derived from plant-based foods and medicinal plants [6]. Several studies [7,8,9] have suggested that plant-derived compounds, such as curcumin, propolis, sulforaphane, betalain, catechins, rhein, emodin, aloe-emodin, flavonoids, and triptolide, and medicinal plants (rhubarb, Astragalus membranaceus, and Tripterygium wilfordii Hook F) show potential bioeffects in CKD and dialysis patients. Antioxidant, anti-inflammatory, anticarcinogenic, and renoprotective activity has been attributed to these bioactive compounds [10] (Table 1, Figure 1).

Table 1.
Bioactive compounds and food sources of plant-based foods and medicinal plants.
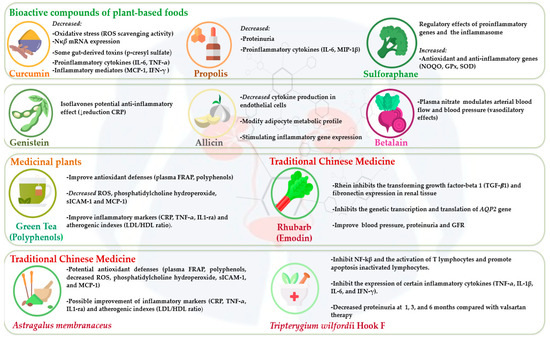
Figure 1.
Effects of bioactive compounds from plant-based foods and medicinal plants in chronic kidney disease and dialysis patients.
Diet is an important pillar in CKD to delay the progression of the disease and initiation of dialysis. A high fruit and vegetable intake is associated with a reduced risk of developing CKD in the early stages [32]. Despite plant-based protein reducing proteinuria, some authors [26,33] showed that bioactive compounds of plant-based foods and medicinal plants could be involved in the pathogenesis, progression, and complications of CKD. Reducing the current global intake of dietary risk factors could help to reduce CKD-treated end-stage renal disease (ESRD) incidence and the burden of CKD by approximately 3.0% by 2040 [2]. Plant-based diets are rich in protective nutrients and phytochemicals. Among bioactive compounds, some substances actively regulate redox reactions, such as flavonoids, phenolic compounds, carotenoids, anthocyanins, and vitamins C and E [6]. These bioactive compounds interact with reactive oxygen species (ROS) and nitrogen species, contributing to the overall antioxidant defense system in the body and preventing cellular metabolism damage caused by oxidative stress [34]. Dietary antioxidants can act as reducing agents, free radical scavengers, and modulators of enzymatic activity, contributing to redox homeostasis and protecting against oxidative damage.
Furthermore, some antioxidants induce a beneficial response to mild oxidative stress by activating cellular protection and detoxification mechanisms [8,10]. This interaction is complex and depends on multiple factors, including the chemical structure of the antioxidants and their bioavailability.
Recent findings [35,36] demonstrated that the kidney–gut axis plays an important role in the pathogenesis of CKD through a bidirectional interaction between CKD and the host gut microbiota. Accumulating research [35,37] indicates that CKD may influence the composition of the gut microbiota, causing intestinal dysbiosis, which in turn, elevates gut-derived uremic toxins (e.g., indoxyl sulfate), contributing to the progression of CKD, inflammation, and CVD.
Considering that a balanced and personalized diet is essential to maintain nutritional status and improve inflammation and oxidative stress, complementary dietary strategies could be of interest to ameliorate uremic toxicity, improve dysbiosis, delay CKD progression, and improve outcomes in dialysis patients. Therefore, this prompted us to explore the effects of plant-based foods and medicinal plants. Given the particular interest in bioactive compounds, the aim was to conduct a critical and quantitative systematic review of randomized clinical trials investigating the potential effects of selected phytochemicals from plant-based foods and medicinal plants in CKD and dialysis patients.
2. Materials and Methods
This systematic review was conducted following the updated Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) (http://prisma-statement.org/prismastatement/Checklist.aspx, accessed on 11 May 2024) guidelines (Figure 2). This review included randomized clinical trials (RCTs) and studies that related plant-based bioactive compounds (curcumin, propolis, sulforaphane, betalain, catechins, rhein, emodin, aloe-emodin, flavonoids, and triptolide), and some medicinal plants (rhubarb, Astragalus membranaceus, and Tripterygium wilfordii Hook F) in CKD and dialysis patients. A protocol with the main elements of the study was developed and registered with PROSPERO (code number: 595162).
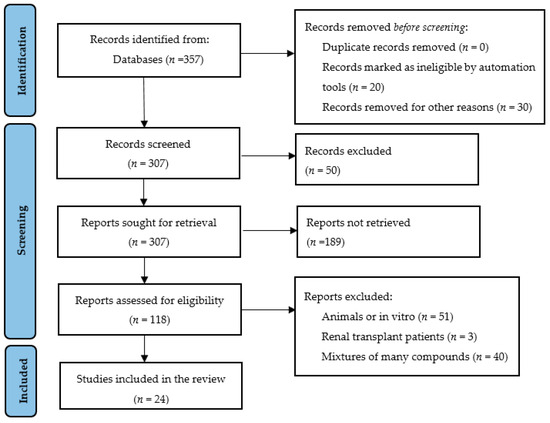
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow diagram of the systematic review.
2.1. Search Strategy
The search for articles was carried out in the PubMed, LILACS, Embase, Web of Science, and Scopus electronic databases. The following descriptors were used for the database search: “plant bioactive compound”, “phytochemicals”, “diet”, “plant-based”, “herb medicinal”, “medicinal plants”, “chronic kidney disease”, and “dialysis”. In addition, RCTs and studies related to inflammation, oxidative stress, and dysbiosis were considered. These health descriptors were combined with the Boolean operators AND and OR and grouped for sensitivity and specificity. All titles and/or abstracts were assessed, and irrelevant studies were excluded. The articles of interest were then retrieved, and the full texts were reviewed.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria included articles published in English and Spanish between December 2020 and October 2024 evaluating human studies with the effects of bioactive compounds obtained from plant-based foods and selected medicinal plants in CKD and dialysis patients. The following exclusion/inclusion criteria were followed using PICO (patient, intervention, comparator, and outcomes): only human subjects with CKD at any stage and dialysis patients were selected, and the full texts of articles without any time filters were considered suitable for inclusion. The following exclusion and inclusion criteria were adopted for the full-text articles: RCT and quasi-RCT clinical trials with a mean duration of ≥ 4 weeks were included; interventions targeting the consumption of animal-derived bioactive nutrients, vitamins, minerals, toxic compounds, or other nutritional oral supplements were excluded; and interventions analyzing the effects of single isolated bioactive compounds (curcumin, etc.) were included. Any diet or study demonstrating a lack of adherence or compliance in the pre-post study was excluded. Review articles, theses, abstracts in scientific congress, letters to the editor, case reports, animal or in vitro studies, and studies on renal transplant patients and mixtures of many compounds were excluded. Articles repeated in different databases and articles that duplicated information from a previously included population were discarded. After duplicates were removed, the titles and abstracts from all reports were screened, full texts were independently examined for eligibility, and data were extracted by the authors individually. A meta-analysis was not applicable because of the heterogeneity of the studies, approaches, and results.
2.3. Study Selection
The research simultaneously and independently identified and selected the articles in all the databases searched between January 2000 and October 2024. Disagreements between the reviewers were resolved by consensus. In this stage, there were no language or period restrictions for choosing the publications. The studies were selected by evaluating the titles, reading the abstracts, and subsequently reading the full texts of the studies, observing the inclusion and exclusion criteria. Additionally, the reference lists of the chosen articles were analyzed to identify any study that had not been captured using the search strategy. The Critical Appraisal Skills Programme (CASP) Systematic Review Checklist (https://casp-uk.net/casp-tools-checklists/systematic-review-checklist/, accessed on 12 May 2024) was used to assess the reliability, relevance, and results of articles included in this systematic review.
Figure 2 shows the PRISMA flow diagram of the systematic review of plant-based foods and medicinal plants. In the first search, 357 articles were identified in the databases. Of these, 50 were initially discarded after reading the titles. On further screening, which consisted of reading and analyzing the abstracts, 189 articles were excluded, of which 118 were assessed for eligibility for full-text reading according to the PICO strategy. After this evaluation, 23 RCTs and 1 clinical trial were included in this systematic review. The results were described in two parts: plant-based bioactive compounds (n = 18 articles) and medicinal plants (n = 6 articles). The results of the studies are summarized in
2.4. Data Extraction
Data were extracted independently by 3 researchers (E.J., G.B., and M.R.) and included in an Excel spreadsheet containing the following items: authors, year of publication, bioactive compound, study design, type and number of patients (CKD 1–5, hemodialysis or peritoneal dialysis), dosage, molecular and therapeutic effects, and major findings associated with bioactive-compound interventions. The information extracted from the articles was used to build an explanatory model regarding the factors associated with plant-based foods and medicinal plants and their potential effects in CKD and dialysis patients.
2.5. Evaluation of the Quality of the Articles
The Jadad scale [38] was used to assess independently the methodological quality of the RCTs included in this systematic review. For each item of the study analyzed, a response and a code were attributed: yes (1) or no (0), with the score of the questionnaire ranging from 0 to 5 points. An RCT was of high methodological quality, assuming a low risk of bias if the Jadad score was ≥ 3 points. In the methodological quality assessment of the 23 included RCTs, 4 articles had a Jadad score of 4 points (21.05%) [39,40,41,42] and 19 articles (78.95%) met all the checklist criteria [12,13,14,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. Studies with Jadad scale scores < 3 points were excluded due to their low quality and high risk of methodological bias. The quality classification was carried out independently by 3 researchers (E.J., G.B., and M.R), and any differences were resolved through discussion.
3. Results
3.1. Potential Effects of Plant-Based Food Bioactive Compounds on Chronic Kidney Disease
3.1.1. Curcumin
Curcumin, found in turmeric (Curcuma Longa), is a phenolic compound with a variety of biological activities in CKD. It is a yellow-colored spice that comes from a flowering plant of the ginger family and is native to Asia and Central America. Previous studies [11,12,13,14] have reported the bioeffects of curcumin on inflammation, oxidative stress, and CKD progression. Table 2 shows the clinical trials of curcumin and its therapeutic effects in CKD and dialysis patients.

Table 2.
Potential effects of curcumin on chronic kidney disease and dialysis patients.
Inflammation per se accounts for 30–50% of CKD patients, contributing significantly to CKD progression and poorer prognosis in dialysis patients [59]. Causative factors, such as oxidative stress and cellular senescence, hypoxia, fluid and sodium overload, immune dysfunction, and intestinal dysbiosis, as well as accumulation of uremic toxins that emerge from molecular and metabolic derangements in the uremic milieu, were reported [60,61].
The effects of curcumin in CKD and dialysis patients have been related to molecular mechanisms such as scavenging superoxide radicals, hydrogen peroxide, and nitric oxide (NO). All of these molecular mechanisms were associated with the amelioration of oxidative stress through increased expression of antioxidant enzymes, activation of nuclear factor erythroid factor 2 (Nrf2), scavenging of ROS, and downregulation of inflammatory cytokines (IL-6 and TNF-α) modulated by nuclear factor κappa β (NF-kβ) [12,13,14]. Two double-blind randomized clinical trials [43,44] demonstrated that curcumin was an effective adjuvant therapy for oxidative and inflammation modulation in hemodialysis (HD) patients. One hundred and nineteen HD patients were either treated with 500 mg turmeric (4.42% curcumin; one capsule/three times per day) or a placebo for three months of follow-up. In the turmeric-supplemented group, a significant reduction in plasma IL-6 and TNF-α concentrations was found compared with the placebo group. In this study, turmeric supplementation in HD patients was safe and effective as an anti-inflammatory agent. Another RCT carried out on 28 HD patients [39] analyzed the expression of inflammatory transcription factors, such as NF-kβ, Nrf2, Nod-like receptor pyrin domain containing-3 (NLRP3), and IL-1β. The intervention group received after HD a juice composed of 100 mL orange juice, 12 g carrot, and 2.5 g turmeric (95% curcumin) administered three times per week. Interestingly, the findings suggested that curcumin was an effective anti-inflammatory supplement by decreasing CRP levels, NF-kβ, and mRNA expression in patients who received HD.
Oxidative stress is a risk factor for morbidity and mortality in CKD and dialysis patients [62]. The mechanisms of action of the kidney’s antioxidant barrier can slow down oxidative stress under physiological conditions. Oxidative stress has been linked to the production of highly reactive intermediate molecules in the presence of inflammation, such as superoxide anion (O2-) and hydroxyl (OH-) free radicals. The hyperproduction of ROS can further enhance the inflammatory response by activating pro-inflammatory mediators through the NF-κβ metabolic pathway [63].
Curcumin, as a phenolic antioxidant compound, has also been studied as an adjuvant therapy to prevent or attenuate oxidative stress and as an anti-inflammatory agent in non-diabetic and diabetic CKD patients. In a randomized double-blind placebo-controlled clinical trial [14], the role of curcumin supplementation was studied in the profiles of redox status and Nrf2 activation in non-diabetic proteinuric CKD patients compared with diabetic proteinuric CKD patients. Fifty-two volunteers were supplemented with 107 mg of curcumin in each meal (320 mg/day) or placebo for 8 weeks. The curcumin-supplemented group showed improved free radical scavenging activity and attenuated serum levels of malondialdehyde (MDA)—an oxidative stress marker in non-diabetic proteinuric CKD patients. Likewise, a double-blind, placebo-controlled RCT carried out in HD patients [12] studied the potential antioxidant and anti-inflammatory response to curcumin supplementation. Forty-three HD patients were randomized into two groups to receive curcuminoid capsules (1 g/day) versus placebo for 12 weeks. As oxidative stress markers, catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) activity, and MDA, together with high-sensitivity CRP, were analyzed. The curcuminoid intervention showed a marked increase in CAT activity, whereas no changes were observed in GPx, GR, MDA, and CRP concentrations.
The plausible explanation for the above results was associated with the heterogeneity of the population analyzed (CKD, dialysis), sample size, the duration of the RCTs, and the dosage of curcumin used.
A CKD stage 3–4 double-blind placebo-controlled clinical trial [40] examined if curcumin supplementation had anti-inflammatory and antioxidant effects in the prevention of contrast-induced nephropathy (CIN) following coronary angiography or angioplasty. Thirty CKD patients in stages 3–4 were selected to be supplemented with curcumin, and thirty were given a placebo. Curcumin supplementation (500 mg/3 times a day) was administered from 2 days before the intervention until 3 days post-procedure. Serum creatinine and urinary neutrophil gelatinase-associated lipocalin were analyzed before the procedure and on days 2 and 3 after angiography. No significant results were obtained with curcumin in ameliorating the possible adverse effects of CIN in patients with stage 3–4 CKD.
Based on the findings of previous studies [12,14,40], curcumin supplementation could be considered a complementary dietary strategy to prevent or ameliorate oxidative stress and inflammatory response in CKD and dialysis. On the other hand, the protective effect of curcumin in reducing the effect of CIN in patients with stage 3–4 CKD remains unclear. Further randomized clinical trials with large sample sizes are needed to determine the dosage of curcumin to attenuate inflammation and reverse oxidative stress in CKD and dialysis patients.
Emerging evidence suggests that gut microbiota dysbiosis is involved in CKD progression and plays a key role in the gut–kidney axis. Therefore, increased attention has been given to gut microbiota as a novel approach to treating CKD. A placebo-controlled double-blind clinical trial [45] was conducted on twenty-four stage 3a–4 CKD patients. The study aimed to evaluate the effect of curcumin supplementation on inflammation, lipid peroxidation, and gut microbiota diversity. The curcumin-supplemented therapeutic arm was given 500 mg of curcumin/twice a day for 6 months compared to placebo (controls). A significant reduction in pro-inflammatory mediators [monocyte chemoattractant protein-1 (MCP-1 or CCL-2), interferon-gamma (IFN-γ), and interleukin-4 (IL-4)] and lipid peroxidation markers (thiobarbituric acid reactive substances (TBARS)) were found to be associated only with curcumin intervention. No significant changes were observed in the placebo group. After 6 months of curcumin supplementation, gut alpha diversity was observed to be similar to that of healthy subjects. At the phylum level, gut diversity was more balanced at the expense of a significant increase in Lachnoclostridium spp. and a decrease in Escherichia spp. and Shigella spp. It is also noteworthy that, at the family level, Lactobacillaceae spp. increased significantly in the last 3 months of curcumin supplementation.
Several potential mechanisms may explain the relationship between gut microbiota diversity and inflammation in CKD. Firstly, gut microbiota imbalance directly influences inflammatory cytokine production, contributing to a progressive decline in renal function and characterized by a gradual accumulation of gut uremic toxins [indoxyl sulfate (IS) and p-cresyl sulfate (pCS)], resulting from abnormal gut microbial metabolism. Additionally, gut microbiota can influence the host immune system. The gut is the largest immune organ, and the gut microbiota plays an important role in immune maturation, homeostasis, and tolerance. Changes in gut microbiota can lead to intestinal inflammation and increased intestinal barrier permeability, contributing to the activation of the immune system. Second, CKD is also associated with changes in the composition of the gut microbiota, and reduced gut microbiota diversity may affect interactions between taxa, leading to an inflammatory response. Third, gut microbiota can regulate the production of indole and indole derivatives, such as indoleacetic acid and tryptamine (TMA) from tryptophan, promoting gut homeostasis, preventing inflammatory responses, and inhibiting CD4+ T cell and B cell activation. Gut microbiota can also produce TMA from choline and carnitine and then convert it to trimethylamine-N-oxide (TMAO) in the liver. This is considered a novel modulator of inflammation, and microbial metabolism of TMAO is associated with the activation of pro-inflammatory factors and the development of various inflammatory conditions. CKD results in very high production of TMAO, which directly influences the development of vascular calcification and atherosclerosis [35,36]. Several gut-derived uremic toxins, such as IS, pCS, indole-3-acetic acid (IAA), TMAO, and phenylacetylglutamine, have been closely linked to inflammation, vascular calcification, CVD, and dysbiosis in CKD and dialysis patients [35,37,64]. A randomized double-blind placebo-controlled clinical trial carried out in HD patients [13] evaluated the effect of curcumin supplementation compared to placebo on reducing plasma levels of gut uremic toxins. Fourteen patients were randomly assigned to the curcumin group and received 100 mL of orange juice and 2.5 g of turmeric, and 14 controls received the orange juice without turmeric three times per week after the HD session. After three months of follow-up, the curcumin-supplemented group exhibited significantly reduced pCS, whereas no differences in IS or IAA were found in either group. The study results suggested that curcumin supplementation three times a week post-HD session helps to mitigate inflammation and oxidative stress. The gut microbiota is a complex ecosystem, and its metabolites can modulate host metabolism, immunity, and pathophysiology by influencing signaling pathways, genes, proteins, and maintenance of gut barrier integrity in CKD and dialysis patients. Increased intestinal permeability has been related to hyperammonemia ions in the intestinal lumen, alteration of tight junction proteins (claudin-1, occludin, and tight junction ZO1 protein) in the colonic mucosa, and translocation of bacterial products of intestinal origin, as shown by the presence of DNA fragments of aerobic and anaerobic pathogens of intestinal origin circulating in patients with CKD and on dialysis [36,65]. An increase in circulating gut-derived bacterial products activates innate immunity and inflammatory cell recruitment, which results in increased production of circulating proinflammatory cytokines. Thus, the interaction between tight junction disruption and inflammation creates a vicious cycle that potentiates intestinal and systemic inflammation and intestinal barrier dysfunction [66].
In the eight RCTs included in this systematic review, curcumin or turmeric supplementation showed no adverse effects in CKD or dialysis patients. The RCTs used doses of curcumin between 367 and 1500 mg/day, while the doses of turmeric supplements were between 500 and 2500 mg/day.
Turmeric longa, with its bioactive compound curcumin, given its low cost and lack of side effects in RCTs, was shown to be a potential anti-inflammatory and antioxidant supplement. Moreover, it was shown to reduce the production of gut-derived uremic toxins through modulation of the gut microbiota in CKD and HD patients. Various studies [67,68] have recommended mixing curcumin powder with black pepper, due to the bioactive compound piperine, which has been shown to enhance absorption and bioavailability. More RCTs are needed to investigate the molecular mechanisms, metabolic pathways, therapeutic dosage, and clinical effects of curcumin in CKD and dialysis patients.
3.1.2. Propolis
Propolis is a natural resin-like substance collected by honeybees from various plant sources, including buds and exudates, and is mixed with wax, honey, and salivary secretions [69]. Over 300 bioactive compounds have been identified in propolis, mainly related to phenolic compounds such as flavonoids, terpenes, aromatic aldehydes, beta-steroids, and alcohols [15]. The composition of propolis varies with geographical location, plant source, season, productivity, bee species, and harvesting method [16]. Propolis has been utilized in studies to evaluate biological properties, such as anti-inflammatory, antioxidant, antifungal, antitumoral, and immunomodulatory activities [15,16,17]. Table 3 shows the RCTs conducted with propolis in CKD and dialysis patients.

Table 3.
Potential effects of propolis and sulforaphane in chronic kidney disease and dialysis patients.
Some RCTs [41,70,71], have reported effects of propolis on CKD or dialysis patients and its nephroprotective properties and/or effects on inflammatory response and oxidative damage. The molecular mechanisms of propolis involved blocking NF-κβ localization, inhibiting ROS, regulating gene expression, and reducing oxidative stress and inflammation by modulating the TNF-α pathway [72]. The anti-inflammatory effects of propolis are mediated by its inhibition of the expression of Toll-like receptors 2 and 4. Also, propolis contains flavonoids such as kaempferol, galangin, pinocembrin, acacetin, isorhamnetin, aurapten, and 5,7-dimethoxy-8-flavonol that have potent antioxidant effects [73].
The effect of green propolis has been a matter of interest in CKD. A randomized placebo-controlled clinical trial in thirty-two CKD stage 2–4 patients [41] analyzed the impact of Brazilian green propolis extract on proteinuria and changes in glomerular filtration rate (GFR). Measurements were performed for albuminuria, proteinuria, changes in GFR, and the urinary level ofMCP-1 as an inflammatory marker. Participants were randomized to receive 500 mg/day of green propolis (intervention group) or placebo (controls) for 12 months follow-up. In the propolis-supplemented group, proteinuria was significantly improved (695 mg/day vs. 1403 mg/day) and there was a significant reduction in urinary excretion of MCP-1 (58 pg/per mg of creatinine vs. 98 pg/per mg of creatinine) post-intervention compared to the controls. Furthermore, the hepatic, muscle, and pancreatic blood markers analyzed in the study showed no significant changes during the 12-month follow-up. This RCT demonstrated that propolis reduced proteinuria and inflammatory status with no adverse effects associated with supplementation in CKD stage 3b patients.
Due to the multifactorial nature of the inflammatory process in CKD, a multimodal approach with propolis could be of interest. Inflammatory markers, such as serum CRP, are higher in dialysis patients than in the general population, which may be a result of increased cytokines IL-1β, IL-6, and TNF-α [60]. A prospective open-label clinical trial [70] evaluated the safety of the inflammatory status of green propolis extract in 37 HD patients. Patients were allocated to receive 200 mg/day of dehydrated Brazilian green propolis extract (EPP-AF®) for 4 weeks followed by a washout period without propolis. Plasma levels of IFN-γ, selected cytokines (IL-1β, IL-1α, IL1RA, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, and TNF-α), and serum CRP were measured as the primary endpoints for the assessment of inflammatory parameters. To evaluate the potential for hepatic, pancreatic, or muscle toxicity, analyses were performed before and after the use of EPP-AF® with AST, ALT, pancreatic amylase, and CPK. The results from the study showed that a significant reduction in IL-6, TNF-α, INF-γ, IL1Ra, IL-17, IL-13, and IL-8 was found after propolis supplementation. Notably, in this clinical trial, EPP-AF® supplementation was shown to be safe and associated with a significant and sustainable reduction in proinflammatory cytokines after a 4-week post-trial period. Therefore, the above findings show the immunomodulatory activity of EPP-AF® in HD patients. Additionally, a double-blind controlled clinical trial in 41 HD patients [46] was randomized to EPP-AF® green propolis dry extract (400 mg/day) or placebo for two-month follow-up. The effect of propolis on plasma TNF-α and macrophage inflammatory protein-1β (MIP-1β) levels was examined. The propolis-supplemented group exhibited significantly reduced concentrations of TNF-α, with a non-significant trend of reduced MIP-1β levels. No substantial changes were observed in the placebo group.
In the aforementioned studies [41,46,70], the results demonstrated the anti-inflammatory and long-lasting effect of supplementation with dehydrated green propolis extract at different dosages in CKD and HD patients. Similarly, the effects of propolis were studied in peritoneal dialysis (PD). A randomized clinical study [47] in 22 PD patients assessed the inflammatory effect of propolis. PD patients were randomly allocated to receive a concentrated standardized dry extract of green propolis (400 mg/day) or a placebo for two months. Plasma levels of proinflammatory cytokines TNF-α and IL-6 were measured, and quantitative polymerase chain reaction analyses were performed to evaluate the transcriptional expression levels of Nrf2 and NF-κB in peripheral blood mononuclear cells (PBMCs). Plasma MDA levels, a lipid peroxidation marker, as well as serum CRP were analyzed. The results showed a significant reduction in TNF-α (p = 0.02) after propolis intervention, but no statistical changes in MDA, IL-6, or CRP concentrations were found. A non-significant trend of fold-change increases in Nrf2 expression after two months of intervention was observed in the propolis group. The supplementation of EPP-AF® green propolis extract (400 mg/day) for two months ameliorated inflammation, decreasing the TNF-α plasma concentrations in PD patients. Further RCTs are required to explore the effect of propolis supplementation on inflammatory and oxidative markers in CKD and dialysis populations.
Propolis, as a bioactive compound, can be proposed for use as a natural adjuvant for its possible nephroprotective and anti-inflammatory and antioxidant effects in CKD and dialysis patients. So far, RCTs conducted with propolis in CKD and dialysis have shown no remarkable adverse effects of green propolis extract supplementation with dosages between 400 and 500 mg/day. Given the limited number of conducted trials at present, more long-term randomized clinical trials are needed to investigate the properties of propolis in the different stages of CKD and dialysis modalities.
3.1.3. Sulforaphane
Sulforaphane is an isothiocyanate naturally found in cruciferous vegetables (Brassicaceae), such as broccoli, cauliflower, cabbage, kale, brussels sprouts, kohlrabi, and collard greens [74]. Hydrolysis of cruciferous vegetables such as broccoli and Brussels sprouts yields immunomodulatory isothiocyanates, most notably sulforaphane (1-isothiocyanato-4-methylsulfinylbutane). Studies [18,74] have shown that sulforaphane-based interventions may represent a novel therapeutic approach in CKD and dialysis patients (Table 1 and Table 3).
Sulforaphane is a potent antioxidant and pro-oxidant with the ability to promote endogenous antioxidants as a natural activator of the Nrf2/Keap1 cytoprotective pathway, considered a master regulator of cellular antioxidant responses [18]. It reduces the accumulation of ROS; restores the renal activities of superoxide dismutase-1, CAT, and GPx; and enhances intracellular glutathione in high-glucose-treated renal glomerular mesangial cells and tubule cells in vitro [18]. There is no acceptable daily intake (ADI) or medium daily intake (MDI), but at least three servings of about 150–200 g per week of cruciferous vegetables are recommended [75]. Recent studies [18,19,20] have demonstrated that sulforaphane has numerous biological properties as an antimicrobial, antioxidant, anti-inflammatory, and anti-oncogenic agent and as an epigenetic modulator. Sulforaphane has been reported to act as an antibacterial agent against Helicobacter pylori by inhibiting bacterial urease synthesis [20], and it has also been reported that Nrf2 activators have a potential role in the treatment of COVID-19 [76] viral pneumonia via benefiting the pulmonary antibacterial defense system. Consequently, there is great interest in sulforaphane as a bioactive compound in CKD. A recent randomized double-blind crossover study in 30 HD patients [42] aimed to evaluate the effects of sulforaphane on the expression of Nrf2 and NF-κβ. Fourteen HD patients were randomly assigned to the sulforaphane intervention (one sachet/day of 2.5 g containing 1% extract and 0.5% myrosinase) and sixteen patients to the placebo group (one sachet/day of 2.5 g containing corn starch colored with chlorophyll) for 2-month follow-up. After the 2-month washout period, the groups were switched. Nrf2 and NF-κβ mRNA expression levels were assessed by polymerase chain reaction as inflammatory parameters and serum MDA was assessed as a marker of lipid peroxidation. The sulforaphane supplementation (150 μmol/day) showed no significant changes in Nrf2 or NF-kβ mRNA expression. Likewise, no significant differences were observed in proinflammatory cytokines (IL-6 and TNF-α) or MDA levels. No adverse effects were found with the sulforaphane extract intervention. The authors pointed out that 150 μmol/day had no anti-inflammatory or antioxidant effect on HD patients. Therefore, the biological properties of sulforaphane remain unclear, and further RCTs are needed to elucidate the efficacy of sulforaphane supplementation in HD patients.
However, another recent randomized placebo-controlled clinical trial in twenty-five CKD stage 3–5 patients [48] evaluated the effects of sulforaphane on the mRNA expression of Nrf2, NF-κβ, and markers of oxidative stress (lipid peroxidation and protein carbonylation). Patients in the sulforaphane group were given 400 μg of L-sulforaphane daily (60 capsules of 200 μg). For one month, including weekends, they were instructed to take two capsules daily and to do so between meals to prevent potential interactions with other nutrients. The placebo group received 400 μg of cornstarch daily. The sulforaphane group showed a significant increase in the mRNA expression of Nrf2 and quinone oxidoreductase 1 (NQO1), as well as improvements in phosphate, glucose, and triglyceride levels. In contrast, the placebo group experienced an increase in plasma levels of low-density lipoproteins (LDLs) and total cholesterol, highlighting the positive effects of sulforaphane on reducing oxidative stress, as well as its ability to improve cardiovascular parameters.
The findings from this systematic review suggest that it is important to standardize the sulforaphane dosage and treatment duration in CKD and dialysis patients in future studies. Although a few non-RCTs have used this compound as a supplement in CKD or dialysis patients, the safety profile of sulforaphane supplementation was considered favorable, with no statistically significant differences in adverse effects between treatment and placebo groups. Based on this background, future interventions with standardized doses of sulforaphane should be tested in longer-term clinical trials to establish its potential as a modulator of inflammatory and oxidative stress in CKD, as well as for delaying the onset of dialysis.
3.1.4. Genistein
Genistein is a small, hydrophobic molecule with a molecular weight of 270 Daltons. It is an isoflavone considered to be a phytoestrogen. Genistein is mostly found in leguminous plants such as soybeans (Glycine max), chickpeas (Cicer arietinum), and broad beans (Vicia faba), which contain approximately 11–35 mg of isoflavones in 100 g of dry beans. The bioavailability of genistein ranges from 0.1 to 4.0%. Exogenous genistein is absorbed in the digestive tract, metabolized, and distributed by attaching itself directly to plasma proteins. More than 30 aromatic hydroxylation and demethylation metabolites of genistein have been detected in urine, plasma, and human tissues. Among the soybean isoflavones, genistein is considered the most predominant in the human diet [21]. To date, the ADI and MDI have not been established. Genistein supplementation has shown promise in the management of CKD. Some studies [21,22] have indicated that genistein supplementation may help regulate blood pressure by affecting renin secretion and inhibiting cellular apoptosis through inhibition of SIRT1 gene expression in common kidney diseases (diabetic nephropathy, hypertensive kidney disease, and renal fibrosis).
Additionally, genistein supplementation has demonstrated effects on calcium and phosphate balance, with decreased urinary calcium content and increased serum vitamin D. Furthermore, genistein has been found to have diuretic effects by reducing vascular resistance and protecting the permeability barrier of the kidney, inhibiting the increase in albumin permeability in acute glomerular inflammatory injury. These findings highlight the multifaceted potential benefits of genistein supplementation in CKD patients [22]. Table 1 and Table 4 show the biological effects of genistein in CKD and dialysis patients.

Table 4.
Potential effects of genistein, allicin, and beetroot in chronic kidney disease and dialysis patients.
Research has suggested that genistein may play a role in modulating the inflammatory response and oxidative stress [22]. A randomized double-blind clinical trial study [49] hypothesized that dietary soy isoflavones improved systemic inflammation in inflamed HD patients. Inflammation was measured by CRP, IL-6, and TNF-α, and nutrition parameters were studied by serum albumin, prealbumin, insulin-like growth factor-1 (IGF-1), and normalized protein catabolic rate (nPCR). Thirty-two inflamed HD adults were administered soy-based nutritional supplements with isoflavones (54 mg in a protein drink during the scheduled dialysis session or 26 mg in a protein bar or something similar on non-dialysis days) (soy group) vs. milk protein without isoflavones (control group) for 8 weeks. The supplemented group showed a positive correlation of total isoflavones and genistein with serum albumin and IGF-1 and an inverse association of total isoflavones with CRP level. Previous findings supported an anti-inflammatory, nutritional effect of dietary soy in HD patients with underlying systemic inflammation. Specifically, the proposed molecular mechanisms are related to an enhancement of the inflammatory response through the downregulation of several inflammatory factors [IL-1, IL-6, NF-κβ, TNF-α, Toll-like receptor 4, and serum CRP]. Of note, genistein also inhibits oxidative stress (protein carbonyl, MDA, and Nrf2), which protects against oxidation of DNA, proteins, and lipids. Moreover, it is involved in angiogenesis through the inhibition of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) in kidney cancer [22].
Also, there is some experimental evidence to suggest that the progression of CKD is slower given diets based on soy protein. A crossover clinical trial [23] compared the effect of a soy-based vegetarian protein diet (VPD) and an animal-based low-protein diet (APD) in CKD stages 3–4. The patients were randomly assigned to either group 1 (VPD) or group 2 (APD). They stayed on one diet for 6 months and then switched to the other diet for a second 6-month period. The VPD was well tolerated; decreased 24 h urinary creatinine and phosphate and serum CRP; and maintained GFR. These potential advantages may have protective effects, specifically in CKD stages 3–4.
Genistein, as an isoflavone and soy derivative, may be useful in slowing the progression of CKD and as an anti-inflammatory agent in patients with CKD, such as those on dialysis. Potential drug interactions are a crucial consideration when incorporating genistein supplementation into the treatment of CKD and dialysis patients. Genistein’s impact on the renin–angiotensin–aldosterone system and blood pressure regulation suggest caution when combined with antihypertensive angiotensin-converting enzyme (ACE) inhibitors targeting similar physiological pathways. The potential use of genistein as a diuretic warrants more in-depth clinical research, particularly to assess its efficacy and safety in CKD and dialysis patients. Additionally, its influence on calcium and phosphate balance may interact with drug targeting. Furthermore, the varying effects of genistein on kidney permeability barriers and tight junctions highlight the complexity of its interactions with medications affecting kidney function, necessitating careful monitoring and assessment of potential drug combinations.
3.1.5. Allicin
Allicin, a sulfur-containing compound, is synthesized from alliin through the action of the enzyme alliinase. The chemical structure of allicin consists of two prop-2-ene-1-sulfinothioic acid S-allyl ester molecules linked by a disulfide bond, giving it its characteristic odor. It is primarily found in garlic (Allium sativum) and other related Allium species, such as ramsons (Allium ursinum) and hooker chives (Allium hookeri). These plants produce allicin as a defense mechanism when their cells are damaged, and it has been reported to be one of the primary substances responsible for garlic’s antiviral activity and immunomodulatory, anti-inflammatory, and antioxidant effects [26]. It is important to note that allicin is particularly active against several multidrug-resistant strains of human pathogens, including methicillin-resistant Staphylococcus aureus and Helicobacter pylori.
The natural sources of allicin, particularly garlic, have long been recognized for their potential health benefits. Allicin’s ability to promote the production of antioxidant enzymes and inhibit the oxidation of LDL particles underscores its potential in ameliorating oxidative stress-related complications often observed in CKD patients. Allicin is considered a superoxide anion radical scavenger that can ameliorate lipid peroxidation. Moreover, it can activate Nrf2, which is responsible for the activation of antioxidant response elements and the production of phase II antioxidant and detoxifying enzymes, such as glutathione (GSH), SOD, CAT, and GPx [77]. Table 4 shows the RCTs of allicin and its bioeffects in CKD and dialysis patients.
Asgharpour et al. [50], in a randomized double-blind clinical trial, evaluated the potential use of garlic extract to improve lipid profiles, reduce inflammation, and modify cardiovascular (CV) risk factors. Seventy HD patients were randomized to receive 300 mg of standardized garlic powder (containing 1.3 milligrams of garlic extract) or a placebo for eight weeks. After a six-week washout period, the two groups were switched so that the group that received garlic powder in the first eight weeks received a placebo for the second eight weeks and vice versa. In this study, garlic extract was useful in reducing triacylglyceride, oxidized LDL, and homocysteine levels. These findings underscore the potential of allicin, as a component of garlic extract, to address the critical aspects of managing CKD and its associated complications, particularly in the context of oxidative stress and inflammation. A further randomized double-blind clinical trial [51] evaluated the effect of garlic extract on the inflammatory markers (IL-6, CRP, and erythrocyte sedimentation rate) of 40 patients undergoing PD. The patients received a dosage of 400 mg of a standardized garlic extract, including 1 mg of alliin or a placebo twice a day for 8 weeks. The garlic-supplemented group showed significantly reduced IL-6, CRP, and erythrocyte sedimentation rates, while in the placebo group a significant decrease was only observed in plasma IL-6. These properties suggest that allicin may hold the potential for mitigating oxidative stress and inflammation, as well as potentially addressing bacterial complications that can arise in dialysis patients. Therefore, the multifaceted properties of allicin present a compelling rationale for further investigation into its potential benefits for individuals with CKD, particularly in the framework of oxidative stress, inflammation, and potential antimicrobial effects.
3.1.6. Betalain from Beetroot (Beta Vulgaris Rubra)
The tuber Beta vulgaris rubra, also known as beet, has aroused great interest as a functional food for its impact on human health [24]. Beetroot is a source of inorganic nitrate, which can be reduced to form nitrite and nitric oxide (NO) after dietary intake. NO is an important endogenous signaling molecule in biological systems and has been implicated in a wide variety of physiological and pathological processes, including blood pressure regulation, immune defense, and neurotransmission. NO was first identified as an endothelial-derived relaxing factor and has well-established vasodilatory properties, playing essential roles in the regulation of renal blood flow, GFR, and blood pressure. It is increasingly being recognized that the reduced bioavailability of NO due to decreased production and/or increased scavenging contributes to the pathogenesis of CVD, including hypertension, atherosclerosis, myocardial ischemia and infarction, and heart failure. It has recently been recognized that the intake of nitrates from vegetables is an important exogenous source of NO with vasodilator activities [24,25,78].
However, excessive or dysregulated NO production can lead to harmful effects. In pathological conditions such as infection, an overproduction of NO may contribute to tissue damage and worsen inflammatory responses. NO can interact with ROS to produce reactive nitrogen species, such as peroxynitrite, which can induce oxidative and nitrosative stress, causing cellular damage and playing a role in the development of various diseases, including CVD [25]. Production of NO in the body is both a positive and negative process, with its effects being highly context-dependent.
Beta vulgaris rubra has emerged as a potential means to prevent and manage diseases associated with diminished NO bioavailability, notably arterial hypertension and endothelial dysfunction [24,25]. Beetroot contains phytochemical compounds that include ascorbic acid, carotenoids, phenolic acids, flavonoids, and highly bioactive pigments known as betalains (beetroot powder) characterized by their high antioxidant and anti-inflammatory activity [24,25]. Betalains have no ADI or MDI, but nitrates have been recommended for an ADI of 3.7 mg/kg [79]. Table 4 shows the RCTs conducted with beetroot.
Betalains also offer health-promoting properties, particularly for disorders characterized by chronic inflammation, such as CKD [24]. In this regard, a randomized crossover study [80] in CKD patients analyzed NO concentrations and renal resistance indexes as prognostic markers of CV mortality. Beetroot juice with a nitrate load of 300 mg was administered to the intervention group compared to the placebo. Hemodynamic parameters as well as plasma nitrate concentration and renal resistance indexes were measured before and 4h after treatment. The dietary nitrate exerted hypotensive effects on patients with diabetic nephropathy or hypertensive kidney disease without any changes in serum potassium and creatinine after they drank the beetroot juice. Likewise, the nitrate dietary load was able to decrease the renal resistance index—a marker of CKD progression and CV mortality. However, there are limited observations on beetroot, and further investigations are warranted. Therapeutic interventions directed toward the improvement of NO production, in addition to management of other CV risk factors, may prevent the development of endothelial dysfunction, in addition to facilitating the proper management of CKD patients at increased risk of CVD.
In the same way, a randomized single-blind placebo-controlled study carried out on HD patients [52] examined the kinetics of plasma nitrate. Eight HD patients and seven healthy volunteers were randomly assigned to receive 300 mL of nitrate-rich concentrated beetroot juice and a placebo. The beetroot juice consumption was safe and well-tolerated, with significantly higher plasma nitrate concentrations in HD patients. Long-term efficacy studies of dietary nitrate supplementation are warranted to investigate clinical benefits in subjects with reduced NO bioactivity, including CKD and HD patients.
Beetroot and its bioactive compounds (betalains, mostly), can modulate mechanisms related to CKD progression and CVD, indicating that it could be a promising nutritional therapy to modulate common CKD-associated complications, such as arterial hypertension, endothelial dysfunction, inflammation, and oxidative stress [81]. As a non-pharmacological intervention, beetroot juice may offer health benefits for CKD and dialysis patients, but further clinical trials are needed.
3.2. Potential Effects of Some Medicinal Plants on Chronic Kidney Disease
3.2.1. Catechins from Green Tea (Camellia Sinensis)
Green tea comes from the plant Camellia sinensis and has high anti-inflammatory, antioxidant, and antimutagenic properties because of its polyphenolic compounds in various biological systems [26]. Furthermore, green tea is distinguished by the presence of catechins, a subgroup of polyphenols, known for their powerful antioxidant activities. Three main catechins have been documented in green tea, epicatechin, epigallocatechin, and epicatechin-3-gallate (EGCG). The catechin EGCG is the most abundant, with an ADI of <800 mg/day and an MDI of 90–300 mg/day obtained from green tea infusions [82].
The health effects of EGCG are mediated by the underlying molecular mechanisms, mainly by direct inhibition of stress- or stimulus-induced ROS overproduction. Moreover, ECGC can influence the Nrf2 complex, leading to nuclear translocation of free Nrf2, which subsequently binds to the antioxidant response element within the promoter region of cytoprotective genes encoding antioxidant enzymes, which are also modulated by the NF-κβ and TNF-α signaling pathways [27]. Table 5 shows the RCTs conducted with green tea or EGCG.

Table 5.
Potential effects of bioactive compounds from medicinal plants in chronic kidney disease and dialysis patients.
Various studies [53,54,55,83] have investigated the relationship between green tea and the mechanisms of CKD. An epidemiological study with Mendelian randomization in CKD researched the potential causal effects of tea intake on albuminuria and GFR [83]. The genome-wide association study summary data set for tea consumption was based on the UK Biobank, and the data on habitual tea consumption were obtained as a baseline from a dietary questionnaire. A total of 2672 single-nucleotide polymorphisms associated with tea consumption were found, 45 of which were independent and usable in CKD gen. Drinking an additional daily cup of tea had a nephroprotective effect by increasing GFR. Alternatively, high tea consumption was also inversely correlated with a lower risk of albuminuria. The study provided new hypotheses about green tea and CKD that should be explored in future clinical trials.
HD patients suffer from high levels of oxidative stress and antioxidant-decreased enzyme activity, e.g., SOD, CAT, and GSH-Px [84]. A clinical trial [53] studied the combination of EGC and Gand Amla extract (AE) in thirteen diabetic HD patients compared to healthy volunteers. The EGCG/AE enhanced antioxidant defense in diabetic HD patients. In addition, no hepatic or renal toxicity, and no inflammatory response were observed. The study showed that catechins improve antioxidant defenses, plasma glucose, CRP, and atherogenic indices (HDL and LDL/HDL ratio) in diabetic HD patients.
Likewise, another clinical study [54] studied the effect of supplementation with 800 mg of EGCG in treating residual albuminuria in 42 patients with diabetic nephropathy. The consumption of green tea significantly reduced albuminuria by diminishing podocyte apoptosis through activation of the wingless pathway. Also, a randomized placebo-controlled trial [55] evaluated the effects of supplementation with decaffeinated green tea extract on induced ROS and proinflammatory cytokines as CV risk factors in HD patients. The study consisted of three phases. Firstly, the researchers measured the plasma catechin concentrations in 6 healthy subjects and 10 HD patients after a single oral dose (455 mg of decaffeinated green tea extracts) that was comparable to four cups of green tea. In the following phase, the antioxidant capacity of catechins (455 mg) was compared with oral vitamin C (500 mg) in 10 HD patients. Lastly, the sample was randomized in a 7-month interventional study, in which 30 patients received a placebo and 14 patients received catechins (455 mg/d) from the third to the fifth month. Supplementation with catechins was more effective than vitamin C by reducing HD-enhanced plasma hypochlorous acid count activities and restoring paraoxonase-1 activity. Furthermore, the post-dialysis plasma concentrations of proapoptotic mediators, proinflammatory cytokines, and leukocyte-mediated anti-inflammatory molecules (the Fas/TNF receptor superfamily, IL-6R, IL-8, NAP-2, IL-1ra, IL-6R, IL-6R, IL-8, NAP-2, and IL-1ra) were downregulated significantly by the catechins. During the three experimental months, the pre-dialysis plasma concentrations of phosphatidylcholine hydroperoxide, TNF-α, sICAM-1, MCP-1, and CRP in the intervention group were lower than their baseline values and those in the control group. This study provided support for the possibility that catechins reduce HD-induced production of hydrogen peroxide and hypochlorous acid, CV risk factors, and proinflammation.
Previous preclinical evidence has suggested that EGCG may exert its protective effects through various mechanisms, including activation of Nrf2/HO-1 signaling, anti-inflammatory activity, and prevention of epithelial-to-mesenchymal transition [85]. Understanding the molecular interactions of EGCG with proteins, as well as its impact on biological processes at the cellular level, could provide valuable insights for the development of potential therapeutic interventions for CKD and dialysis patients. Moreover, causal research on the association between tea consumption and kidney function, particularly CKD, remains limited. This highlights the need for further investigation into the causal relationship between tea consumption, catechin intake, and kidney function, providing a compelling direction for future research in this area.
3.2.2. Rhein, Emodin, and Aloe-Emodin from Rhubarb
In Asia, especially in Japan, not only traditional herbologists but also nephrologists have occasionally used herbal medicines for the treatment of CKD patients as part of a multi-approach therapy. Rheum officinale has long been used by practitioners of traditional Chinese medicine for its strong cathartic action and, more recently, to delay the progression of CKD. Identified active constituents of processed Rheum officinale include rhein, emodin, and aloe-emodin, each of which confers different pharmacologic actions [9]. In CKD, rhubarb contributes to the elimination of nitrogenous waste products through the gastrointestinal tract and the regulation of water and electrolyte balance. It has been reported that the abnormal expression of aquaporins could lead to less absorption of water in the colon or more secretion of intestinal juice. Emodin inhibits the genetic transcription and translation of the aquaporin 2 gene and decreases the gluconeogenesis of renal tubular cells and the adenosine triphosphate (ATP) content of epithelial mitochondria [56].
To date, a few studies that used rhubarb in CKD patients have been reported (Table 5). A randomized clinical trial [56] was conducted on 144 patients with CKD stage 3–4 to evaluate the efficacy and safety of rhubarb supplementation. Patients received conservative CKD management along with a rhubarb capsule (350 mg/thrice daily) or a placebo for 12 weeks. The results showed a reduction in levels of glucose, urea, creatinine, and 24 h total urine protein, whereas hemoglobin and GFR were increased. As for side effects, there was no statistical difference between the two groups.
The findings on rhubarb or Rheum officinale seem promising, but the lack of studies means that it not possible yet to draw strong conclusions about its use in patients with CKD.
3.2.3. Astragalus Membranaceus (Huangqi)
Astragalus, known as Huangqi in China, a perennial herb in the Fabaceae family, is widely used in traditional Chinese medicine. The dried root of this plant is traditionally used as a tonic herb in CKD with a GFR less than 60 mL/min/1.73 m2. More than 100 compounds have been isolated and identified from Astragalus, such as flavonoids, saponins, polysaccharides, amino acids, and other compounds (ribobioside, sphingolipids, oligosaccharides, acids, enzymes, and trace elements).
Huangqi has been demonstrated to exhibit immunomodulatory effects, anti-inflammatory properties, and antioxidant activities [28,29] (Table 1 and Table 5). Various studies [86,87] have shown that Huangqi extracts modulate either pro- or anti-inflammatory immune responses depending on doses and cultural conditions. Administration of Huangqi extracts to palmitate-treated macrophages decreased the expression of proinflammatory cytokines and enhanced the production of anti-inflammatory cytokines. Similarly, Huangqi treatment was shown to promote T-helper (Th) cell differentiation toward Th1 and Th2 cell types [88]. In a recent metanalysis [89], the use of Astragalus membranaceus was associated with decreased plasma IL-6.
A controlled before-and-after clinical trial [57] studied the effect of Astragalus membranaceus on GFR in patients with advanced CKD stage 4–5 to assess the efficacy of Astragalus membranaceus in CKD progression combined with conventional therapy. Thirty-five CKD patients were treated with 2.5 g Astragalus twice a day, together with conventional therapy for more than 3 months. The supplementation of astragalus significantly increased the GFR in patients with CKD stage 4 during the first three months and maintained the GFR during the remaining eight months. The supplementation with Astragalus was effective in delaying CKD progression in this study, but new RCTs are needed to confirm the effects of this herbal therapy.
The effects of Astragalus have also been studied in PD patients. A retrospective single-center case–control study [88] measured the effects of astragalus on residual renal function and total daily urine volume. Eight PD patients and sixteen controls were included in this analysis. The Astragalus membranaceus formulae were ingested as decoctions prepared from raw herbs or concentrated Chinese medicine granules. Results showed that the supplemented group had significantly higher daily urine volumes and residual renal function at 2 years. The findings of the study highlighted the potential of Astragalus membranaceus in maintaining residual renal function in PD patients, but causal relationships cannot be established given the methodological design of the study.
Currently, there are significant adverse consequences associated with the utilization of Astragalus, including allergic response and gastrointestinal disorders [90]. However, further well-designed studies, particularly RCTs, are warranted to confirm these findings and elucidate the underlying mechanisms.
3.2.4. Triptolide from Tripterygium Wilfordii Hook F
Tripterygium wilfordii Hook F (TwHF) is a perennial herb belonging to the family Celastraceae, and it is a traditional Chinese herbal medicine commonly used to treat inflammatory diseases [30]. The active products of TwHF include a variety of alkaloids, flavonoids, sterols, phenols, glycosides, triterpenoids, and proteins. Triptolide is one of the bioactive compounds in TwHF that has been implicated in the suppression of T cell proliferation and the induction of T cell apoptosis in the development of type 1 DM [31].
Nevertheless, research into the relationship between TwHF and CKD has been scarce (Table 1 and Table 5). A randomized clinical trial [58] was conducted to evaluate the efficacy and safety of TwHF for reducing proteinuria in sixty-five patients with diabetic nephropathy. Patients were given 120 mg of TwHF per day for three months, followed by 60 mg of TwHF/day for the remaining 3 months or 160 mg of valsartan daily for 6 months. Proteinuria in the TwHF group decreased at 1, 3, and 6 months. In contrast, in the valsartan group, proteinuria was not significantly attenuated at 1, 3, or 6 months post-intervention.
A systematic review of TwHF-derived treatments for CKD and dialysis patients is warranted. Various bioactive compounds in TwHF can act on CKD using a multitargeting pattern, thus providing insight into understanding herbal medicines and their mechanisms of action. However, the lack of clinical trials and the consistency of the results highlight that more studies are still needed to ensure the efficacy and safety of TwHF extract as an herbal medicine in CKD.
4. Conclusions
Considering that the rising prevalence of CKD imposes a significant burden on global healthcare systems due to high medical costs, incorporating nutrition-based therapies, such as those involving plant bioactive compounds, could serve as an affordable and manageable supplementary treatment for CKD and dialysis patients. Turmeric acts as an antioxidant and anti-inflammatory agent, and a potential modulator of the microbiota in CKD stages 3 and 4 and in HD patients. Likewise, propolis was shown to improve proteinuria in stage 2–4 CKD, as well as anti-inflammatory and antioxidant effects in dialysis patients. Additionally, green tea, as a medicinal plant, had antioxidant and anti-inflammatory effects in CKD and dialysis patients. This systematic review highlights the current impact of certain bioactive compounds and medicinal plants on the management and progression of CKD and in dialysis patients.
Many of these bioactive compounds have been found to mitigate inflammation by modulating the NF-κβ pathway, which lowers levels of cytokines and pro-inflammatory compounds. Others exhibit direct ROS scavenging properties due to their molecular structure or have indirect antioxidative effects through the upregulation of antioxidant enzymes. A diet tailored to CKD that includes natural foods containing these compounds may delay disease progression and quality of life while reducing health damage and economic costs related to complications.
Based on these studies, traditional Chinese medicine could play a role in a comprehensive approach to managing CKD and dialysis patients. However, there remains insufficient evidence to endorse its use in clinical practice. Further clinical and observational studies with larger patient populations are therefore needed to better understand the effects of bioactive compounds and medicinal plants, the safe and effective dosages, as well as possible side effects in larger trials.
Author Contributions
Conceptualization, E.J. and M.R.; Writing—Original Draft Preparation, E.J. and M.R.; Writing—Review and Editing, E.J., G.B. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–2040 for 195 countries and territories. Lancet 2018, 10, 2052–2090. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update. Kidney Int. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Lefevre, M.; Beecher, G.R.; Gross, M.D.; Keen, C.L.; Etherton, T.D. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: The antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu. Rev. Nutr. 2004, 24, 511–538. [Google Scholar] [CrossRef] [PubMed]
- Quintela, B.C.S.F.; Carioca, A.F.; de Oliveira, J.G.R.; Fraser, S.D.; da Silva Junior, G.B. Dietary patterns and chronic kidney disease outcomes: A systematic review. Nephrology 2021, 26, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 11–12. [Google Scholar] [CrossRef]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean diet as an antioxidant: The impact on metabolic health and overall wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, H.; Yue, J.; Li, J.; Hou, Y.B.; Deng, J.L. Rheum officinale (a traditional Chinese medicine) for chronic kidney disease. Cochrane Database Syst. Rev. 2012, 7, CD008000. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Villazana, J. Advances in the determination of bioactive compounds in foods. Cienc. Tecnol. Agrollanía 2020, 19, 7–17. [Google Scholar]
- Martins-de-Souza, D. Studies on Biomarkers and New Targets in Aging Research in Iran Focus on Turmeric and Curcumin; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2021; Available online: http://www.springer.com/series/15040 (accessed on 16 September 2024).
- Rodrigues, H.C.; Martins, T.F.P.; Santana, N.C.e.S.; Braga, C.C.; Silva, M.C.; da Cunha, L.C.; Sugizaki, C.S.A.; Freitas, A.T.V.S.; Costa, N.A.; Peixoto, M.D.R.G. Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2021, 1, 136–142. [Google Scholar] [CrossRef]
- Salarolli, R.T.; Alvarenga, L.; Cardozo, L.F.M.F.; Teixeira, K.T.R.; de Moreira, L.S.G.; Lima, J.D.; Rodrigues, S.D.; Nakao, L.S.; Fouque, D.; Mafra, D. Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int. Urol. Nephrol 2021, 1, 1231–1238. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverri, J. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J. Ren. Nutr. 2016, 4, 237–244. [Google Scholar] [CrossRef]
- Gheflati, A.; Dehnavi, Z.; Yazdi, A.G.; Khorasanchi, Z.; Raeisi-Dehkordi, H.; Ranjbar, G. The effects of propolis supplementation on metabolic parameters: A systematic review and meta-analysis of randomized controlled clinical trials. Avicenna J. Phytomed. 2021, 11, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Forma, E.; Bryś, M. Anticancer activity of propolis and its compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 2, 8473. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.F.M.F.; Alvarenga, L.A.; Ribeiro, M.; Dai, L.; Shiels, P.G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cruciferous vegetables: Rationale for exploring potential salutary effects of sulforaphane-rich foods in patients with chronic kidney disease. Nutr. Rev. 2021, 79, 1204–1224. [Google Scholar] [CrossRef] [PubMed]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid. Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed]
- Helen, K.; Peterson, T.G.; Barnes, S. Mechanisms of action of the soy isoflavone genistein: Emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 1998, 68, 1418–1425. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; Shang, J.; Huang, H.; Zhang, Y.; Ding, Y.; Liang, Y.; Xie, Z.; Chen, C. Effects of Genistein on Common Kidney Diseases. Nutrients 2022, 14, 3768. [Google Scholar] [CrossRef] [PubMed]
- Soroka, N.; Silverberg, D.S.; Greemland, M.; Birk, Y.; Blum, M.; Peer, G.; Iaina, A. Comparison of a Vegetable-Based (Soya) and an Animal-Based Low-Protein Diet in Predialysis Chronic Renal Failure Patients. Nephron 1998, 79, 173–180. [Google Scholar] [CrossRef]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 801–822. [Google Scholar] [CrossRef]
- Griffiths, A.; Alhulaefi, S.; Hayes, E.J.; Matu, J.; Brandt, K.; Watson, A. Exploring the Advantages and Disadvantages of a Whole Foods Approach for Elevating Dietary Nitrate Intake: Have Researchers Concentrated Too Much on Beetroot Juice? Appl. Sci. 2023, 13, 7319. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; García-Mayorga, E.A.; Díaz-Avila, D.; Garza-Veloz, I.; Martinez-Fierro, M.L.; González-Mateo, G.T. Potential therapeutic effects of natural plant compounds in kidney disease. Molecules 2021, 26, 96. [Google Scholar] [CrossRef]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Ji, B.; Xuan, L.; Zhang, Y.; Zhang, G.; Meng, J.; Mu, W.; Liu, J.; Paek, K.Y.; Park, S.Y.; Wang, J.; et al. Advances in Biotechnological Production and Metabolic Regulation of Astragalus membranaceus. Plants 2023, 12, 1858. [Google Scholar] [CrossRef]
- Song, C.Y.; Xu, Y.G.; Lu, Y.Q. Use of Tripterygium wilfordii Hook F for immune-mediated inflammatory diseases: Progress and future prospects. J. Zhejiang Univ. Sci. B 2020, 21, 280–290. [Google Scholar] [CrossRef]
- Sravanthi, V.; Sampath Kumar, H.M. Immunomodulators in Prophylaxis and Therapy of Type-1 Diabetes. In Discovery and Development of Antidiabetic Agents from Natural Products, 1st ed.; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 229–249. [Google Scholar] [CrossRef]
- Khan, M.A.; Kassianos, A.J.; Hoy, W.E.; Alam, A.K.; Healy, H.G.; Gobe, G.C. Promoting Plant-Based Therapies for Chronic Kidney Disease. J. Evid. Based. Integr. Med. 2022, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Fouque, D. Gut microbiota and inflammation in chronic kidney disease patients. Clin. Kidney J. 2015, 1, 332–334. [Google Scholar] [CrossRef]
- Borges, N.A.; Barros, A.F.; Nakao, L.S.; Dolenga, C.J.; Fouque, D.; Mafra, D. Protein-Bound Uremic Toxins from Gut Microbiota and Inflammatory Markers in Chronic Kidney Disease. J. Ren. Nutr. 2016, 1, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Alvarenga, L.; Salarolli, R.; Cardozo, L.F.M.F.; Santos, R.S.; de Brito, J.S.; Kemp, J.A.; Reis, D.; de Paiva, B.R.; Stenvinkel, P.; Lindholm, B.; et al. Impact of curcumin supplementation on expression of inflammatory transcription factors in hemodialysis patients: A pilot randomized, double-blind, controlled study. Clin Nut. 2020, 39, 3594–3600. [Google Scholar] [CrossRef]
- Hami, M.; Bigdeli, A.; Khameneh-Bagheri, R.; Rajabi, O.; Salehi, M.; Zahedi-Avval, F. The Effect of Curcumin in Prevention of Contrast Nephropathy Following Coronary Angiography or Angioplasty in CKD Patients. Iran. J. Kidney Dis. 2019, 13, 304–309. [Google Scholar] [PubMed]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: A randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019, 25, 140. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Cardozo, L.F.M.F.; Paiva, B.R.; Baptista, B.G.; Fanton, S.; Alvarenga, L.; Lima, L.S.; Britto, I.; Nakao, L.S.; Fouque, D.; et al. Sulforaphane supplementation did not modulate NRF2 and NF-kB mRNA expressions in hemodialysis patients. J. Ren. Nut. 2023, 1, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Akmali, M.; Malekmakan, L.; Dabaghimanesh, M.; Khorsand, M. Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial. Int. 2015, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Samadian, F.; Dalili, N.; Gholi, F.P.R.; Fattah, M.; Malih, N.; Nafar, M.; Firoozan, A.; Ahmadpoor, P.; Samavat, S.; Ziaie, S. Evaluation of Curcumin’s effect on inflammation in hemodialysis patients. Clin. Nutr. ESPEN 2017, 1, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 1, 231. [Google Scholar] [CrossRef]
- Chermut, T.R.; Fonseca, L.; Figueiredo, N.; de Oliveira Leal, V.; Borges, N.A.; Cardozo, L.F.; Correa Leite, P.E.; Alvarenga, L.; Regis, B.; Delgado, A.; et al. Effects of propolis on inflammation markers in patients undergoing hemodialysis: A randomized, double-blind controlled clinical trial. Complement. Ther. Clin. Pract. 2023, 51, 101732. [Google Scholar] [CrossRef]
- Baptista, B.G.; Fanton, S.; Ribeiro, M.; Cardozo, L.F.; Regis, B.; Alvarenga, L.; Ribeiro-Alves, M.; Berretta, A.A.; Shiels, P.G.; Mafra, D. The effect of Brazilian Green Propolis extract on inflammation in patients with chronic kidney disease on peritoneal dialysis: A randomised double-blind controlled clinical trial. Phytomedicine 2023, 114, 154731. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Coutinho-Wolino, K.S.; Nakao, L.S.; Cardozo, L.F.; Mafra, D. Sulforaphane upregulates the mRNA expression of NRF2 and NQO1 in non-dialysis patients with chronic kidney disease. Free Radic. Biol. Med. 2024, 221, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fanti, P.; Asmis, R.; Stephenson, T.J.; Sawaya, B.P.; Franke, A.A. Positive effect of dietary soy in ESRD patients with systemic inflammation—Correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol. Dial. Transplant. 2006, 8, 2239–2246. [Google Scholar] [CrossRef]
- Asgharpour, M.; Khavandegar, A.; Balaei, P.; Enayati, N.; Mardi, P.; Alirezaei, A.; Bakhtiyari, M. Efficacy of Oral Administration of Allium sativum Powder “garlic Extract” on Lipid Profile, Inflammation, and Cardiovascular Indices among Hemodialysis Patients. Evid. Based Complement. Alternat. Med. 2021, 2021, 6667453. [Google Scholar] [CrossRef]
- Zare, E.; Alirezaei, A.; Bakhtiyari, M.; Mansouri, A. Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: A randomized double-blind clinical trial study. BMC Nephrol. 2019, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Martinez, A.; Rosa-Diez, G.; Ferraris, J.R.; Sohlenius-Sternbeck, A.K.; Nihlen, C.; Olsson, A.; Lundberg, J.O.; Weitzberg, E.; Carlström, M.; Krmar, R.T. Plasma Nitrate and Nitrite Kinetics after Single Intake of Beetroot Juice in Adult Patients on Chronic Hemodialysis and in Healthy Volunteers: A Randomized, Single-Blind, Placebo-Controlled, Crossover Study. Nutrients 2022, 14, 2480. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Liou, S.Y.; Wu, H.C.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y. Efficacy of Epigallocatechin-3-Gallate and Amla (Emblica officinalis) Extract for the Treatment of Diabetic-Uremic Patients. J. Med. Food 2011, 7–8, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; Lopes De Faria, J.M.; Lopes De Faria, J.B. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.P.; Wu, M.S.; Yang, C.C.; Huang, K.C.; Liou, S.Y.; Hsu, S.M.; Chien, C.T. Chronic green tea extract supplementation reduces hemodialysis-enhanced production of hydrogen peroxide and hypochlorous acid, atherosclerotic factors, and proinflammatory cytokines 1–3. Am. J. Clin. Nutr. 2007, 86, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Nasiruddin, M.; Haque, S.F.; Khan, R.A. Evaluation of Rhubarb Supplementation in Stages 3 and 4 of Chronic Kidney Disease: A Randomized Clinical Trial. Int. J. Chronic Dis. 2014, 2014, 789340. [Google Scholar] [CrossRef]
- Okuda, M.; Horikoshi, S.; Matsumoto, M.; Tanimoto, M.; Yasui, H.; Tomino, Y. Beneficial effect of Astragalus membranaceus on estimated glomerular filtration rate in patients with progressive chronic kidney disease. Hong Kong J. Nephrol. 2012, 14, 17–23. [Google Scholar] [CrossRef][Green Version]
- Ge, Y.; Xie, H.; Li, S.; Jin, B.; Hou, J.; Zhang, H.; Hi, M.; Liu, Z. Treatment of diabetic nephropathy with Tripterygium wilfordii Hook F extract: A prospective, randomized, controlled clinical trial. J. Transl. Med. 2013, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, J.; Zhang, F.; Huang, C.; Wu, Y.; Han, Y.; Zhang, W.; Zhao, Y. Prognostic role of C-reactive protein and Interleukin-6 in dialysis patients: A systematic review and meta-analysis. J. Nephrol. 2013, 26, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Tranplant. 2018, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Cobo, G.; Lindholm, B.; Stenvinkel, P. Inflammation and Protein-Energy Wasting in the Uremic Milieu. Contrib. Nephrol. 2017, 191, 58–71. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2020, 1, 263. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; Desantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wang, F.; Jiang, H.; Liu, H.; Wei, M.; Wang, Z. Gut Bacterial Translocation May Aggravate Microinflammation in Hemodialysis Patients. Dig. Dis. Sci. 2014, 1, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Cabała, S.; Ożgo, M.; Herosimczyk, A. The Kidney–Gut Axis as a Novel Target for Nutritional Intervention to Counteract Chronic Kidney Disease Progression. Metabolites 2024, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 4, 353–356. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.; Cardozo, L.F.M.F.; Borges, N.A.; Chermut, T.R.; Ribeiro, M.; Leite, M.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. To bee or not to bee? The bee extract propolis as a bioactive compound in the burden of lifestyle diseases. Nutrition 2021, 83, 111094. [Google Scholar] [CrossRef]
- Duarte Silveira, M.A.; Malta-Santos, H.; Rebouças-Silva, J.; Teles, F.; Batista Dos Santos Galvão, E.; Pinto De Souza, S.; Dantas Dutra, F.R.; Dantas Gomes, M.M.; Teixeira, M.B.; Miranda Rebelo da Conceição, L.F.; et al. Effects of Standardized Brazilian Green Propolis Extract (EPP-AF®) on Inflammation in Haemodialysis Patients: A Clinical Trial. Int. J. Nephrol. 2022, 22, 1035475. [Google Scholar] [CrossRef]
- Anvarifard, P.; Ostadrahimi, A.; Ardalan, M.; Anbari, M.; Ghoreishi, Z. The effects of propolis on pro-oxidant–antioxidant balance, glycemic control, and quality of life in chronic kidney disease: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2023, 1, 9884. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Chaudhari, A.Z.; Teli, D.; Balar, P.; Vora, L. Propolis and Their Active Constituents for Chronic Diseases. Biomedicines 2023, 11, 259. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Liebman, S.E.; Le, T.H. Eat your broccoli: Oxidative stress, nrf2, and sulforaphane in chronic kidney disease. Nutrients 2021, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- García, E.L.; Lesmes, I.B.; Perales, A.D.; Moreno, V.; Baquedano, M.P.P.; Velasco, A.M.R.; Salvo, U.F.; Romero, L.T.; Porcel, F.B.O.; Laín, S.A.; et al. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) sobre recomendaciones dietéticas sostenibles y recomendaciones de actividad física para la población española. Rev. Com. Científico AESAN 2022, 36, 11–70. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_comite/INFORME_RECOMENDACIONES_DIETETICAS.pdf (accessed on 27 September 2024).
- Lin, C.Y.; Yao, C.A. Potential role of NRF2 activators with dual antiviral and anti-inflammatory properties in the management of viral pneumonia. Infect. Drug. Resist. 2020, 13, 1735–1741. [Google Scholar] [CrossRef]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Chermut, T.R.; Sequeira, J.; de Souza Gouveia Moreira, L.; Teixeira, K.T.R.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. From the distinctive smell to therapeutic effects: Garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin. Nutr. 2021, 40, 4807–4819. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Chandra, M.; Tuteja, R.; Misra, M.K. Nitric Oxide as a Unique Bioactive Signaling Messenger in Physiology and Pathophysiology. J. Biomed. Biotechnol. 2004, 2004, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Reglamento (UE) 2023/915 de la Comisión. Relativo a los Límites Máximos de Determinados Contaminantes en los Alimentos. 2023. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2023-80614 (accessed on 25 September 2024).
- Kemmner, S.; Lorenz, G.; Wobst, J.; Kessler, T.; Wen, M.; Günthner, R.; Stock, K.; Heemann, U.; Burkhardt, K.; Baumann, M.; et al. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: A pilot study. Nitric Oxide 2017, 64, 7–15. [Google Scholar] [CrossRef]
- Moreira, L.D.S.G.; Fanton, S.; Cardozo, L.; Borges, N.A.; Combet, E.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. Pink pressure: Beetroot (Beta vulgaris rubra) as a possible novel medical therapy for chronic kidney disease. Nutr. Rev. 2022, 80, 1041–1061. [Google Scholar] [CrossRef]
- Reglamento (UE) 2022/2340 de la Comisión Respecta a los Extractos de Té Verde que Contienen (-) 3-Galato de Epigalocatequina. 2022. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2022-81764 (accessed on 25 September 2024).
- Zhang, Y.; Xiong, Y.; Shen, S.; Yang, J.; Wang, W.; Wu, T.; Chen, L.; Yu, Q.; Zuo, H.; Wang, X.; et al. Causal Association Between Tea Consumption and Kidney Function: A Mendelian Randomization Study. Front. Nutr. 2022, 9, 801591. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Dursun, B.; Suleymanlar, G.; Ozben, T. Effect of haemodialysis on the oxidative stress and antioxidants in diabetes mellitus. Acta Diabetol. 2005, 42, 123–128. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Molecular Mechanisms of Epigallocatechin-3-Gallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Curr. Dev. Nutr. 2019, 3, nzz101. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Deng, Y.; Chen, Y.; Chuang, P.Y.; He, J.C. Therapeutic use of traditional Chinese herbal medications for chronic kidney diseases. Kidney Int. 2013, 84, 1108–1118. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, P.; Zhang, Z.; Youn, J.Y.; Wang, C.; Zhang, H. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol. Ther. 2021, 225, 107843. [Google Scholar] [CrossRef] [PubMed]
- Lui, S.L.; Zhu, D.; Cheng, S.W.; Ng, F.; Hui, P.C.; Yip, T. Effects of astragalus membranaceus-based chinese medicine formulae on residual renal function in patients on peritoneal dialysis. Perit. Dial. Int. 2015, 35, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Q.; Yu, F.; Chen, H.J.; Deng, Y.R.; Lin, J.; Xu, Y.; Zheng, X.; Zhang, J.W.; Liu, J.F. Efficacy of astragalus combined with renin-angiotensin-aldosterone system blockers in the treatment of stage III diabetic nephropathy: A systematic review and meta-analysis. Ren. Fail. 2024, 46, 2359033. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Y.; Chu, S.W.F.; Chow, L.Y.; Yeam, C.T.; Low, L.L.; Quah, J.H.M. Role of Alternative Medical Systems in Adult Chronic Kidney Disease Patients: A Systematic Review of Literature. Cureus 2022, 14, e32874. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).