From Tea to Functional Foods: Exploring Caryopteris mongolica Bunge for Anti-Rheumatoid Arthritis and Unraveling Its Potential Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
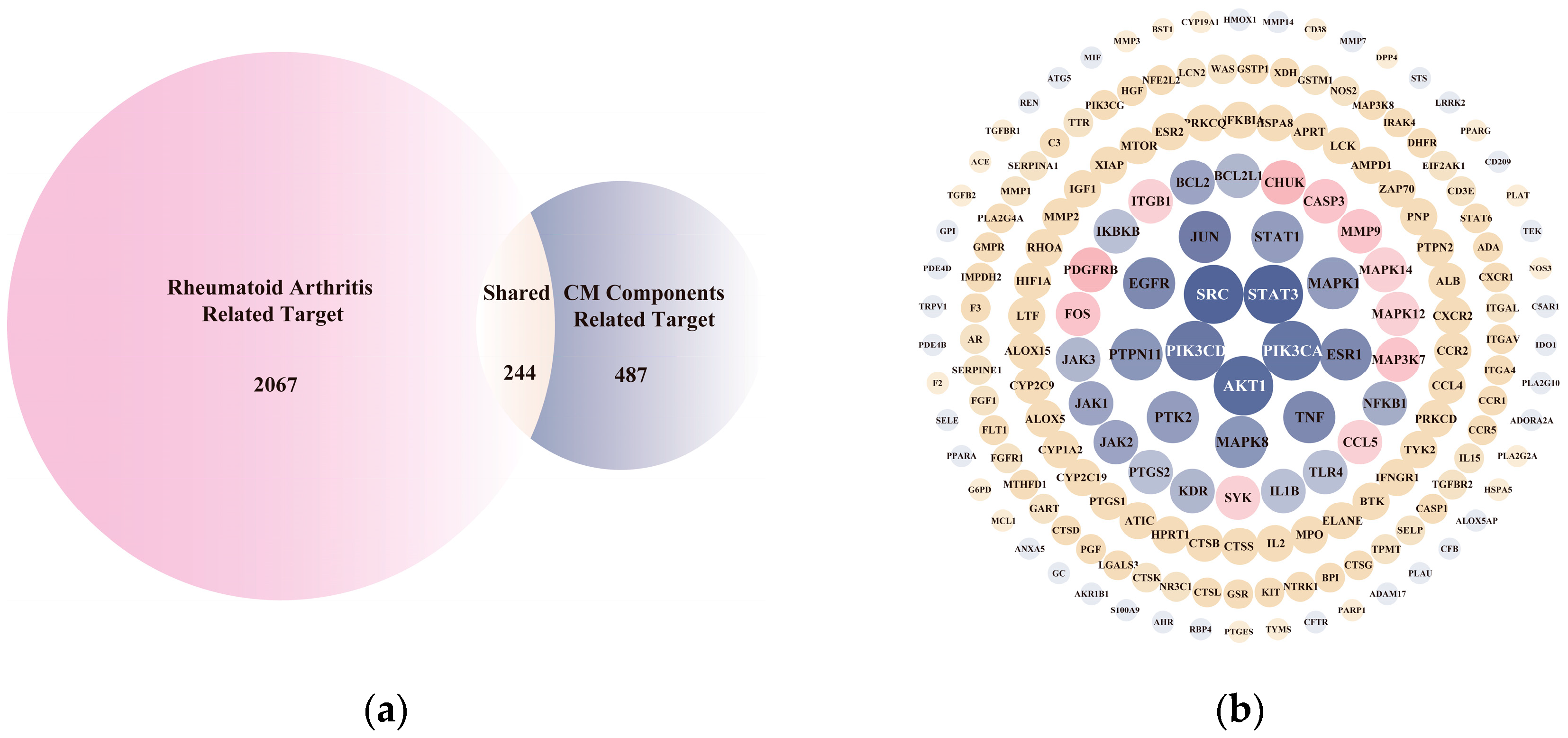
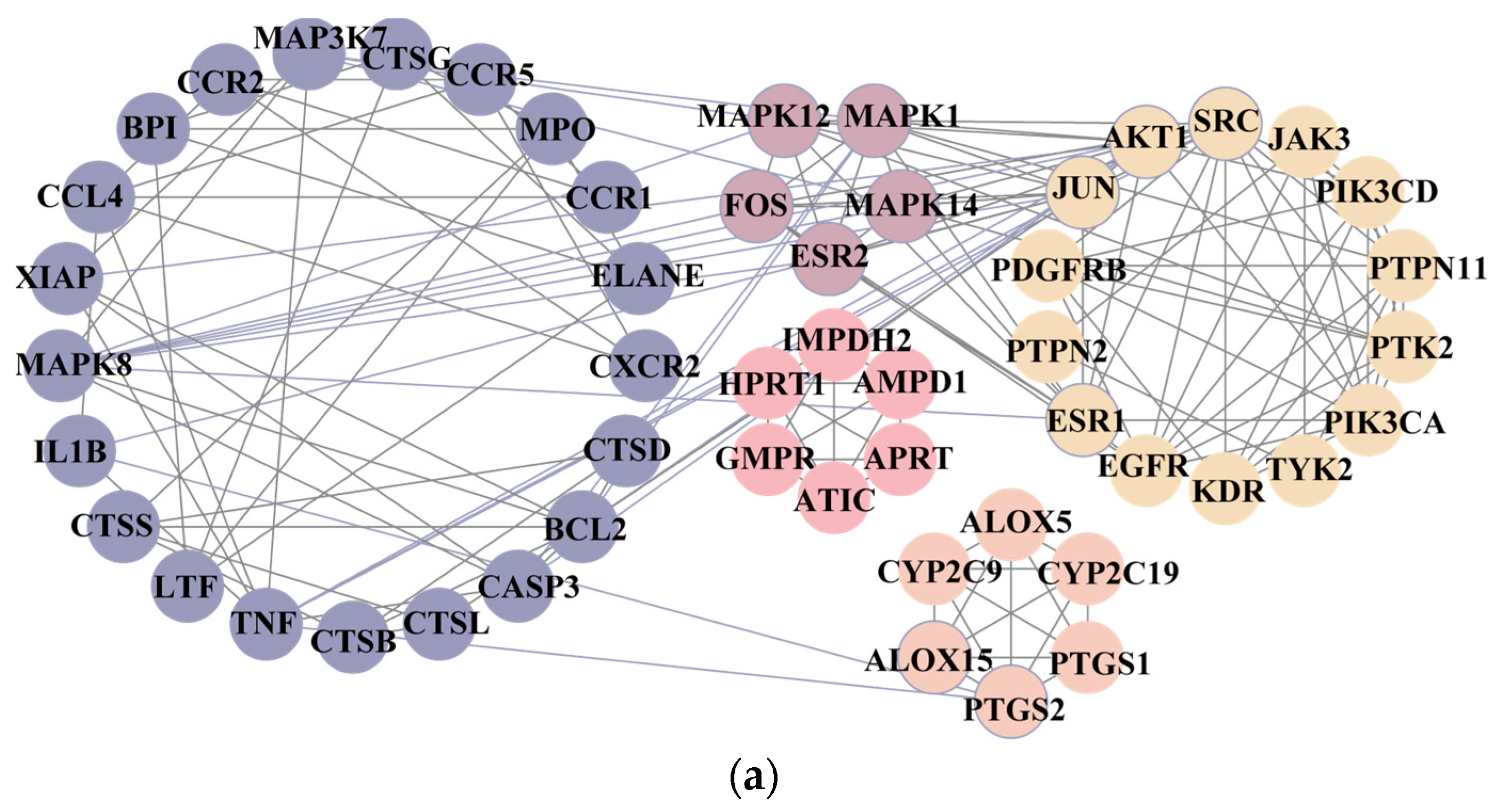
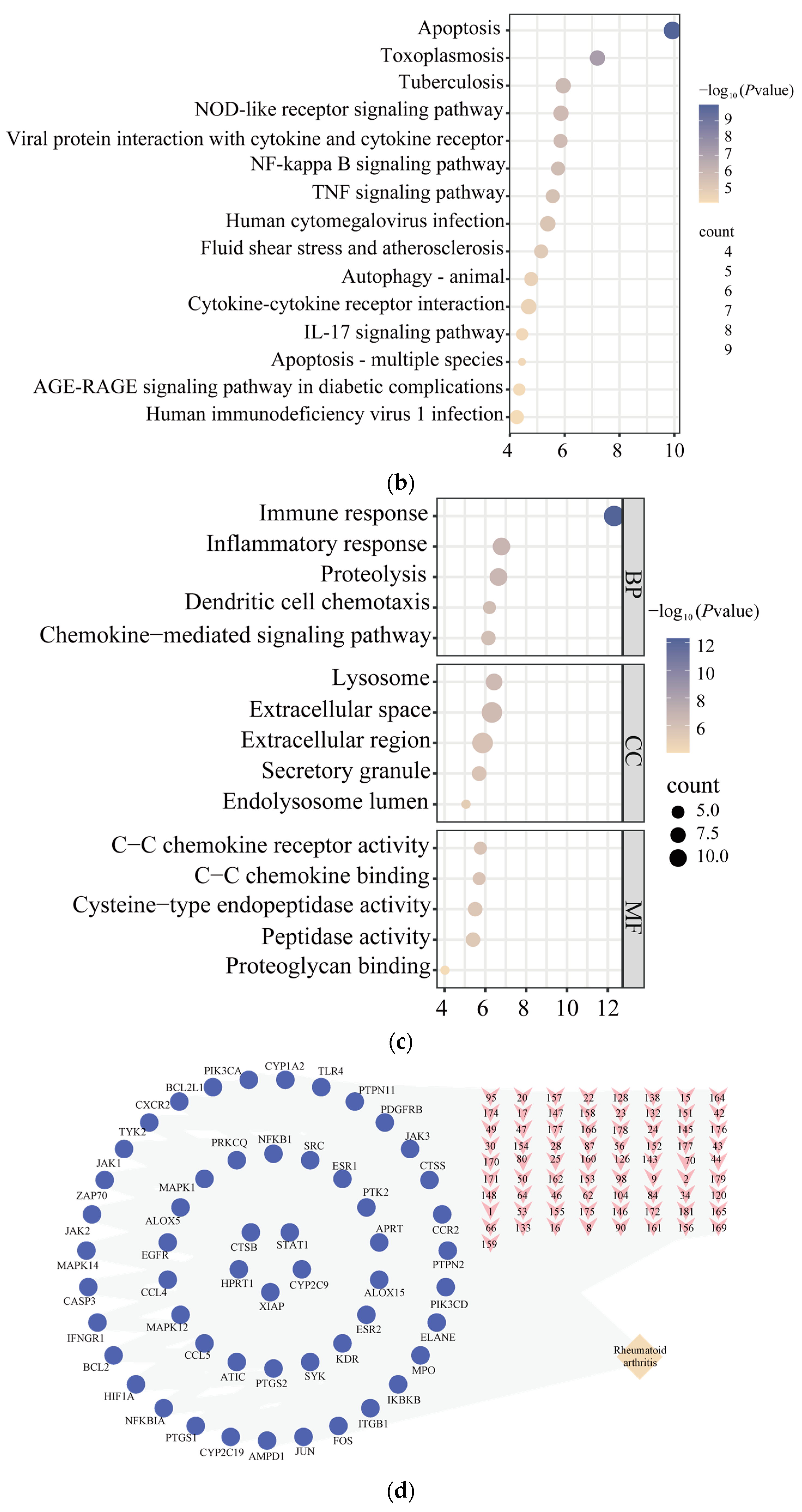
2.2. Network Pharmacology Analysis
2.3. Molecular Docking
2.4. CM Extraction Preparation and Determination
2.5. Animals
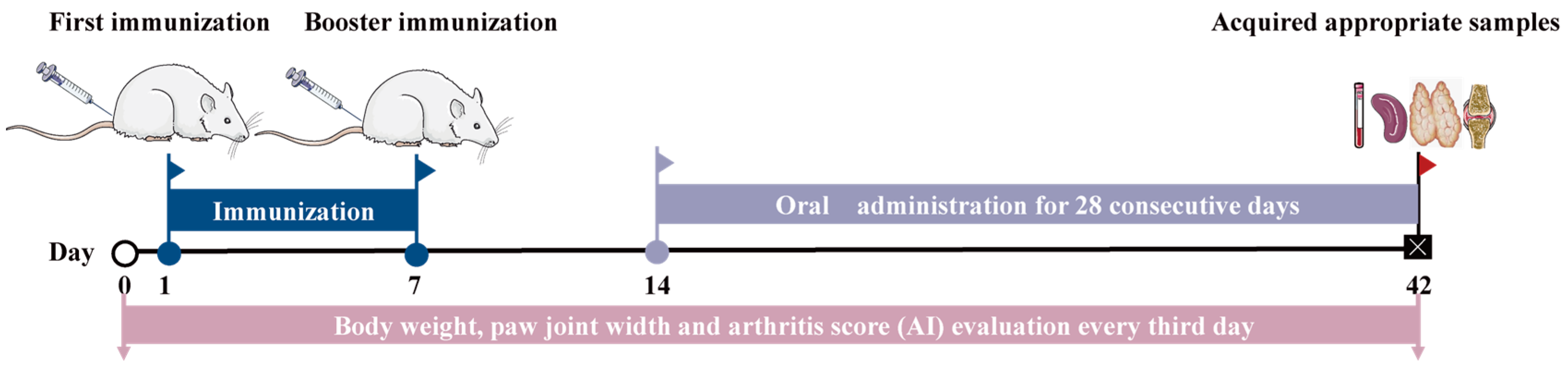
2.6. Induction, Treatment, and Evaluation of Collagen-Induced Arthritis (CIA) in Rats
2.7. Measurement of Inflammatory Cytokine Production and Organ Index
2.8. Histological and Immunofluorescence Analyses
2.9. Metabonomic Analysis
2.10. Transcriptional Sequencing and Bioinformatics Analysis
2.11. RNA Isolation and Real-Time Polymerase Chain Reaction (PCR)
2.12. Statistical Analysis
3. Results
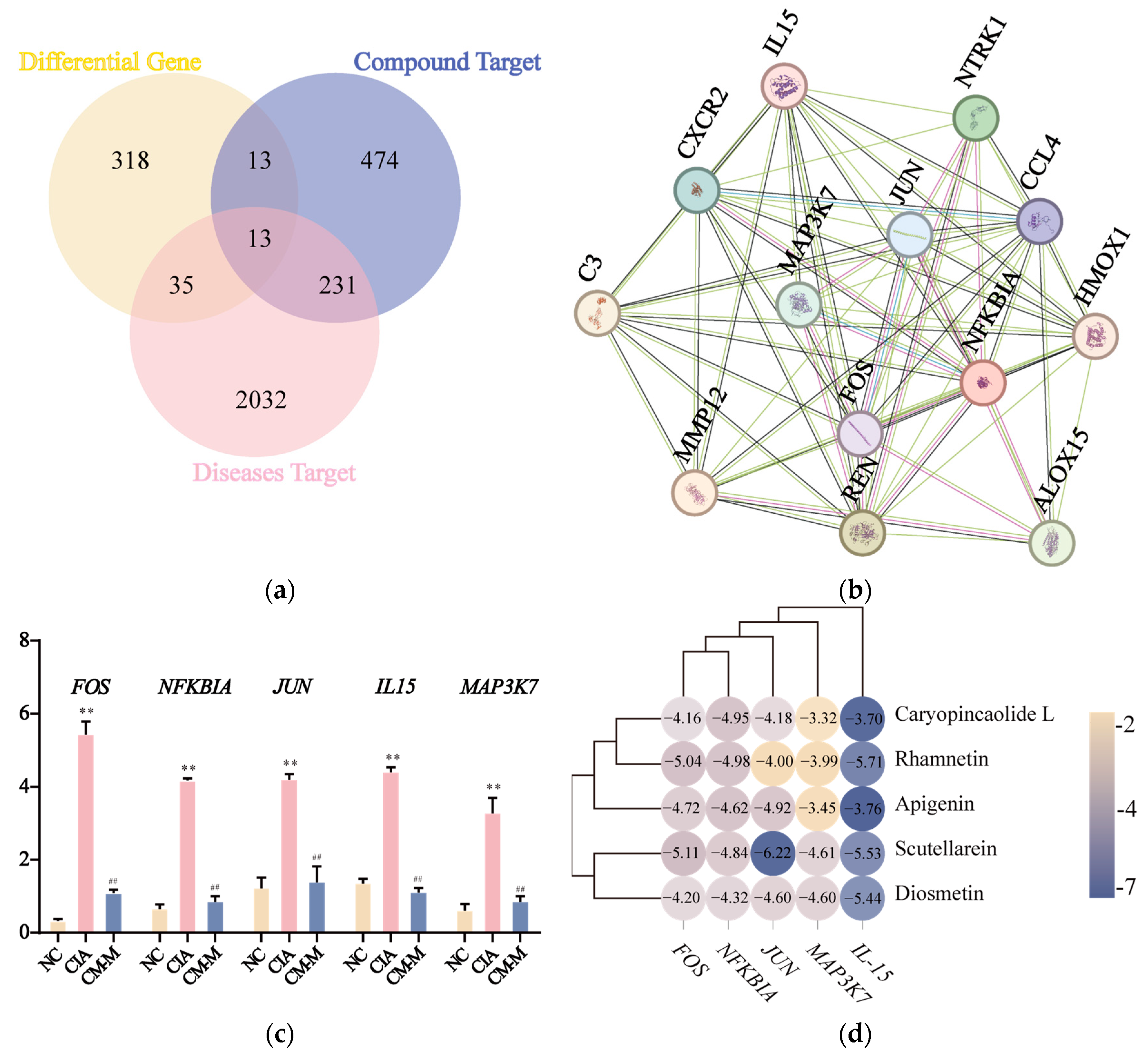
3.1. Inflammatory-Response-Related Biological Processes Involved in CM on Anti-RA Process
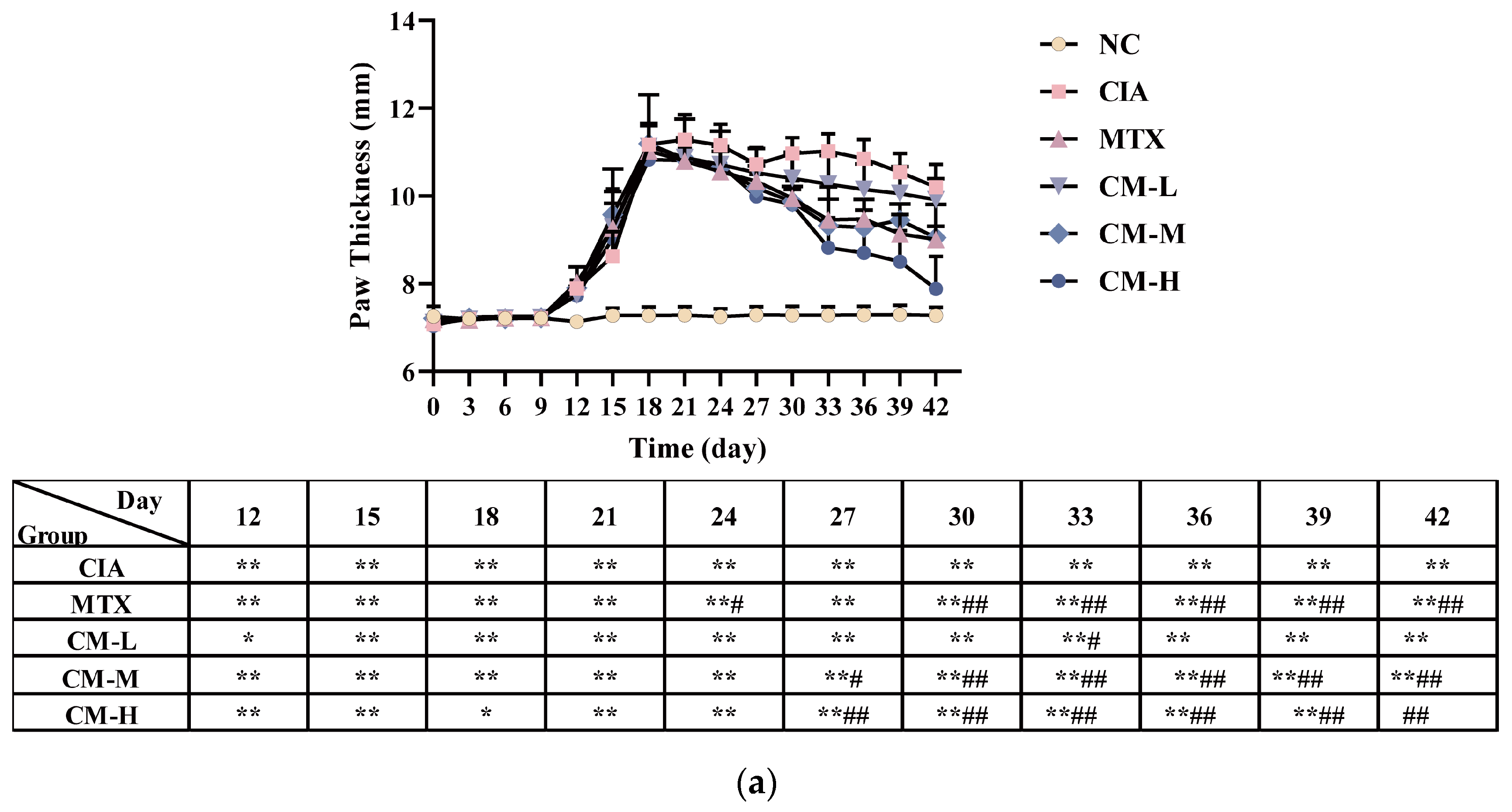
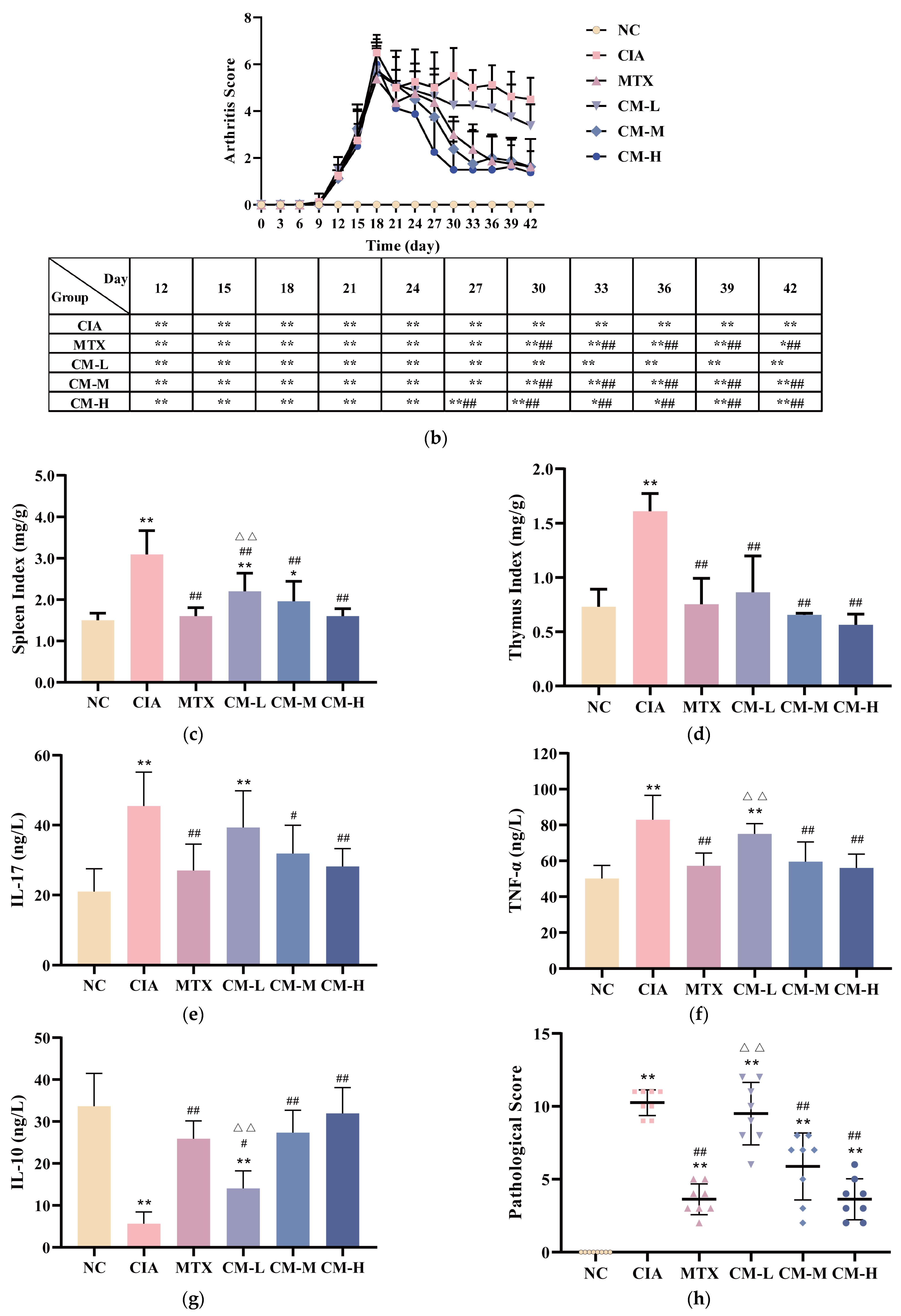
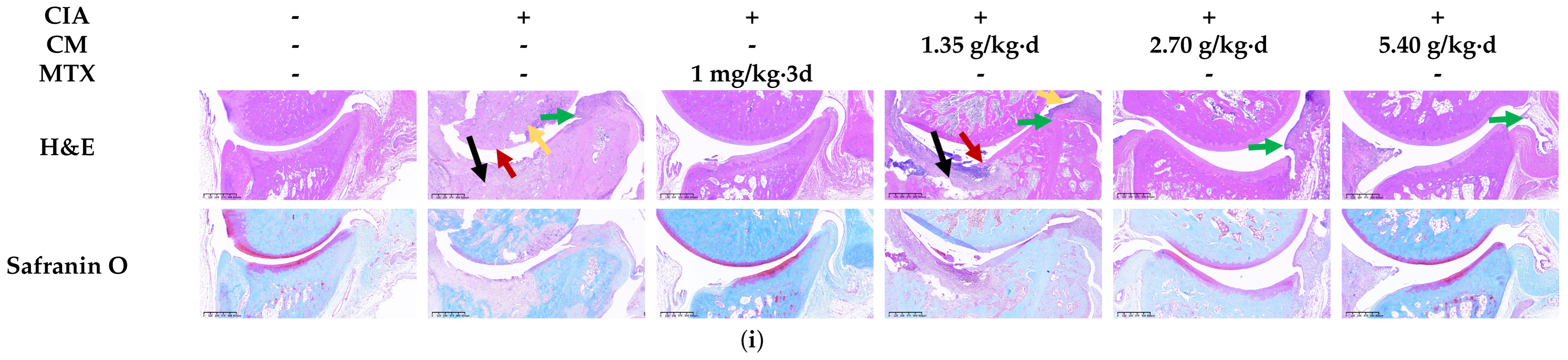
3.2. CM Alleviated Symptomatic Progression in CIA Rats
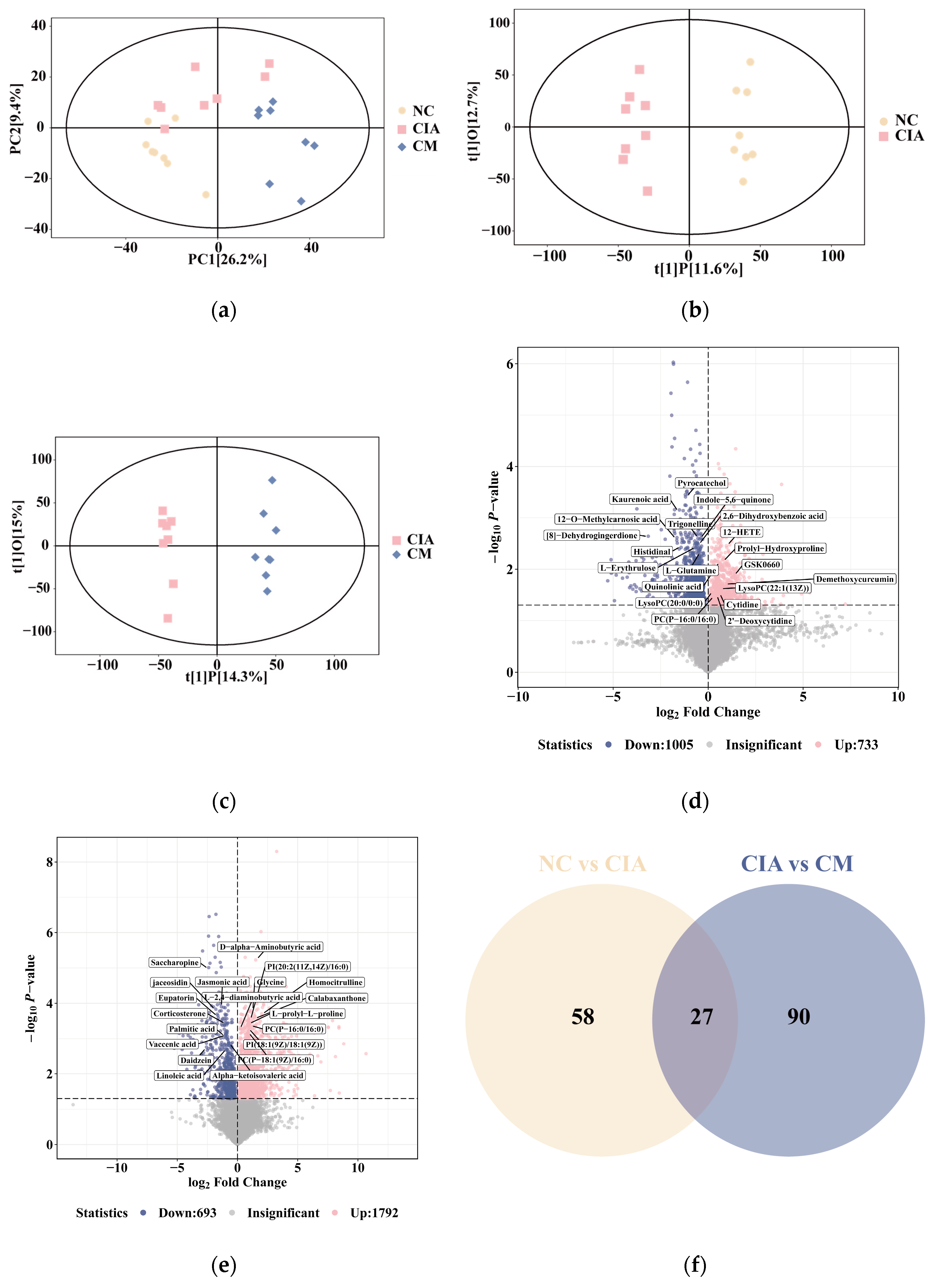
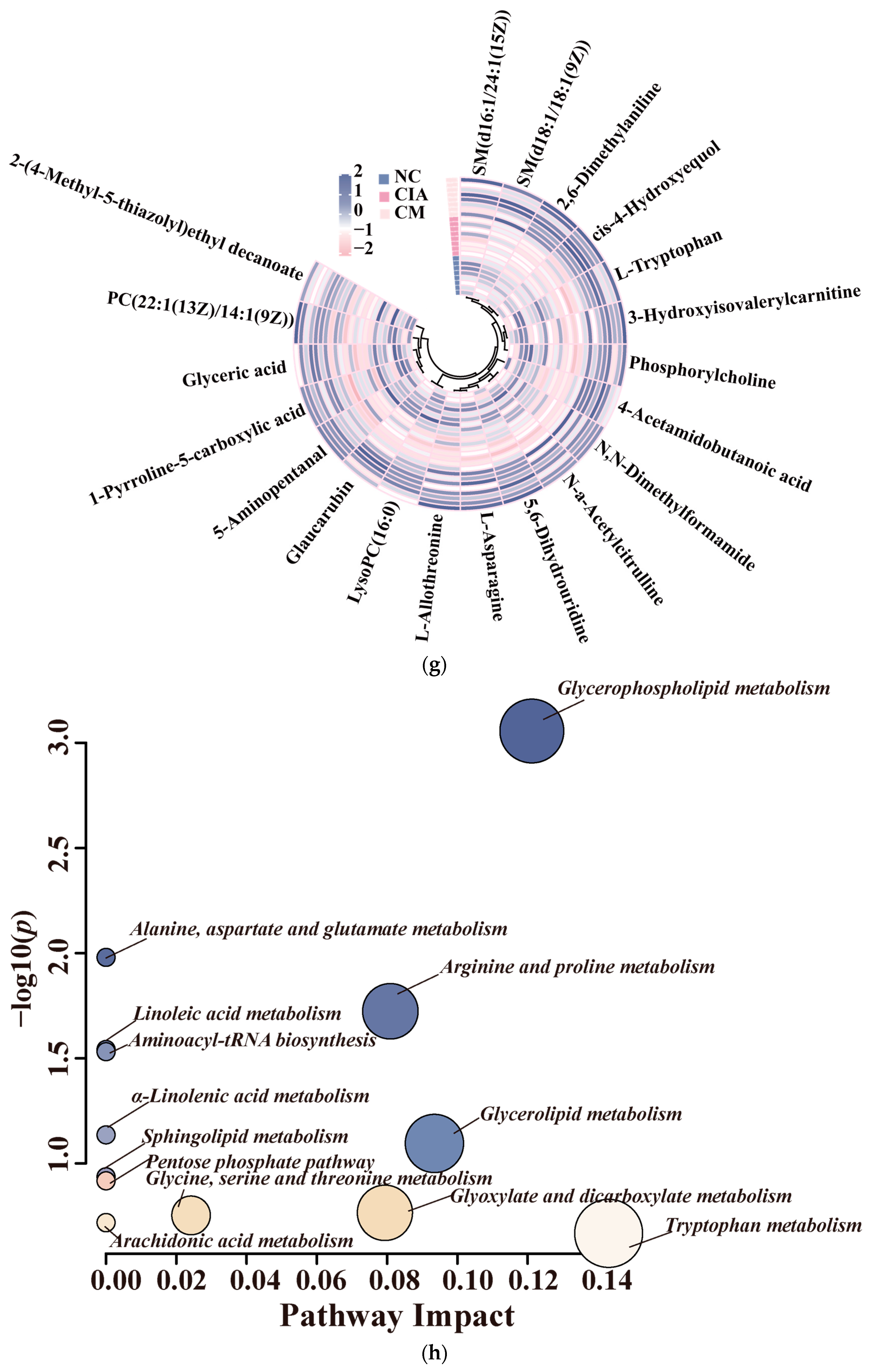
3.3. CM Rebalanced Metabolism by Promoting Glycerophospholipid and Amino Acid Turnover in CIA Rats
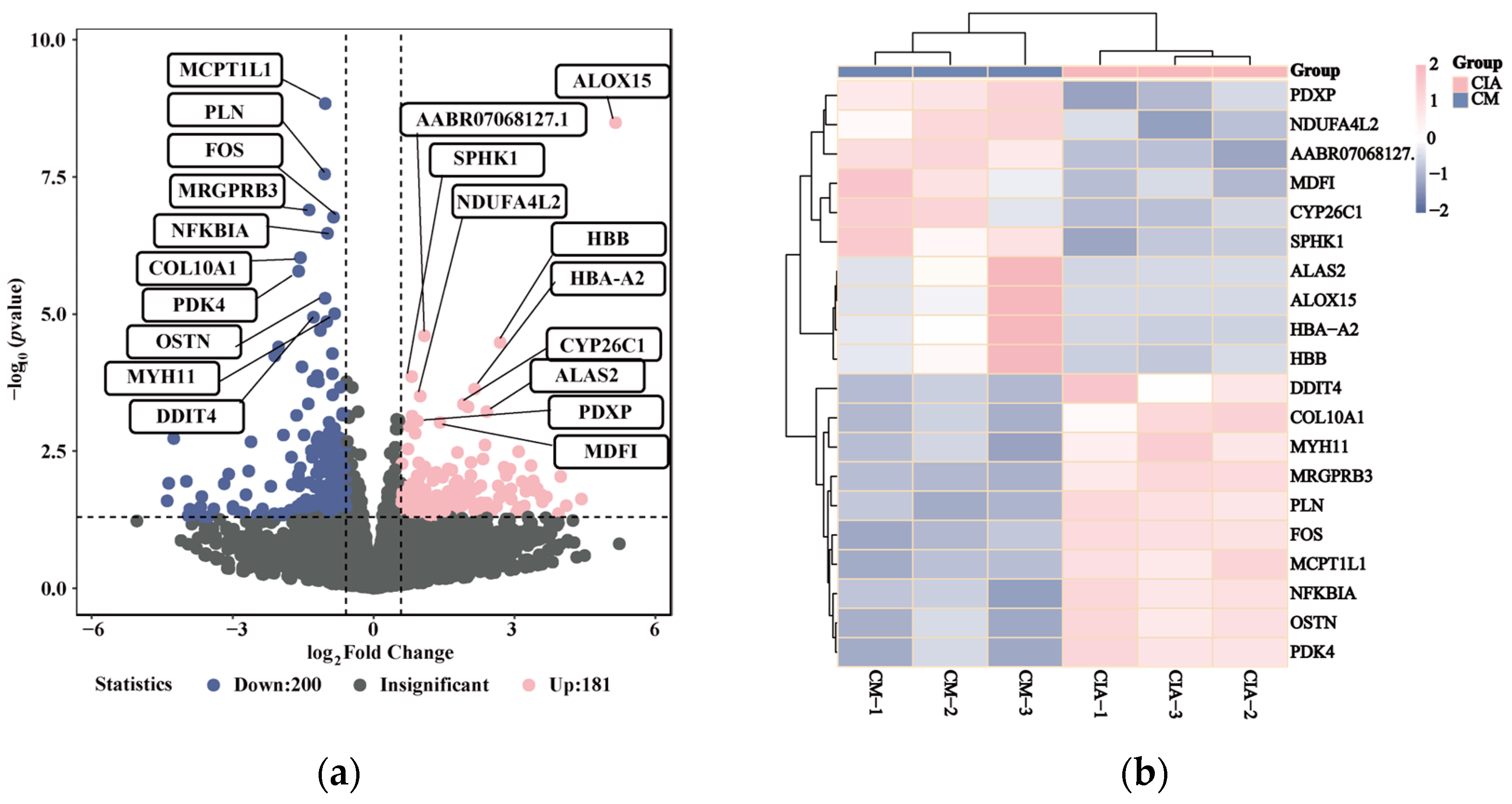
3.4. Inflammatory-Related Transcriptional Profiles Respond to CM Treatment in CIA Rats
3.5. CM Exhibited a Down-Regulatory Effect on Inflammatory-Related Gene Expression with Its Active Components
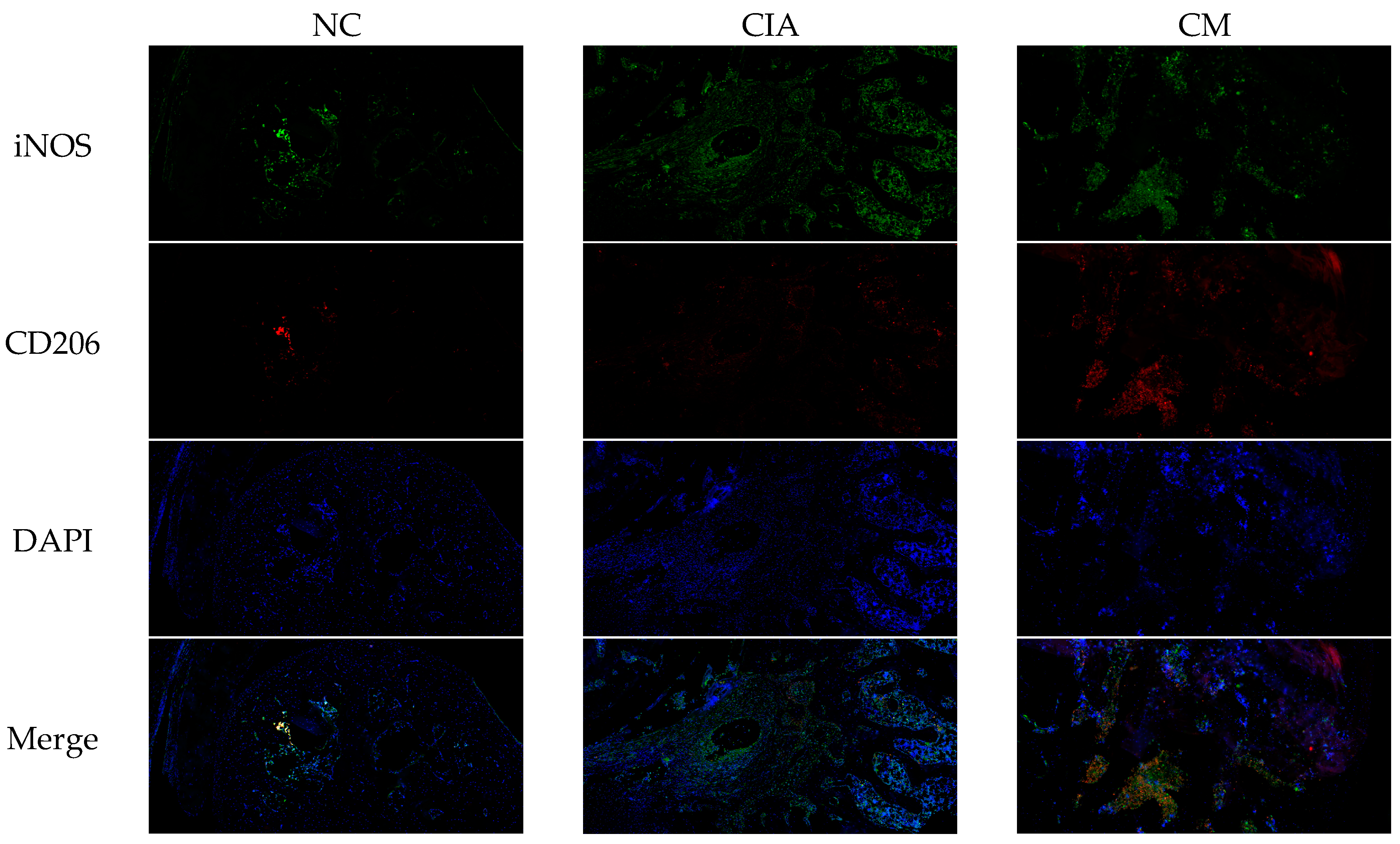
3.6. CM Induced M2 Macrophage Polarization In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef]
- Alivernini, S.; Firestein, G.S.; McInnes, I.B. The pathogenesis of rheumatoid arthritis. Immunity 2022, 55, 2255–2270. [Google Scholar] [CrossRef]
- Wallace, B.I.; Cooney, L.; Fox, D.A. New molecular targets in the treatment of rheumatoid arthritis. Curr. Opin. Rheumatol. 2024, 36, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Venetsanopoulou, A.I.; Voulgari, P.V.; Drosos, A.A. Advances in non-biological drugs for the treatment of rheumatoid arthritis. Expert Opin. Pharmacother. 2024, 25, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C. Pain in the joints and beyond; the challenge of rheumatoid arthritis. Lancet Rheumatol. 2023, 5, e351–e360. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, M.; Cao, J.; Fang, T.; Zhang, J.; Zhen, Y.; Wu, F.; Yu, X.; Liu, Y.; Li, J.; et al. Applications and recent advances in transdermal drug delivery systems for the treatment of rheumatoid arthritis. Acta Pharm. Sin. B 2023, 13, 4417–4441. [Google Scholar] [CrossRef]
- Ligaa, U. Medicinal Plants of Mongolia Used in Mongolian Traditional Medicine; KCA Press: Seoul, Republic of Korea, 1996. [Google Scholar]
- Inner Mongolia Food and Drug Administration. Inner Mongolia Mongolian Medicinal Materials Standard 2015 (Supplement); Inner Mongolia People’s Publishing House: Hohhot, China, 2018. [Google Scholar]
- Mishig, D.; Gruner, M.; Lubken, T.; Ganbaatar, C.; Regdel, D.; Knolker, H.J. Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge. Sci. Rep. 2021, 11, 13740. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, X.; Xu, H.; Wei, X.; Chou, G. Abietane diterpenoids and iridoids from Caryopteris mongolica. Chin. J. Nat. Med. 2023, 21, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Saruul, E.; Murata, T.; Selenge, E.; Sasaki, K.; Yoshizaki, F.; Batkhuu, J. An antibacterial ortho-quinone diterpenoid and its derivatives from Caryopteris mongolica. Bioorg. Med. Chem. Lett. 2015, 25, 2555–2558. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Ishikawa, Y.; Saruul, E.; Selenge, E.; Sasaki, K.; Umehara, K.; Yoshizaki, F.; Batkhuu, J. Abietane-type diterpenoids from the roots of Caryopteris mongolica and their cholinesterase inhibitory activities. Phytochemistry 2016, 130, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Selenge, E.; Oikawa, S.; Ageishi, K.; Batkhuu, J.; Sasaki, K.; Yoshizaki, F. Cholinesterase-inhibitory diterpenoids and chemical constituents from aerial parts of Caryopteris mongolica. J. Nat. Med. 2015, 69, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Tsevelmaa, N.; Narangerel, B.; Odgerel, O.; Dariimaa, D.; Batkhuu, J. Anti-Brucella activity of Caryopteris mongolica Bunge root extract against Brucella melitensis infection in mice. BMC Complement. Altern. Med. 2018, 18, 144. [Google Scholar] [CrossRef]
- Ahsan, H.; Haider, I.; Mushtaq, M.N.; Qaisar, M.N.; Naqvi, F.; Asif, A. Pharmacological support to anti-arthritic prospective of physostigmine: A new approach. Inflammopharmacology 2021, 29, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Gowayed, M.A.; Rothe, K.; Rossol, M.; Attia, A.S.; Wagner, U.; Baerwald, C.; El-Abhar, H.S.; Refaat, R. The role of α7nAChR in controlling the anti-inflammatory/anti-arthritic action of galantamine. Biochem. Pharmacol. 2019, 170, 113665. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Steigerova, M.; Sima, M.; Slanar, O. Pathogenesis of Collagen-Induced Arthritis: Role of Immune Cells with Associated Cytokines and Antibodies, Comparison with Rheumatoid Arthritis. Folia Biol. 2023, 69, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Aqsa; Ali, S.; Summer, M.; Yousaf, S.; Nazakat, L.; Noor, S. Pharmacological and immunomodulatory modes of action of medically important phytochemicals against arthritis: A molecular insight. Mol. Biol. Rep. 2024, 51, 448. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Barone, A.; Seriolo, B.; Accardo, S. Macrophages, synovial tissue and rheumatoid arthritis. Clin. Exp. Rheumatol. 1993, 11, 331–339. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef] [PubMed]
- Gallo, K.; Goede, A.; Preissner, R.; Gohlke, B.O. SuperPred 3.0: Drug classification and target prediction—A machine learning approach. Nucleic Acids Res. 2022, 50, W726–W731. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lian, X.; Li, F.; Wang, C.; Zhu, F.; Qiu, Y.; Chen, Y. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022, 50, D1398–D1407. [Google Scholar] [CrossRef] [PubMed]
- Harahap, I.A.; Schmidt, M.; Pruszynska-Oszmalek, E.; Sassek, M.; Suliburska, J. Impact of Lactobacillus acidophilus and Its Combination with Isoflavone Products on Calcium Status, Calcium Transporters, and Bone Metabolism Biomarkers in a Post-Menopausal Osteoporotic Rat Model. Nutrients 2024, 16, 2524. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, R.S.; Bello, S.; Biering-Sorensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, S.; Wang, Y.; Pu, W.; Liu, Q.; Zhang, Y.; Liu, Y.; Hao, H. Fangji Huangqi Decoction alleviates rheumatoid arthritis through regulating HIF-1α mediated the angiogenesis and the balance between autophagy and apoptosis. J. Ethnopharmacol. 2024, 329, 118061. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Deng, L.; Yang, H.; Gong, Z.; Wang, Q.; Pan, D.; Zeng, S.; Chen, J. 6-Shogaol inhibits the proliferation, apoptosis, and migration of rheumatoid arthritis fibroblast-like synoviocytes via the PI3K/AKT/NF-κB pathway. Phytomedicine 2023, 109, 154562. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, X.; Ma, F.; Li, C.; Bu, R.; Lu, J.; Gao, J.; Xue, P. Metabolomics profiling reveals Echinops latifolius Tausch improves the trabecular micro-architecture of ovariectomized rats mainly via intervening amino acids and glycerophospholipids metabolism. J. Ethnopharmacol. 2020, 260, 113018. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar] [CrossRef]
- Worley, B.; Halouska, S.; Powers, R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal. Biochem. 2013, 433, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.B.; Ma, R.; Wang, M.N.; He, J.; Wang, P.P.; Tao, Q.W.; Xu, Y. Jolkinolide B ameliorates rheumatoid arthritis by regulating the JAK2/STAT3 signaling pathway. Phytomedicine 2024, 124, 155311. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chen, Z.; Li, T.; Nie, Z.; Han, H.; Zhong, S.; Yin, Z.; Sun, S.; Xie, J.; Shen, J.; et al. Role and Therapeutic Potential for Targeting Fibroblast Growth Factor 10/FGFR1 in Relapsed Rheumatoid Arthritis. Arthritis Rheumatol. 2024, 76, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Tewari, D.; Kumar, S.; Chandra, S. Network pharmacology-based approach to investigate the molecular targets and molecular mechanisms of Rosmarinus officinalis L. for treating aging-related disorders. Biogerontology 2024, 25, 793–808. [Google Scholar] [CrossRef]
- Chen, K.M.; Lan, K.P.; Lai, S.C. Neuroprotective effects of CysLT2R antagonist on Angiostrongylus cantonensis-induced edema and meningoencephalitis. Mol. Biochem. Parasitol. 2024, 260, 111649. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yoshida, K.; Nishizawa, T.; Otani, K.; Yamashita, Y.; Okabe, H.; Hadano, Y.; Kayama, T.; Kurosaka, D.; Saito, M. Inflammation and Bone Metabolism in Rheumatoid Arthritis: Molecular Mechanisms of Joint Destruction and Pharmacological Treatments. Int. J. Mol. Sci. 2022, 23, 2871. [Google Scholar] [CrossRef]
- Koper-Lenkiewicz, O.M.; Sutkowska, K.; Wawrusiewicz-Kurylonek, N.; Kowalewska, E.; Matowicka-Karna, J. Proinflammatory Cytokines (IL-1, -6, -8, -15, -17, -18, -23, TNF-α) Single Nucleotide Polymorphisms in Rheumatoid Arthritis-A Literature Review. Int. J. Mol. Sci. 2022, 23, 2106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Wu, J.; Chen, S.; Hu, T.; Liu, Y.; Li, X.; Li, X.; Wu, Y.; Yu, J.; et al. Potential significance of changes in serum levels of IL-17, TNF-α and DKK-1 in the progression of the rheumatoid arthritis. Autoimmunity 2023, 56, 2276068. [Google Scholar] [CrossRef] [PubMed]
- Selimov, P.; Karalilova, R.; Damjanovska, L.; Delcheva, G.; Stankova, T.; Stefanova, K.; Maneva, A.; Selimov, T.; Batalov, A. Rheumatoid arthritis and the proinflammatory cytokine IL-17. Folia Med. 2023, 65, 53–59. [Google Scholar] [CrossRef]
- Mielle, J.; Audo, R.; Hahne, M.; Macia, L.; Combe, B.; Morel, J.; Daien, C. IL-10 Producing B Cells Ability to Induce Regulatory T Cells Is Maintained in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, S.; Chen, J.; Jian, C.; Hu, J.; Du, H.; Hai, H.; Wu, J.; Zeng, F.; Zhu, J.; et al. Glycerophospholipid metabolism is involved in rheumatoid arthritis pathogenesis by regulating the IL-6/JAK signaling pathway. Biochem. Biophys. Res. Commun. 2022, 600, 130–135. [Google Scholar] [CrossRef]
- Engel, K.M.; Schiller, J.; Galuska, C.E.; Fuchs, B. Phospholipases and Reactive Oxygen Species Derived Lipid Biomarkers in Healthy and Diseased Humans and Animals—A Focus on Lysophosphatidylcholine. Front. Physiol. 2021, 12, 732319. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, B.; Plewa, S.; Klupczynska, A.; Sikorska, D.; Samborski, W.; Kokot, Z.J. Serum free amino acid levels in rheumatoid arthritis according to therapy and physical disability. Cytokine 2019, 113, 332–339. [Google Scholar] [CrossRef]
- Wang, S.; Tian, S.; Li, M.; Li, Z. Methionine attenuates the intensity of rheumatoid arthritis by downregulating NF-κB and iNOS expression in neonatal rats. 3 Biotech 2018, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Deutz, N.E. Biomarkers of arginine and lysine excess. J. Nutr. 2007, 137 (Suppl. 2), 1662S–1668S. [Google Scholar] [CrossRef]
- Jian, C.; Wei, L.; Wu, T.; Li, S.; Wang, T.; Chen, J.; Chang, S.; Zhang, J.; He, B.; Wu, J.; et al. Comprehensive multi-omics analysis reveals the core role of glycerophospholipid metabolism in rheumatoid arthritis development. Arthritis Res. Ther. 2023, 25, 246. [Google Scholar] [CrossRef]
- Srivastava, M.; Baig, M.S. NOS1 mediates AP1 nuclear translocation and inflammatory response. Biomed. Pharmacother. 2018, 102, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Lin, C.C.; Hsiao, L.D. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef]
- van Woerden, G.M.; Senden, R.; de Konink, C.; Trezza, R.A.; Baban, A.; Bassetti, J.A.; van Bever, Y.; Bird, L.M.; van Bon, B.W.; Brooks, A.S.; et al. The MAP3K7 gene: Further delineation of clinical characteristics and genotype/phenotype correlations. Hum. Mutat. 2022, 43, 1377–1395. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Leung, B.P.; Sturrock, R.D.; Field, M.; Liew, F.Y. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat. Med. 1997, 3, 189–195. [Google Scholar] [CrossRef]
- Lee, C.; Park, D.W.; Lee, J.; Lee, T.I.; Kim, Y.J.; Lee, Y.S.; Baek, S.H. Secretory phospholipase A2 induces apoptosis through TNF-α and cytochrome c-mediated caspase cascade in murine macrophage RAW 264.7 cells. Eur. J. Pharmacol. 2006, 536, 47–53. [Google Scholar] [CrossRef]
- Cutolo, M.; Campitiello, R.; Gotelli, E.; Soldano, S. The Role of M1/M2 Macrophage Polarization in Rheumatoid Arthritis Synovitis. Front. Immunol. 2022, 13, 867260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, W. Isorhamnetin attenuates collagen-induced arthritis via modulating cytokines and oxidative stress in mice. Int. J. Clin. Exp. Med. 2015, 8, 16536–16542. [Google Scholar]
- Liu, X.R.; Li, S.F.; Mei, W.Y.; Liu, X.D.; Zhou, R.B. Isorhamnetin Downregulates MMP2 and MMP9 to Inhibit Development of Rheumatoid Arthritis through SRC/ERK/CREB Pathway. Chin. J. Integr. Med. 2024, 30, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, B.; Bai, J.Y.; Xia, S.; Mao, M.; Li, X.; Li, N.; Chen, L. The roles of synovial hyperplasia, angiogenesis and osteoclastogenesis in the protective effect of apigenin on collagen-induced arthritis. Int. Immunopharmacol. 2019, 73, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Y.; Zhou, Q.; Jie, H.; He, Y.; Han, J.; He, J.; Jiang, Y.; Sun, E. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J. Cell. Mol. Med. 2016, 20, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Liu, M.; Zhou, B.; Yang, G. Diosmetin exhibits anti-proliferative and anti-inflammatory effects on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes through regulating the Akt and NF-κB signaling pathways. Phytother. Res. 2020, 34, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Du, W.; Che, W.; Wang, L.; Zhao, L. Apigenin Inhibits the Progression of Osteoarthritis by Mediating Macrophage Polarization. Molecules 2023, 28, 2915. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, H.; Zhang, H.; Jia, W.; Lu, B.; Zhang, J.; Ji, L. Scutellarin suppresses the metastasis of triple-negative breast cancer via targeting TNFα/TNFR2-RUNX1-triggered G-CSF expression in endothelial cells. Biochem. Pharmacol. 2023, 217, 115808. [Google Scholar] [CrossRef]




| NO | Compounds | Formula | Accurate Mass | Rt (min) | Error (ppm) | MS/MS | Structure | |
|---|---|---|---|---|---|---|---|---|
| Measured | Predicted | |||||||
| 1 | Caryopincaolide L | C20H24O4 | 329.1761 | 329.1747 | 74.72 | 4.25 | 311.1655, 293.1548, 283.1343, 243.1024, 227.1074, 151.1123, 133.1019, 119.0864 | 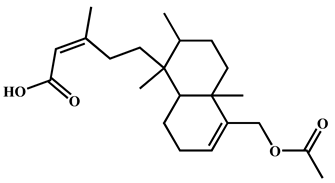 |
| 2 | Scutellarein | C15H10O6 | 285.0406 | 285.0404 | 42.97 | 0.7 | 267.0303, 257.0462, 241.0501, 213.0555, 148.9880, 117.0336 |  |
| 3 | Rhamnetin | C16H12O7 | 315.0512 | 315.051 | 50.08 | 0.63 | 300.028 | 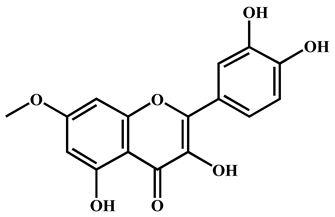 |
| 4 | Apigenin | C15H10O5 | 269.0457 | 269.0455 | 61.05 | 0.74 | 241.0506, 225.0549, 151.0027, 117.0325 |  |
| 5 | Diosmetin | C16H12O6 | 299.0562 | 299.0561 | 63.73 | 0.33 | 284.0328, 256.0378, 151.0000, 117.0318 | 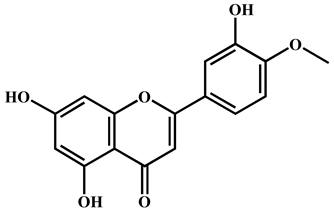 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, Z.; Fu, Y.; Tian, Y.; Xue, P.; Wang, Y.; Yang, F.; Li, G.; Wang, R. From Tea to Functional Foods: Exploring Caryopteris mongolica Bunge for Anti-Rheumatoid Arthritis and Unraveling Its Potential Mechanisms. Nutrients 2024, 16, 4311. https://doi.org/10.3390/nu16244311
Dong X, Wang Z, Fu Y, Tian Y, Xue P, Wang Y, Yang F, Li G, Wang R. From Tea to Functional Foods: Exploring Caryopteris mongolica Bunge for Anti-Rheumatoid Arthritis and Unraveling Its Potential Mechanisms. Nutrients. 2024; 16(24):4311. https://doi.org/10.3390/nu16244311
Chicago/Turabian StyleDong, Xin, Zhi Wang, Yao Fu, Yuxin Tian, Peifeng Xue, Yuewu Wang, Feiyun Yang, Guojing Li, and Ruigang Wang. 2024. "From Tea to Functional Foods: Exploring Caryopteris mongolica Bunge for Anti-Rheumatoid Arthritis and Unraveling Its Potential Mechanisms" Nutrients 16, no. 24: 4311. https://doi.org/10.3390/nu16244311
APA StyleDong, X., Wang, Z., Fu, Y., Tian, Y., Xue, P., Wang, Y., Yang, F., Li, G., & Wang, R. (2024). From Tea to Functional Foods: Exploring Caryopteris mongolica Bunge for Anti-Rheumatoid Arthritis and Unraveling Its Potential Mechanisms. Nutrients, 16(24), 4311. https://doi.org/10.3390/nu16244311





