Using Machine Learning to Fight Child Acute Malnutrition and Predict Weight Gain During Outpatient Treatment with a Simplified Combined Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Assessment
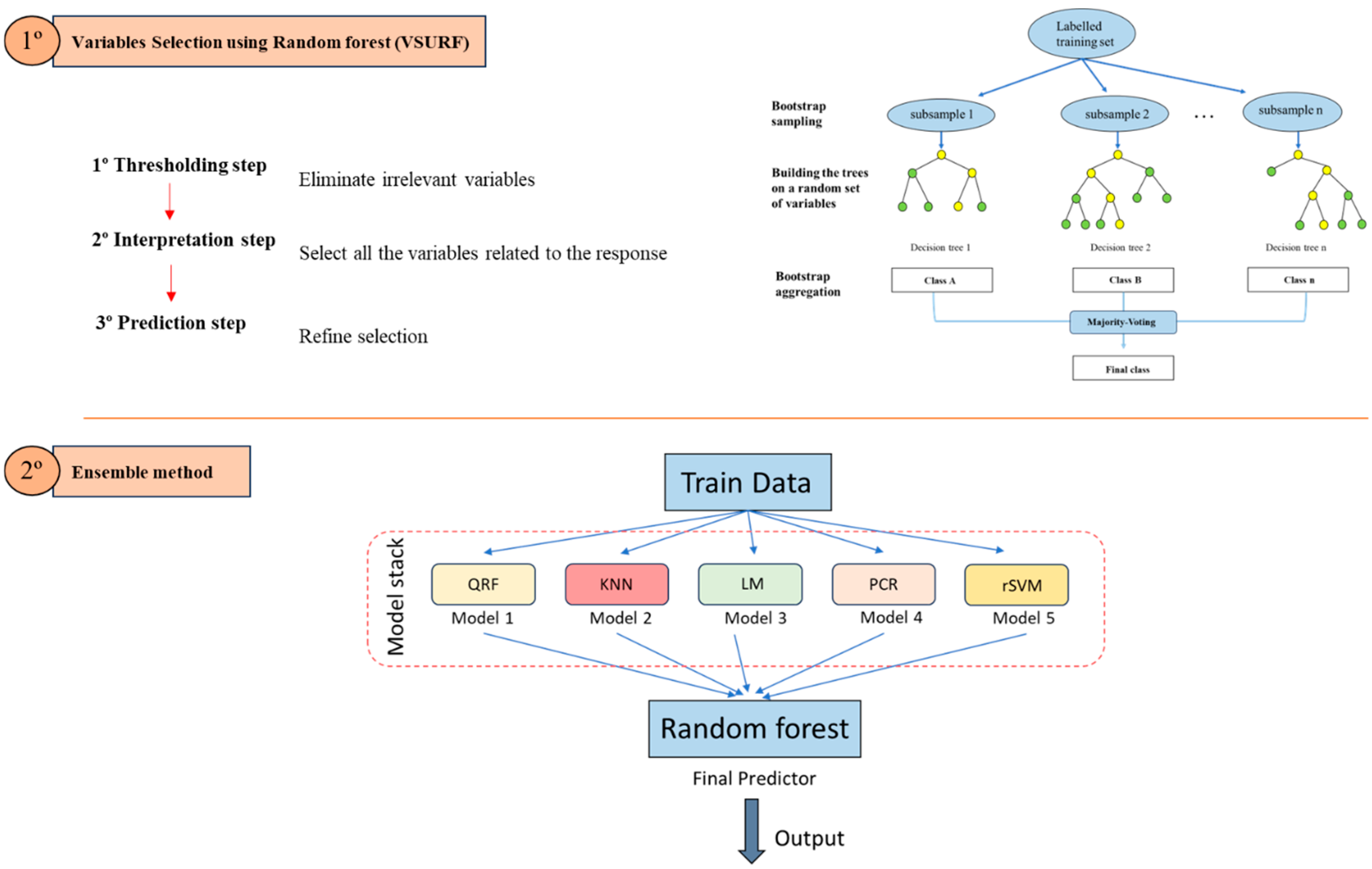
2.2. Data Cleaning and Variable Selection
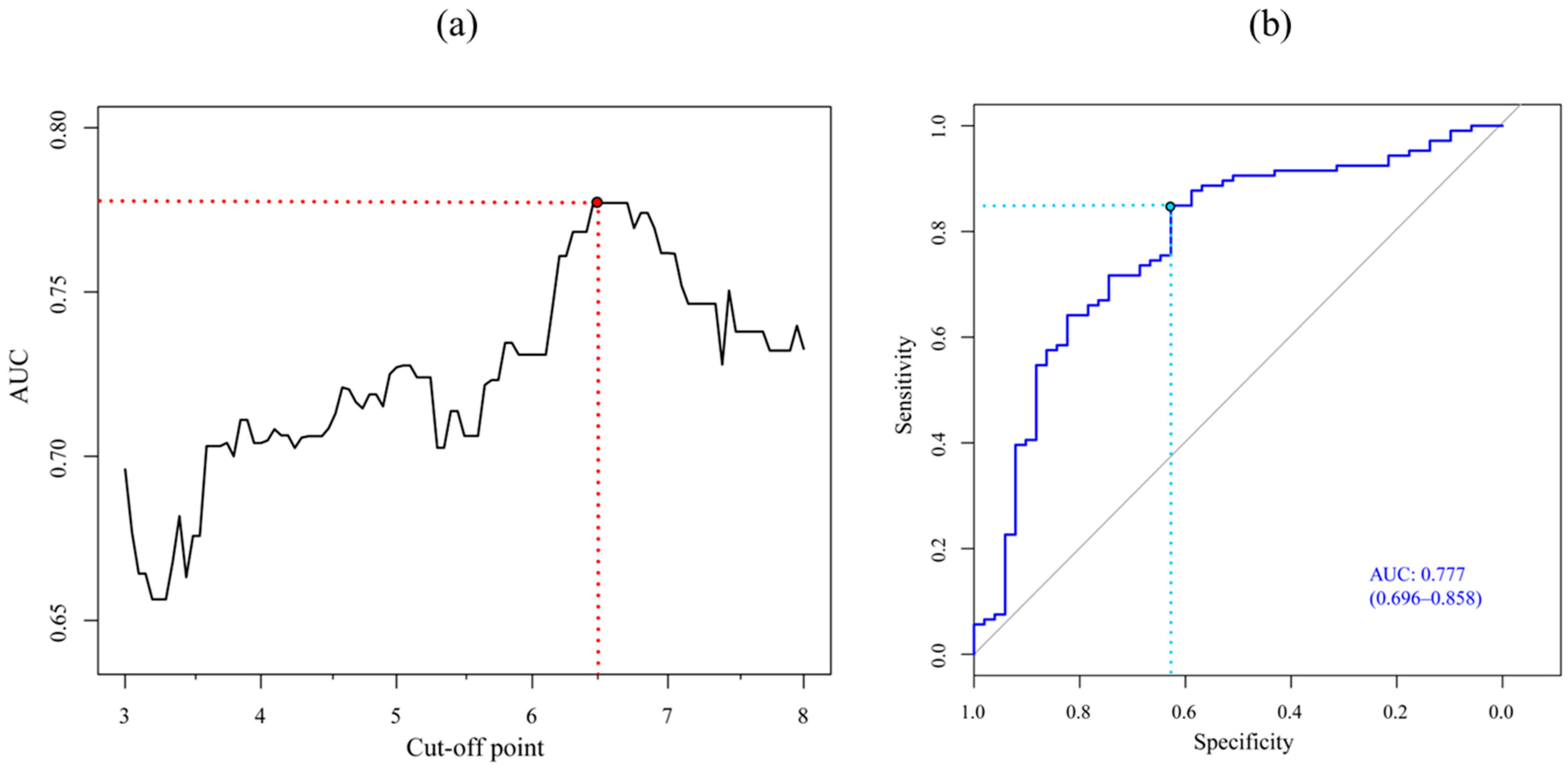
2.3. Ensemble Model and Cut-Off Point Determination
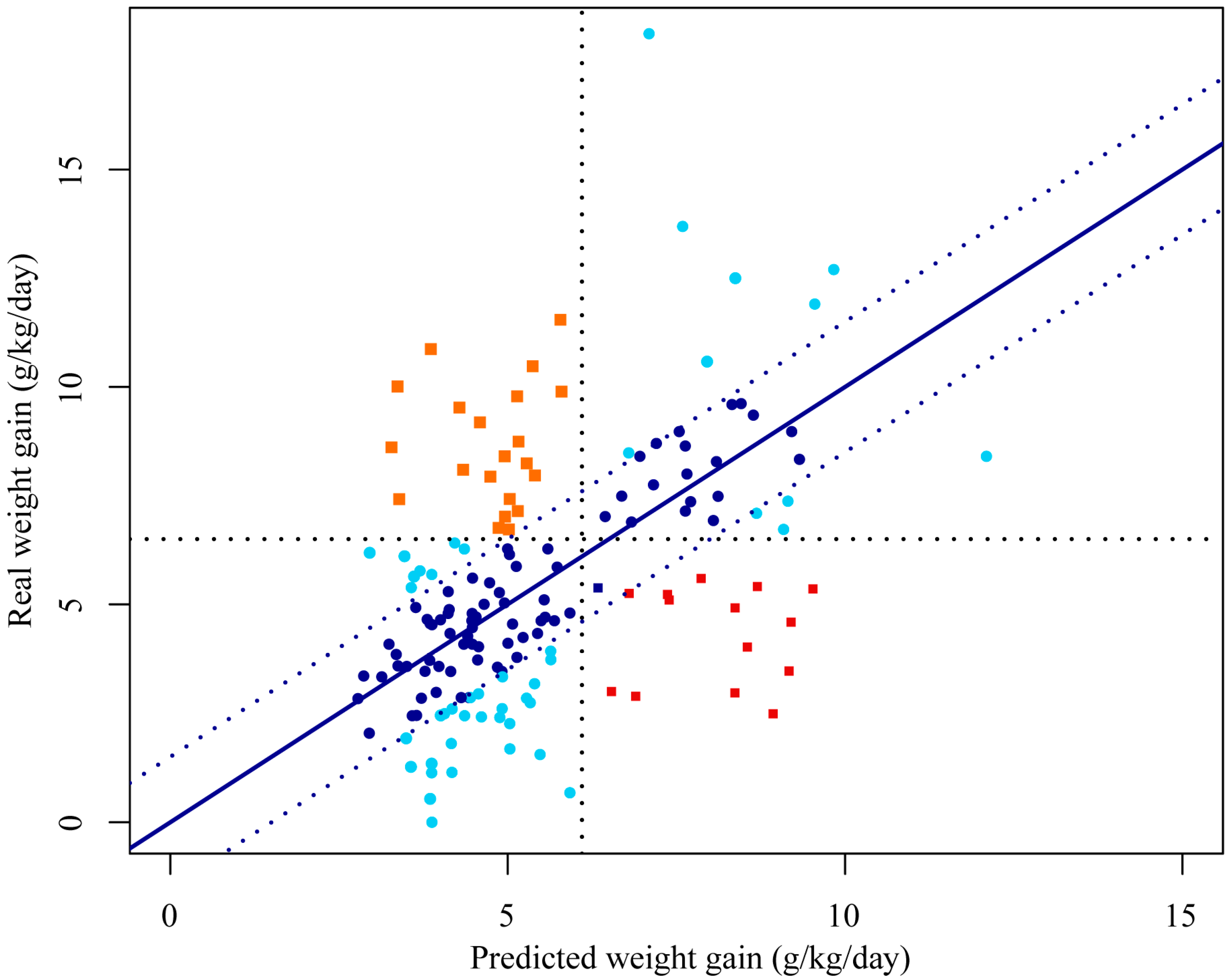
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF; WHO; World Bank Group. Levels and Trends in Child Malnutrition. 2023. Available online: https://www.who.int/publications/i/item/9789240073791 (accessed on 11 September 2024).
- Rodríguez, L.; Cervantes, E.; Ortiz, R. Malnutrition and gastrointestinal and respiratory infections in children: A public health problem. Int. J. Environ. Res. Public Health 2011, 8, 1174–1205. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Olofin, I.; McDonald, C.M.; Ezzati, M.; Flaxman, S.; Black, R.E.; Fawzi, W.W.; Caulfield, L.E.; Danaei, G.; Nutrition Impact Model Study. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: A pooled analysis of ten prospective studies. PLoS ONE 2013, 8, e64636. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.; Ben Ameur, A.; Anderson, A.; Holte-McKenzie, M.; Papastavrou, S.; Tse, C.; Riddle, A.; Pentlow, S.; Schofield, D.; Ali Ahmed, H.A. Gender-Transformative Framework for Nutrition Advancing Nutrition and Gender Equality Together. World Vision. 2024. Available online: https://gendernutritionframework.org/ (accessed on 21 October 2024).
- Agostoni, C.; Baglioni, M.; La Vecchia, A.; Molari, G.; Berti, C. Interlinkages between Climate Change and Food Systems: The Impact on Child Malnutrition-Narrative Review. Nutrients 2023, 15, 416. [Google Scholar] [CrossRef] [PubMed]
- López-Ejeda, N.; Charle-Cuéllar, P.; Vargas, A.; Guerrero, S. Can Community Health Workers manage uncomplicated severe acute malnutrition? A review of operational experiences in delivering SAM treatment through community health platforms. Matern. Child Nutr. 2019, 15, e12719. [Google Scholar] [CrossRef]
- UNICEF. Treatment of Wasting Using Simplified Approaches. A Rapid Evidence Review. 2021. Available online: https://www.unicef.org/documents/rapid-review-treatment-wasting-using-simplified-approaches (accessed on 6 September 2024).
- World Health Organization (WHO). Guideline: WHO Guideline on the Prevention and Management of Wasting and Nutritional Oedema (Acute Malnutrition) in Infants and Children Under 5 Years. 2023. Available online: https://iris.who.int/handle/10665/376075 (accessed on 14 September 2024).
- Sphere. The Sphere Handbook. Humanitarian Charter and Minimum Standards in Humanitarian Response. 2018. Available online: https://www.spherestandards.org/handbook-2018/ (accessed on 19 September 2024).
- Das, J.K.; Salam, R.A.; Saeed, M.; Kazmi, F.A.; Bhutta, Z.A. Effectiveness of Interventions for Managing Acute Malnutrition in Children under Five Years of Age in Low-Income and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 116. [Google Scholar] [CrossRef]
- Maust, A.; Koroma, A.S.; Abla, C.; Molokwu, N.; Ryan, K.N.; Singh, L.; Manary, M.J. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. J. Nutr. 2015, 145, 2604–2609. [Google Scholar] [CrossRef]
- Sánchez-Martínez, L.J.; Charle-Cuéllar, P.; Gado, A.A.; Dougnon, A.O.; Sanoussi, A.; Ousmane, N.; Lazoumar, R.H.; Toure, F.; Vargas, A.; Hernández, C.L.; et al. Impact of a simplified treatment protocol for moderate acute malnutrition with a decentralized treatment approach in emergency settings of Niger. Front. Nutr. 2023, 10, 1253545. [Google Scholar] [CrossRef]
- Jangam, E.; Annavarapu, C.S.R. A stacked ensemble for the detection of COVID-19 with high recall and accuracy. Comput. Biol. Med. 2021, 135, 104608. [Google Scholar] [CrossRef]
- Hwangbo, L.; Kang, Y.J.; Kwon, H.; Lee, J.I.; Cho, H.J.; Ko, J.K.; Sung, S.M.; Lee, T.H. Stacking ensemble learning model to predict 6-month mortality in ischemic stroke patients. Sci. Rep. 2022, 12, 17389. [Google Scholar] [CrossRef]
- Khan, N.A.; Yunus, R.M. A hybrid ensemble approach to accelerate the classification accuracy for predicting malnutrition among under-five children in sub-Saharan African countries. Nutrition 2023, 108, 111947. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.C. Data Mining: The Textbook; Springer: London, UK, 2015. [Google Scholar] [CrossRef]
- Charle-Cuéllar, P.; Lopez-Ejeda, N.; Gado, A.A.; Dougnon, A.O.; Sanoussi, A.; Ousmane, N.; Lazoumar, R.H.; Sánchez-Martínez, L.J.; Touré, F.; Vargas, A.; et al. Effectiveness and Coverage of Severe Acute Malnutrition Treatment with a Simplified Protocol in a Humanitarian Context in Diffa, Niger. Nutrients 2023, 15, 1975. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Opondo, C.; Lelijveld, N.; Marron, B.; Onyo, P.; Musyoki, E.N.; Adongo, S.W.; Manary, M.; Briend, A.; Kerac, M. A simplified, combined protocol versus standard treatment for acute malnutrition in children 6–59 months (ComPAS trial): A cluster-randomized controlled non-inferiority trial in Kenya and South Sudan. PLoS Med. 2020, 17, e1003192. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). The Niger: Response Overview; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 5 September 2024).
- Shah, A.D.; Bartlett, J.W.; Carpenter, J.; Nicholas, O.; Hemingway, H. Comparison of Random Forest and Parametric Imputation Models for Imputing Missing Data Using MICE: A CALIBER Study. Am. J. Epidemiol. 2014, 179, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Rajput, D.; Wang, W.J.; Chen, C.C. Evaluation of a decided sample size in machine learning applications. BMC Bioinform. 2023, 24, 48. [Google Scholar] [CrossRef]
- Genuer, R.; Poggi, J.M.; Tuleau-Malot, C. VSURF: An R Package for Variable Selection Using Random Forests. R J. 2015, 7, 19–33. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.H.J.; Kerac, M.; Trehan, I.; Briend, A. Severe acute malnutrition. Nat. Rev. Dis. Prim. 2017, 3, 17067. [Google Scholar] [CrossRef]
- Isanaka, S.; Hitchings, M.D.T.; Berthé, F.; Briend, A.; Grais, R.F. Linear growth faltering and the role of weight attainment: Prospective analysis of young children recovering from severe wasting in Niger. Matern. Child Nutr. 2019, 15, e12817. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Inpatient Treatment of Severely Malnourished Children. 2003. Available online: https://apps.who.int/iris/bitstream/handle/10665/205172/B0003.pdf;sequence=1 (accessed on 12 September 2024).
- Ashworth, A. Efficacy and effectiveness of community-based treatment of severe malnutrition. Food Nutr. Bull. 2006, 27 (Suppl. S3), S24–S48. [Google Scholar] [CrossRef]
- Daures, M.; Phelan, K.; Issoufou, M.; Kouanda, S.; Sawadogo, O.; Issaley, K.; Cazes, C.; Séri, B.; Ouaro, B.; Akpakpo, B.; et al. New approach to simplifying and optimising acute malnutrition treatment in children aged 6–59 months: The OptiMA single-arm proof-of-concept trial in Burkina Faso. Br. J. Nutr. 2020, 123, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Cazes, C.; Phelan, K.; Hubert, V.; Boubacar, H.; Bozama, L.I.; Sakubu, G.T.; Thsiala, B.K.; Tusuku, T.; Alitanou, R.; Kouamé, A.; et al. Simplifying and optimising the management of uncomplicated acute malnutrition in children aged 6–59 months in the Democratic Republic of the Congo (OptiMA-DRC): A non-inferiority, randomised controlled trial. Lancet Glob. Health 2022, 10, e510–e520. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, G.; Tausanovitch, Z.; Christian, L.G.; Bebelou, M.S.M.; Mbeng, B.T.; Dembele, A.M.; Fossi, A.; Bansimba, T.; Coulibaly, I.N.; Nikièma, V.; et al. Effectiveness of acute malnutrition treatment with a simplified, combined protocol in Central African Republic: An observational cohort study. Matern. Child Nutr. 2024, 20, e13691. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.T.; Marron, B.; Tausanovitch, Z.; Radin, E.; Andrianarisoa, J.; Dembele, S.; Ouédraogo, C.T.; Coulibaly, I.N.; Biotteau, M.; Ouologuem, B.; et al. Effectiveness of Acute Malnutrition Treatment at Health Center and Community Levels with a Simplified, Combined Protocol in Mali: An Observational Cohort Study. Nutrients 2022, 14, 4923. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Ritz, C.; Friis, H.; Briend, A.; Kaestel, P. Impact of reduced dose of ready-to-use therapeutic foods in children with uncomplicated severe acute malnutrition: A randomised non-inferiority trial in Burkina Faso. PLoS Med. 2019, 16, e1002887. [Google Scholar] [CrossRef]
- Dah, C.; Ourohire, M.; Sié, A.; Ouédraogo, M.; Bountogo, M.; Boudo, V.; Lebas, E.; Nyatigo, F.; Arnold, B.F.; O'Brien, K.S.; et al. How does baseline anthropometry affect anthropometric outcomes in children receiving treatment for severe acute malnutrition? A secondary analysis of a randomized controlled trial. Matern. Child Nutr. 2022, 18, e13329. [Google Scholar] [CrossRef]
- Sanghvi, J.; Mehta, S.; Kumar, R. Predicators for weight gain in children treated for severe acute malnutrition: A prospective study at nutritional rehabilitation center. ISRN Pediatr. 2014, 2014, 808756. [Google Scholar] [CrossRef]
- Thompson, D.S.; McKenzie, K.; Opondo, C.; Boyne, M.S.; Lelijveld, N.; Wells, J.C.; Cole, T.J.; Anujuo, K.; Abera, M.; Berhane, M.; et al. Faster rehabilitation weight gain during childhood is associated with risk of non-communicable disease in adult survivors of severe acute malnutrition. PLoS Glob. Public Health 2023, 3, e0002698. [Google Scholar] [CrossRef]
- Olga, L.; McKenzie, K.; Kerac, M.; Boyne, M.; Badaloo, A.; Bandsma, R.H.J.; Koulman, A.; Thompson, D.S. Weight gain during nutritional rehabilitation post-childhood malnutrition may influence the associations between adulthood desaturases activity and anthro-cardiometabolic risk factors. Clin. Nutr. 2024, 43, 747–755. [Google Scholar] [CrossRef]
- Kangas, S.T.; Kaestel, P.; Salpéteur, C.; Nikièma, V.; Talley, L.; Briend, A.; Ritz, C.; Friis, H.; Wells, J.C. Body composition during outpatient treatment of severe acute malnutrition: Results from a randomised trial testing different doses of ready-to-use therapeutic foods. Clin. Nutr. 2020, 39, 3426–3433. [Google Scholar] [CrossRef]
- Fabiansen, C.; Yaméogo, C.W.; Iuel-Brockdorf, A.; Cichon, B.; Rytter, M.J.H.; Kurpad, A.; Wells, J.C.; Ritz, C.; Ashorn, P.; Filteau, S.; et al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Med. 2017, 14, e1002387. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.J.; Potani, I.; Singh, A.; Griswold, S.; Wong, W.W.; Langlois, B.; Shen, Y.; Chui, K.H.K.; Rosenberg, I.H.; Webb, P.; et al. Body Composition Changes in Children during Treatment for Moderate Acute Malnutrition: Findings from a 4-Arm Cluster-Randomized Trial in Sierra Leone. J. Nutr. 2021, 151, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Goosens, S.; Bekele, Y.; Yun, O.; Harczi, G.; Ouannes, M.; Shepherd, S. Mid-Upper Arm Circumference Based Nutrition Programming: Evidence for a New Approach in Regions with High Burden of Acute Malnutrition. PLoS ONE 2012, 07, e49320. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Mahajan, R.; Marino, E.; Sunyoto, T.; Shandilya, C.; Tabrez, M.; Kumari, K.; Mathew, P.; Jha, A.; Salse, N.; et al. Community-based management of severe acute malnutrition in India: New evidence from Bihar. Am. J. Clin. Nutr. 2015, 101, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Kamugisha, J.G.K.; Lanyero, B.; Nabukeera-Barungi, N.; Nambuya-Lakor, H.; Ritz, C.; Mølgaard, C.; Michaelsen, K.F.; Briend, A.; Mupere, E.; Friis, H.; et al. Weight and mid-upper arm circumference gain velocities during treatment of young children with severe acute malnutrition, a prospective study in Uganda. BMC Nutr. 2021, 7, 26. [Google Scholar] [CrossRef]
- Binns, P.; Dale, N.; Hoq, M.; Banda, C.; Myatt, M. Relationship between mid upper arm circumference and weight changes in children aged 6–59 months. Arch. Public Health 2015, 73, 54. [Google Scholar] [CrossRef]
- Mwangome, M.K.; Fegan, G.; Prentice, A.M.; Berkley, J.A. Are diagnostic criteria for acute malnutrition affected by hydration status in hospitalized children? A repeated measures study. Nutr. J. 2011, 10, 92. [Google Scholar] [CrossRef]
- Khara, T.; Myatt, M.; Sadler, K.; Bahwere, P.; Berkley, J.A.; Black, R.E.; Boyd, E.; Garenne, M.; Isanaka, S.; Lelijveld, N.; et al. Anthropometric criteria for best-identifying children at high risk of mortality: A pooled analysis of twelve cohorts. Public Health Nutr. 2023, 26, 803–819. [Google Scholar] [CrossRef]
- Bitew, F.H.; Sparks, C.S.; Nyarko, S.H. Machine learning algorithms for predicting undernutrition among under-five children in Ethiopia. Public Health. Nutr. 2022, 25, 269–280. [Google Scholar] [CrossRef]
- Qasrawi, R.; Sgahir, S.; Nemer, M.; Halaikah, M.; Badrasawi, M.; Amro, M.; Polo, S.V.; Al-Halawa, D.A.; Mujahed, D.; Nasreddine, L.; et al. Machine Learning Approach for Predicting the Impact of Food Insecurity on Nutrient Consumption and Malnutrition in Children Aged 6 Months to 5 Years. Children 2024, 11, 810. [Google Scholar] [CrossRef]
- Zemariam, A.B.; Yimer, A.; Abebe, G.K.; Wondie, W.T.; Abate, B.B.; Alamaw, A.W.; Yilak, G.; Melaku, T.M.; Ngusie, H.S. Employing supervised machine learning algorithms for classification and prediction of anemia among youth girls in Ethiopia. Sci. Rep. 2024, 14, 9080. [Google Scholar] [CrossRef] [PubMed]
- Brauner-Otto, S.; Baird, S.; Ghimire, D. Maternal employment and child health in Nepal: The importance of job type and timing across the child's first five years. Soc. Sci. Med. 2019, 224, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Ameer, W.; Malik, N.I.; Alam, M.B.; Ahmed, F.; Qureshi, M.G.; Zhao, H.; Yang, J.; Zia, S. Distance to Healthcare Facility and Lady Health Workers' Visits Reduce Malnutrition in under Five Children: A Case Study of a Disadvantaged Rural District in Pakistan. Int. J. Environ. Res. Public Health 2022, 19, 8200. [Google Scholar] [CrossRef] [PubMed]
- van Cooten, M.H.; Bilal, S.M.; Gebremedhin, S.; Spigt, M. The association between acute malnutrition and water, sanitation, and hygiene among children aged 6–59 months in rural Ethiopia. Matern. Child Nutr. 2019, 15, e12631. [Google Scholar] [CrossRef]
| Variable | Possible Responses | VSURF Variable Selected | VSURF Variable of Interpretation | VSURF Variable of Prediction |
|---|---|---|---|---|
| Caregiver’s relationship to the child. | Grandmother, Mother, Father, Sister, Aunt, Other | No | No | No |
| Age of caregiver. | numeric | Yes | No | No |
| Sex of caregiver. | Male, Female | No | No | No |
| Age of mother when pregnant. | numeric | Yes | No | No |
| Number of children < five years. | numeric | Yes | No | No |
| Number of prenatal visits. | None, 1, 2, 3, 4 or more, DKW | No | No | No |
| Supplements during pregnancy (iron and/or folic acid)? | Yes, No, DKW | No | No | No |
| Has the child ever been breastfed? | Yes, No, DKW | No | No | No |
| Is the child currently being breastfed? | Yes, No, DKW | No | No | No |
| Main source of water supply. | Purchased water, rainwater, surface water, unprotected well, protected well, home tap, community tap, water tank truck, other | Yes | Yes | Yes |
| Distance to get your water supply? | Less than 100 m, between 100 and 300 m, between 300 and 500 m, more than 500 m, DKW | Yes | No | No |
| Time to get your water supplies? | Less than 15 min, 15–30 min, 30 min–1 h, 1–2 h, more than 2 h, DKW | Yes | Yes | Yes |
| Debugging water. | Yes, No, DKW | No | No | No |
| Where do you go to satisfy your physiological needs? | In the fields/bushes/rivers, latrine, DKW | Yes | Yes | Yes |
| Main occupation of the head of the household. | Agriculture, house employee, office employee, cattle raising, transport, unskilled manual labor, skilled manual labor, sales and service, other, DKW | Yes | No | No |
| Main occupation of the child’s caregiver. | Agriculture, house employee, office employee, cattle raising, transport, unskilled manual labor, skilled manual labor, sales and service, other, DKW | Yes | Yes | Yes |
| How many days of the week does the caregiver perform this job? | Between 1 and 5 days, between 6 and 7 days, DKW | Yes | No | No |
| How many hours does the child’s caregiver perform this job? | Less than 8 h, more than 8 h, DKW | Yes | No | No |
| How many people live in the child’s household? | numeric | Yes | No | No |
| What is the status of your household? | In propriety, for rent, on loan, other, DKW | Yes | No | No |
| Main construction material for the roof of your household. | Concrete/cement/sheet metal, dung/mud and grass, branches/grass/leaves, other, no roof, DKW | Yes | No | No |
| Main construction material for the floor of your household. | Concrete/cement/sheet metal, dung/mud and grass, branches/grass/leaves, other, no floor, DKW | No | No | No |
| Does your household have a separate room for the kitchen? | Yes, No, DKW | No | No | No |
| Do you have a mattress? | Yes, No, DKW | Yes | No | No |
| Do you have a mobile phone? | Yes, No, DKW | Yes | No | No |
| Do you have a refrigerator? | Yes, No, DKW | No | No | No |
| Do you have a television? | Yes, No, DKW | No | No | No |
| Do you have a radio? | Yes, No, DKW | Yes | No | No |
| Do you have a sewing machine? | Yes, No, DKW | No | No | No |
| Do you have a table? | Yes, No, DKW | No | No | No |
| Do you have chairs or benches? | Yes, No, DKW | No | No | No |
| Do you have access to electricity? | Yes, No, DKW | Yes | No | No |
| Do you have access to livestock? | Yes, No, DKW | No | No | No |
| Do you have access to arable land? | No, less than 1 ha, between 1 and 5 has, more than 5 has, DKW | Yes | No | No |
| Household’s main food source. | Purchase on credit, purchase with money, purchase in kind, help from relatives, NGO food assistance, gathering/hunting or fishing, own production, DKW | No | No | No |
| Enrolled in a food assistance program? | Yes, No, DKW | No | No | No |
| Enrolled in a cash assistance program? | Yes, No, DKW | No | No | No |
| Enrolled in a water, hygiene, or sanitation assistance program? | Yes, No, DKW | No | No | No |
| Enrolled in any assistance program? | Yes, No, DKW | No | No | No |
| During the year, has the number of meals at home been reduced? | Yes, No, DKW | Yes | No | No |
| In the last 4 weeks, have you been worried about a lack of food? | No, Rarely (1 or 2 times), Sometimes (3 to 10 times), Often (more than 10 times), DKW | Yes | No | No |
| In the last 4 weeks, have you reduced your usual portion of food? | No, Rarely (1 or 2 times), Sometimes (3 to 10 times), Often (more than 10 times), DKW | Yes | No | No |
| In the last 4 weeks, have you reduced the number of usual meals? | No, Rarely (1 or 2 times), Sometimes (3 to 10 times), Often (more than 10 times), DKW | Yes | No | No |
| In the last 4 weeks, have you gone an entire day without eating? | No, Rarely (1 or 2 times), Sometimes (3 to 10 times), Often (more than 10 times), DKW | Yes | No | No |
| What do you usually do when your child is sick? | Healer, self-medication, health post/CHW, traditional medicine, nothing, other, DKW | No | No | No |
| Do you think there are barriers to access to treatment for malnutrition? | Yes, No, DKW | No | No | No |
| What is your means of transportation to get to the health center? | Car, Motorbike, Bike, Donkey, Charrette, Walking, Other | Yes | No | No |
| How long does it take to get to the health center? | Less than 1 h, between 1 and 2 h, more than 1 h, DKW | Yes | No | No |
| Can you make the round trip to the health center in one day? | Yes, No, DKW | No | No | No |
| Other things you have to pay for when you go to the health center? | Yes, No, DKW | Yes | Yes | Yes |
| Food Diversity Index. | Poor, Limited, Acceptable | No | No | No |
| Model | MAE | RMSE | R2 | |
|---|---|---|---|---|
| Individual models | QRF | 1.99 | 2.89 | 0.27 |
| KNN | 2.31 | 3.11 | 0.14 | |
| LM | 2.23 | 3.12 | 0.18 | |
| PCR | 2.15 | 2.93 | 0.22 | |
| rSVM | 2.15 | 2.95 | 0.23 | |
| Ensemble model | General | 1.58 | 2.26 | 0.55 |
| SAM | 1.53 | 2.02 | 0.61 | |
| MAM | 1.58 | 2.28 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Martínez, L.J.; Charle-Cuéllar, P.; Gado, A.A.; Ousmane, N.; Hernández, C.L.; López-Ejeda, N. Using Machine Learning to Fight Child Acute Malnutrition and Predict Weight Gain During Outpatient Treatment with a Simplified Combined Protocol. Nutrients 2024, 16, 4213. https://doi.org/10.3390/nu16234213
Sánchez-Martínez LJ, Charle-Cuéllar P, Gado AA, Ousmane N, Hernández CL, López-Ejeda N. Using Machine Learning to Fight Child Acute Malnutrition and Predict Weight Gain During Outpatient Treatment with a Simplified Combined Protocol. Nutrients. 2024; 16(23):4213. https://doi.org/10.3390/nu16234213
Chicago/Turabian StyleSánchez-Martínez, Luis Javier, Pilar Charle-Cuéllar, Abdoul Aziz Gado, Nassirou Ousmane, Candela Lucía Hernández, and Noemí López-Ejeda. 2024. "Using Machine Learning to Fight Child Acute Malnutrition and Predict Weight Gain During Outpatient Treatment with a Simplified Combined Protocol" Nutrients 16, no. 23: 4213. https://doi.org/10.3390/nu16234213
APA StyleSánchez-Martínez, L. J., Charle-Cuéllar, P., Gado, A. A., Ousmane, N., Hernández, C. L., & López-Ejeda, N. (2024). Using Machine Learning to Fight Child Acute Malnutrition and Predict Weight Gain During Outpatient Treatment with a Simplified Combined Protocol. Nutrients, 16(23), 4213. https://doi.org/10.3390/nu16234213






