The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer
Abstract
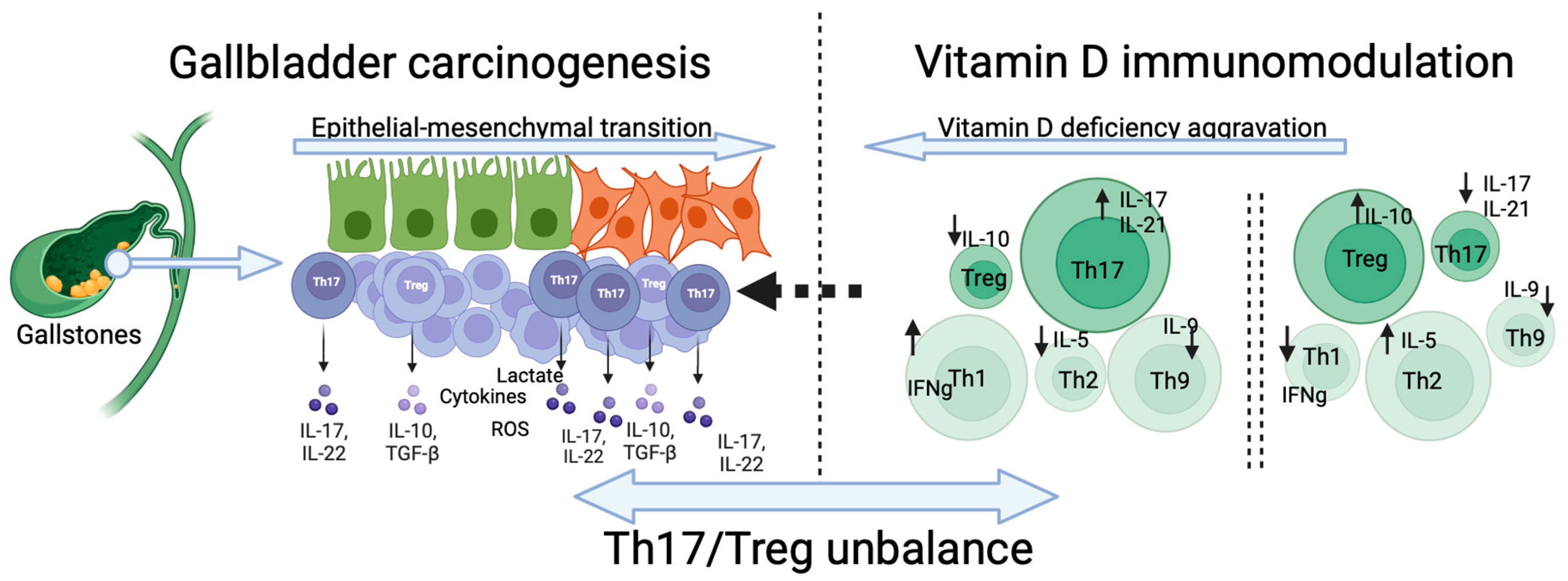
1. Introduction
2. Gallbladder Cancer Risk Factors
3. Vitamin D Deficiency/Supplementation and Inflammation
4. Vitamin D and Gallbladder Cancer
5. Vitamin D Reduces the Th17/Treg Ratio and Suppresses Cancer Cells’ Invasiveness
6. The Influence of Th17/Treg Balance on the Immune Cells Landscape and Carcinogenesis
7. The Dichotomic Role of Th17/Treg Balance in GBC Carcinogenesis
8. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG | ATP-binding cassette transporter |
| AID | autoimmune diseases |
| ALK | anaplastic lymphoma kinase |
| APC | adenomatous polyposis coli |
| BA | bile acids |
| BMI | body mass index |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| BTCs | biliary tract cancers |
| CAC | colitis-associated cancer |
| CCK | cholecystokinin |
| CRC | colorectal cancer |
| CRP | C-reactive protein |
| CVD | cardiovascular disease (CVD) |
| CYP1A1 | cytochrome P450 1A1 |
| DCs | dendritic cells |
| DFS | disease-free survival |
| EMT | epithelial–mesenchymal transition |
| FGF | fibroblast growth factor |
| FoxP3 | forkhead box protein 3. |
| FXR | farnesoid X receptor |
| GBC | gallbladder cancer |
| GSTT1 | glutathione S-transferase theta-1 |
| HCC | hepatocellular carcinoma |
| HER | human epidermal growth factor receptor |
| HNSCC | head and neck squamous cell carcinoma |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| IDH | isocitrate dehydrogenase |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| JNK | Jun N-terminal kinase |
| KRAS | Kristen Rat Sarcoma Viral oncogene homolog |
| LAG3 | lymphocyte activation gene 3 |
| MDSCs | myeloid-derived suppressor cells |
| MLH1 | MutL protein homolog 1 |
| NF-κB | nuclear factor-κB |
| NK | natural killers |
| NLR | neutrophil-to-lymphocyte ratio |
| NT-proBNP | N-terminal pro–B-type natriuretic peptide |
| OS | overall survival |
| OXPHOS | oxidative phosphorylation |
| PBMCs | peripheral blood mononuclear cells |
| PD-1 | programmed death-1 |
| PDAC | pancreatic ductal adenocarcinoma |
| PFS | progression-free survival |
| PI3K | phosphoinositide 3-kinases |
| PTEN | Phosphatase and tensin homolog deleted on chromosome 10 |
| RCTs | randomized clinical trials |
| RORγt | related orphan receptor γt |
| RXR | retinoid X receptor |
| SNPs | single nucleotide polymorphisms |
| SPP1 | secreted phosphoprotein 1 |
| SRC | steroid receptor coactivator |
| STAT3 | signal transducer and activator of transcription 3 |
| T2D | type 2 diabetes |
| TAMs | tumor-associated macrophages |
| TCGA | the cancer genome atlas |
| TGF | transforming growth factor |
| TGR | G protein-coupled receptor |
| Th17 | T-helper 17 cells |
| TILs | tumor-infiltrating lymphocytes |
| TIM3 | T-cell immunoglobulin and mucin domain 3 |
| TLR | toll-like receptors |
| TNF | tumor necrosis factor |
| TP53 | tumor protein p53 |
| Treg | regulatory T cells |
| UDCA | ursodeoxycholic acid |
| UV | ultraviolet |
| VDD | vitamin D deficiency |
| VDR | vitamin D receptor |
| VDREs | vitamin D responsive elements |
| VDS | vitamin D supplementation |
| Wnt | wingless-related integration site |
| YTE-17 | garcinia yunnanensis |
References
- Jones, M.W.; Small, K.; Kashyap, S.; Deppen, J.G. Physiology, Gallbladder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Xiao, R.; Lei, K.; Kuok, H.; Deng, W.; Zhuang, Y.; Tang, Y.; Guo, Z.; Qin, H.; Bai, L.-P.; Li, T. Synthesis and identification of lithocholic acid 3-sulfate as RORγt ligand to inhibit Th17 cell differentiation. J. Leukoc. Biol. 2022, 112, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y. An overview of bile acid synthesis and its physiological and pathological functions. Yi Chuan Hered. 2019, 41, 365–374. [Google Scholar]
- Parra-Soto, S.; Petermann-Rocha, F.; Martínez-Sanguinetti, M.A.; Leiva-Ordeñez, A.M.; Troncoso-Pantoja, C.; Ulloa, N.; Diaz-Martínez, X.; Celis-Morales, C. Cancer in Chile and worldwide: An overview of the current and future epidemiological context. Rev. Med. Chil. 2020, 148, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.A.; Marcano-Bonilla, L.; Roberts, L.R. Gallbladder cancer: Epidemiology and genetic risk associations. Chin. Clin. Oncol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Pérez-Moreno, P.; Riquelme, I.; García, P.; Brebi, P.; Roa, J.C. Environmental and Lifestyle Risk Factors in the Carcinogenesis of Gallbladder Cancer. J. Pers. Med. 2022, 12, 234. [Google Scholar] [CrossRef]
- Feo, C.F.; Ginesu, G.C.; Fancellu, A.; Perra, T.; Ninniri, C.; Deiana, G.; Scanu, A.M.; Porcu, A. Current management of incidental gallbladder cancer: A review. Int. J. Surg. 2022, 98, 106234. [Google Scholar] [CrossRef]
- Koshiol, J.; Bellolio, E.; Vivallo, C.; Cook, P.; Roa, J.C.; McGee, E.E.; Losada, H.; Van Dyke, A.L.; Van De Wyngard, V.; Prado, R.; et al. Distribution of dysplasia and cancer in the gallbladder: An analysis from a high cancer-risk population. Hum. Pathol. 2018, 82, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Z.; Zhang, T.; Chen, M.Y.; Shen, J.L. Novel biomarkers for the diagnosis and prognosis of gallbladder cancer. J. Dig. Dis. 2021, 22, 62–71. [Google Scholar] [CrossRef]
- Mady, M.; Prasai, K.; Tella, S.H.; Yadav, S.; Hallemeier, C.L.; Rakshit, S.; Roberts, L.; Borad, M.; Mahipal, A. Neutrophil to lymphocyte ratio as a prognostic marker in metastatic gallbladder cancer. HPB 2020, 22, 1490–1495. [Google Scholar] [CrossRef]
- Okumura, K.; Gogna, S.; Gachabayov, M.; Felsenreich, D.M.; McGuirk, M.; Rojas, A.; Quintero, L.; Seshadri, R.; Gu, K.; Dong, X.D. Gallbladder cancer: Historical treatment and new management options. World J. Gastrointest. Oncol. 2021, 13, 1317–1335. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef]
- Campbell, F.C.; Xu, H.; El-Tanani, M.; Crowe, P.; Bingham, V. The Yin and Yang of vitamin D receptor (VDR) signaling in neoplastic progression: Operational networks and tissue-specific growth control. Biochem. Pharmacol. 2010, 79, 1–9. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020; 74, 1498–1513. [Google Scholar]
- Grant, W.B. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer?: An examination using Hill’s criteria for causality. Dermatoendocrinol 2009, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Global differences in vitamin D status and dietary intake: A review of the data. Endocr. Connect. 2022, 11, e210282. [Google Scholar] [CrossRef]
- Tuohimaa, P.; Pukkala, E.; Scélo, G.; Olsen, J.H.; Brewster, D.H.; Hemminki, K.; Tracey, E.; Weiderpass, E.; Kliewer, E.V.; Pompe-Kirn, V.; et al. Does solar exposure, as indicated by the non-melanoma skin cancers, protect from solid cancers: Vitamin D as a possible explanation. Eur. J. Cancer 2007, 43, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.S.; Yilmaz, M.K.; Falkmer, U.G.; Poulsen, L.Ø.; Bøgsted, M.; Christensen, H.S.; Bojesen, S.E.; Jensen, B.V.; Chen, I.M.; Johansen, A.Z.; et al. Pre-treatment serum vitamin D deficiency is associated with increased inflammatory biomarkers and short overall survival in patients with pancreatic cancer. Eur. J. Cancer 2021, 144, 72–80. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, G.; Xu, Y.; Zheng, S.; Tang, B.; Huang, H.; Wu, I.X.Y.; Huang, D.; Liu, Y.; Zhang, X. Association Between Vitamin D Exposure and Head and Neck Cancer: A Systematic Review with Meta-Analysis. Front. Immunol. 2021, 12, 627226. [Google Scholar] [CrossRef]
- Lopez-Caleya, J.F.; Ortega-Valín, L.; Fernández-Villa, T.; Delgado-Rodríguez, M.; Martín-Sánchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case-control studies. Cancer Causes Control 2022, 33, 167–182. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, H.; Yang, S.; Cao, H.; Zhang, Z.; Wen, B.; Zhou, S. SDC1 knockdown induces epithelial–mesenchymal transition and invasion of gallbladder cancer cells via the ERK/Snail pathway. J. Int. Med. Res. 2020, 48, 0300060520947883. [Google Scholar] [CrossRef]
- Kim, H.-N.; Lee, S.Y.; Kim, B.-H.; Kim, C.-Y.; Kim, A.; Kim, H. Prognostic value of tumor budding in gallbladder cancer: Application of the International Tumor Budding Consensus Conference scoring system. Virchows Arch. Int. J. Pathol. 2021, 478, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Kshattry, M.; Mandal, S.; Jolly, M.K.; Bhattacharyya, D.K.; Barah, P. An Integrative Systems Biology Approach Identifies Molecular Signatures Associated with Gallbladder Cancer Pathogenesis. J. Clin. Med. 2021, 10, 3520. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.-C.; Chen, S.-C.; Yeh, C.-N.; Pang, J.-H.S.; Shen, S.-C.; Hsu, J.-T.; Liu, Y.-Y.; Chen, L.-W.; Kuo, S.-F.; Takano, M.; et al. MART-10, a less calcemic vitamin D analog, is more potent than 1α,25-dihydroxyvitamin D3 in inhibiting the metastatic potential of MCF-7 breast cancer cells in vitro. J. Steroid Biochem. Mol. Biol. 2014, 139, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Nikolopoulos, G.K.; Aghemo, A.; Lleo, A.; Alqahtani, S.A.; Hassan, C.; Repici, A.; Bonovas, S. Environmental risk factors for gallbladder cancer: Field-wide systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2024, in press. [Google Scholar] [CrossRef]
- Halaseh, S.A.; Halaseh, S.; Shakman, R. A Review of the Etiology and Epidemiology of Gallbladder Cancer: What You Need to Know. Cureus 2022, 14, e28260. [Google Scholar] [CrossRef]
- Bojan, A.; Pricop, C.; Ciocoiu, M.; Vladeanu, M.C.; Bararu Bojan, I.; Badulescu, O.V.; Badescu, M.C.; Plesoianu, C.E.; Halitchi, D.I.; Foia, L.G. Environmental and Metabolic Risk Factors Linked to Gallbladder Dysplasia. Metabolites 2024, 14, 273. [Google Scholar] [CrossRef]
- Salazar, M.; Ituarte, C.; Abriata, M.G.; Santoro, F.; Arroyo, G. Gallbladder cancer in South America: Epidemiology and prevention. Chin. Clin. Oncol. 2019, 8, 32. [Google Scholar] [CrossRef]
- Zollner, L.; Boekstegers, F.; Barahona Ponce, C.; Scherer, D.; Marcelain, K.; Gárate-Calderón, V.; Waldenberger, M.; Morales, E.; Rojas, A.; Munoz, C.; et al. Gallbladder Cancer Risk and Indigenous South American Mapuche Ancestry: Instrumental Variable Analysis Using Ancestry-Informative Markers. Cancers 2023, 15, 4033. [Google Scholar] [CrossRef]
- Boekstegers, F.; Scherer, D.; Barahona Ponce, C.; Marcelain, K.; Gárate-Calderón, V.; Waldenberger, M.; Morales, E.; Rojas, A.; Munoz, C.; Retamales, J.; et al. Development and internal validation of a multifactorial risk prediction model for gallbladder cancer in a high-incidence country. Int. J. Cancer 2023, 153, 1151–1161. [Google Scholar] [CrossRef]
- Canale, M.; Monti, M.; Rapposelli, I.G.; Ulivi, P.; Sullo, F.G.; Bartolini, G.; Tiberi, E.; Frassineti, G.L. Molecular Targets and Emerging Therapies for Advanced Gallbladder Cancer. Cancers 2021, 13, 5671. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, H.; de Bitter, T.J.J.; de Boer, M.T.; van der Post, R.S.; Nijkamp, M.W.; de Reuver, P.R.; Fehrmann, R.S.N.; Hoogwater, F.J.H. Gallbladder Cancer: Current Insights in Genetic Alterations and Their Possible Therapeutic Implications. Cancers 2021, 13, 5257. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Javle, M.; Pang, F.; Zhao, W.; Abdel-Wahab, R.; Chen, X.; Meric-Bernstam, F.; Chen, H.; Borad, M.J.; Liu, Y.; et al. Somatic genetic aberrations in gallbladder cancer: Comparison between Chinese and US patients. Hepatobiliary Surg. Nutr. 2019, 8, 604–614. [Google Scholar] [CrossRef]
- Lin, J.; Dong, K.; Bai, Y.; Zhao, S.; Dong, Y.; Shi, J.; Shi, W.; Long, J.; Yang, X.; Wang, D.; et al. Precision oncology for gallbladder cancer: Insights from genetic alterations and clinical practice. Ann. Transl. Med. 2019, 7, 467. [Google Scholar] [CrossRef]
- García, P.; Lamarca, A.; Díaz, J.; Carrera, E.; Roa, J.C. Current and New Biomarkers for Early Detection, Prognostic Stratification, and Management of Gallbladder Cancer Patients. Cancers 2020, 12, 3670. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumari, N.; Krishnani, N. Molecular pathogenesis of gallbladder cancer: An update. Mutat. Res. Mol. Mech. Mutagen. 2019, 816–818, 111674. [Google Scholar] [CrossRef] [PubMed]
- Feroz, Z.; Tiwari, S.; Vijayaraghavalu, S.; Kumar, M. GSTs genetic polymorphism, gene–environment interaction and association with gallbladder cancer risk in North Indian population: A case-controlled study. J. Cancer Res. Ther. 2023, 19, 1908. [Google Scholar] [CrossRef]
- Feroz, Z.; Gautam, P.; Tiwari, S.; Shukla, G.C.; Kumar, M. Survival analysis and prognostic factors of the carcinoma of gallbladder. World J. Surg. Oncol. 2022, 20, 403. [Google Scholar] [CrossRef]
- Arshad, A.; Mahmood, S.B.Z.; Ayaz, A.; Al Karim Manji, A.; Ahuja, A.K. Association of vitamin D deficiency and disease activity in systemic lupus erythematosus patients: Two-year follow-up study. Arch. Rheumatol. 2021, 36, 101–106. [Google Scholar] [CrossRef]
- Khamisi, S.; Lundqvist, M.; Rasmusson, A.J.; Engström, B.E.; Karlsson, F.A.; Ljunggren, Ö. Vitamin D and bone metabolism in Graves’ disease: A prospective study. J. Endocrinol. Investig. 2023, 46, 425–433. [Google Scholar] [CrossRef]
- He, L.-P.; Song, Y.-X.; Zhu, T.; Gu, W.; Liu, C.-W. Progress in the Relationship between Vitamin D Deficiency and the Incidence of Type 1 Diabetes Mellitus in Children. J. Diabetes Res. 2022, 2022, 5953562. [Google Scholar] [CrossRef] [PubMed]
- Hajeer, S.; Nasr, F.; Nabha, S.; Saab, M.-B.; Harati, H.; Desoutter, A.; Al Ahmar, E.; Estephan, E. Association between vitamin D deficiency and multiple sclerosis- MRI significance: A scoping review. Heliyon 2023, 9, e15754. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Liu, Y.; Luo, J.; Huang, Z.-D.; Zhang, C.; Fu, Y. Vitamin D deficiency associated with Crohn’s disease and ulcerative colitis: A meta-analysis of 55 observational studies. J. Transl. Med. 2019, 17, 323. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B.; Hajian-Tilaki, K.; Babaei, M. Vitamin D Deficiency and Rheumatoid Arthritis: Epidemiological, Immunological, Clinical and Therapeutic Aspects. Mediterr. J. Rheumatol. 2019, 30, 94–102. [Google Scholar] [PubMed]
- de Carvalho, J.F.; Shoenfeld, Y. High frequency of vitamin D insufficiency in polymyalgia rheumatica and giant cell arteritis: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 574–575. [Google Scholar]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Dolin, T.G.; Christensen, I.J.; Lund, C.M.; Bojesen, S.E.; Lykke, J.; Nielsen, D.L.; Larsen, J.S.; Johansen, J.S. Preoperative plasma vitamin D in patients with localized colorectal cancer: Age-dependent association with inflammation, postoperative complications, and survival. Eur. J. Surg. Oncol. 2023, 49, 244–251. [Google Scholar] [CrossRef]
- Vaughan-Shaw, P.G.; Zgaga, L.; Ooi, L.Y.; Theodoratou, E.; Timofeeva, M.; Svinti, V.; Walker, M.; O’Sullivan, F.; Ewing, A.; Johnston, S.; et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut 2020, 69, 103–111. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Palaniswamy, S.; Gill, D.; De Silva, N.M.; Lowry, E.; Jokelainen, J.; Karhu, T.; Mutt, S.J.; Dehghan, A.; Sliz, E.; Chasman, D.I.; et al. Could vitamin D reduce obesity-associated inflammation? Observational and Mendelian randomization study. Am. J. Clin. Nutr. 2020, 111, 1036–1047. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2023, 52, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, T.; Zhu, A.; Schrotz-King, P.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Effects of vitamin D supplementation on inflammatory response in patients with cancer and precancerous lesions: Systematic review and meta-analysis of randomized trials. Clin. Nutr. 2023, 42, 1142–1150. [Google Scholar] [CrossRef]
- Haidari, F.; Abiri, B.; Iravani, M.; Ahmadi-Angali, K.; Vafa, M. Randomized Study of the Effect of Vitamin D and Omega-3 Fatty Acids Cosupplementation as Adjuvant Chemotherapy on Inflammation and Nutritional Status in Colorectal Cancer Patients. J. Diet. Suppl. 2020, 17, 384–400. [Google Scholar] [CrossRef]
- Kazemian, E.; Akbari, M.E.; Moradi, N.; Gharibzadeh, S.; Mondul, A.M.; Jamshidi-Naeini, Y.; Khademolmele, M.; Zarins, K.R.; Ghodoosi, N.; Amouzegar, A.; et al. Vitamin D Receptor Genetic Variation and Cancer Biomarkers among Breast Cancer Patients Supplemented with Vitamin D3: A Single-Arm Non-Randomized Before and After Trial. Nutrients 2019, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Aragaki, A.K.; Prentice, R.L.; Stefanick, M.L.; Manson, J.E.; Wactawski-Wende, J.; Watts, N.B.; Van Horn, L.; Shikany, J.M.; Rohan, T.E.; et al. Long-Term Effect of Randomization to Calcium and Vitamin D Supplementation on Health in Older Women. Ann. Intern. Med. 2024, 177, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Okereke, O.I.; Reynolds, C.F.; Mischoulon, D.; Chang, G.; Vyas, C.M.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; et al. Effect of Long-term Vitamin D3 Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores. JAMA 2020, 324, 471–480. [Google Scholar] [CrossRef]
- Hu, Z.; Zhi, X.; Li, J.; Li, B.; Wang, J.; Zhu, J.; Zhang, Z. Effects of long-term vitamin D supplementation on metabolic profile in middle-aged and elderly patients with type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2023, 225, 106198. [Google Scholar] [CrossRef]
- Wu, Z.; Camargo, C.A.; Sluyter, J.; Waayer, D.; Toop, L.; Scragg, R. Effect of monthly vitamin D supplementation on antibiotic prescribing in older adults: A post hoc analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 314–321. [Google Scholar] [CrossRef]
- Limonte, C.P.; Zelnick, L.R.; Ruzinski, J.; Hoofnagle, A.N.; Thadhani, R.; Melamed, M.L.; Lee, I.-M.; Buring, J.E.; Sesso, H.D.; Manson, J.E.; et al. Effects of long-term vitamin D and n-3 fatty acid supplementation on inflammatory and cardiac biomarkers in patients with type 2 diabetes: Secondary analyses from a randomised controlled trial. Diabetologia 2021, 64, 437–447. [Google Scholar] [CrossRef]
- Sandboge, S.; Räikkönen, K.; Lahti-Pulkkinen, M.; Hauta-alus, H.; Holmlund-Suila, E.; Girchenko, P.; Kajantie, E.; Mäkitie, O.; Andersson, S.; Heinonen, K. Effect of Vitamin D3 Supplementation in the First 2 Years of Life on Psychiatric Symptoms at Ages 6 to 8 Years. JAMA Netw. Open 2023, 6, e2314319. [Google Scholar] [CrossRef]
- Ganmaa, D.; Bromage, S.; Khudyakov, P.; Erdenenbaatar, S.; Delgererekh, B.; Martineau, A.R. Influence of Vitamin D Supplementation on Growth, Body Composition, and Pubertal Development Among School-aged Children in an Area with a High Prevalence of Vitamin D Deficiency. JAMA Pediatr. 2023, 177, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, M.; Jankowska, A.; Słomińska-Frączek, M.; Metelska, P.; Wiśniewski, P.; Socha, P.; Szlagatys-Sidorkiewicz, A. Long-Term Effects of Vitamin D Supplementation in Obese Children During Integrated Weight–Loss Programme—A Double Blind Randomized Placebo–Controlled Trial. Nutrients 2020, 12, 1093. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, M.M.; Alyousef, R.; Alturki, A.; Kofi, M.A. A systematic review: The relationship of COVID-19 to iron, zinc, and vitamin D. J. Fam. Med. Prim. Care 2023, 12, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.A.; de Silva, N.L.; Sumanatilleke, M.; de Silva, S.D.N.; Gamage, K.K.K.; Dematapitiya, C.; Kuruppu, D.C.; Ranasinghe, P.; Pathmanathan, S.; Katulanda, P. Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021, 107, 1484–1502. [Google Scholar] [CrossRef]
- Todosenko, N.; Vulf, M.; Yurova, K.; Khaziakhmatova, O.; Mikhailova, L.; Litvinova, L. Causal Links between Hypovitaminosis D and Dysregulation of the T Cell Connection of Immunity Associated with Obesity and Concomitant Pathologies. Biomedicines 2021, 9, 1750. [Google Scholar] [CrossRef]
- Koshiol, J.; Van De Wyngard, V.; McGee, E.E.; Cook, P.; Pfeiffer, R.M.; Mardones, N.; Medina, K.; Olivo, V.; Pettit, K.; Jackson, S.S.; et al. The Chile Biliary Longitudinal Study: A Gallstone Cohort. Am. J. Epidemiol. 2021, 190, 196–206. [Google Scholar] [CrossRef]
- Shabanzadeh, D.M. New determinants for gallstone disease? Dan. Med. J. 2018, 65, B5438. [Google Scholar]
- Singla, R.; Dutta, U.; Aggarwal, N.; Bhadada, S.K.; Kochhar, R.; Dhaliwal, L.K. Vitamin-D Deficiency Is Associated with Gallbladder Stasis Among Pregnant Women. Dig. Dis. Sci. 2015, 60, 2793–2799. [Google Scholar] [CrossRef]
- Jaffey, J.A.; Matheson, J.; Shumway, K.; Pacholec, C.; Ullal, T.; Van den Bossche, L.; Fieten, H.; Ringold, R.; Lee, K.J.; DeClue, A.E. Serum 25-hydroxyvitamin D concentrations in dogs with gallbladder mucocele. PLoS ONE 2020, 15, e0244102. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Raimondi, S.; Aristarco, V.; Johansson, H.A.; Bellerba, F.; Corso, F.; Gandini, S. Vitamin D Receptor Polymorphisms and Cancer. In Sunlight, Vitamin D and Skin Cancer; Reichrath, J., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–114. [Google Scholar] [CrossRef]
- El-attar, A.Z.; Hussein, S.; Salama, M.F.A.; Ibrahim, H.M.; AlKaramany, A.S.; Elsawi, M.K.; Hemeda, M.; Algazeery, A. Vitamin D receptor polymorphism and prostate cancer prognosis. Curr. Urol. 2022, 16, 246. [Google Scholar] [CrossRef]
- Latacz, M.; Rozmus, D.; Fiedorowicz, E.; Snarska, J.; Jarmołowska, B.; Kordulewska, N.; Savelkoul, H.; Cieślińska, A. Vitamin D Receptor (VDR) Gene Polymorphism in Patients Diagnosed with Colorectal Cancer. Nutrients 2021, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.M.; Esmaieli-bandboni, A.; Malekshahi, Z.V.; Sardood, M.S.; Hashemi, M.; Majidzadeh, K.; Kadkhodazadeh, M.; Esmaili, R.; Negahdari, B. Vitamin D receptor gene polymorphisms and risk of breast cancer in Iranian women. Ann. Med. Surg. 2022, 73, 103150. [Google Scholar] [CrossRef] [PubMed]
- Hochrath, K.; Stokes, C.S.; Geisel, J.; Pollheimer, M.J.; Fickert, P.; Dooley, S.; Lammert, F. Vitamin D modulates biliary fibrosis in ABCB4-deficient mice. Hepatol. Int. 2014, 8, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Yang, F.; Yan, P.; Ma, L.; Yang, L.; Li, L. Paricalcitol inhibits oxidative stress-induced cell senescence of the bile duct epithelium dependent on modulating Sirt1 pathway in cholestatic mice. Free Radic. Biol. Med. 2021, 169, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Anand, A.; Vijayashankar, A.; Sonkar, A.A.; Husain, N.; Chandra, A. Vitamin D Receptor and Role of Vitamin D Supplementation in Advanced Gallbladder Cancer: A Prospective Study from Northern India. Gulf J. Oncolog. 2019, 1, 14–20. [Google Scholar] [PubMed]
- Guo, G.-Y.; Shi, Y.-Q.; Wang, L.; Ren, X.; Han, Z.-Y.; Guo, C.-C.; Cui, L.-N.; Wang, J.-B.; Zhu, J.; Wang, N.; et al. Serum vitamin D level is associated with disease severity and response to ursodeoxycholic acid in primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2015, 42, 221–230. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Filippi, R.; Rimini, M.; Rapposelli, I.G.; Fornaro, L.; Silvestris, N.; Aldrighetti, L.; Aimar, G.; Rovesti, G.; Bartolini, G.; et al. Effects of Metformin and Vitamin D on Clinical Outcome in Cholangiocarcinoma Patients. Oncology 2021, 99, 292–299. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Soares, M.J.; Rowlands, J.; Newsholme, P. Prevailing vitamin D status influences mitochondrial and glycolytic bioenergetics in peripheral blood mononuclear cells obtained from adults. Redox Biol. 2016, 10, 243–250. [Google Scholar] [CrossRef]
- Oraei, M.; Bitarafan, S.; Mesbah-Namin, S.A.; Noori-Zadeh, A.; Mansouri, F.; Parastouei, K.; Anissian, A.; Yekaninejad, M.S.; Hajizadeh, M.; Saboor-Yaraghi, A.A. Immunomodulatory Effects of Calcitriol through DNA Methylation Alteration of FOXP3 in the CD4+ T Cells of Mice. Iran. J. Allergy Asthma Immunol. 2020, 19, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.-S.; Eelen, G.; Ferreira, G.B.; Ghesquière, B.; Cook, D.P.; Nikolic, T.; Roep, B.; Carmeliet, P.; Telang, S.; Mathieu, C.; et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism. J. Steroid Biochem. Mol. Biol. 2019, 187, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Luo, F.; Xing, J.-C.; Zhang, F.; Xu, J.-Z.; Zhang, Z.-H. 1,25(OH)2 D3 inhibited Th17 cells differentiation via regulating the NF-κB activity and expression of IL-17. Cell Prolif. 2018, 51, e12461. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Baek, S.; Hong, S.M.; Lee, J.; Jung, S.M.; Lee, J.; Cho, M.L.; Kwok, S.K.; Park, S.H. 1,25-dihydroxy Vitamin D3 and Interleukin-6 Blockade Synergistically Regulate Rheumatoid Arthritis by Suppressing Interleukin-17 Production and Osteoclastogenesis. J. Korean Med. Sci. 2020, 35, e40. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, E.; El Mourabit, H.; Jager, M.; Clavel, M.; Moog, S.; Vaquero, J.; Ledent, T.; Cadoret, A.; Gautheron, J.; Fouassier, L.; et al. Cholangiopathy aggravation is caused by VDR ablation and alleviated by VDR-independent vitamin D signaling in ABCB4 knockout mice. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166067. [Google Scholar] [CrossRef]
- Reiter, F.P.; Hohenester, S.; Nagel, J.M.; Wimmer, R.; Artmann, R.; Wottke, L.; Makeschin, M.-C.; Mayr, D.; Rust, C.; Trauner, M.; et al. 1,25-(OH)2-vitamin D₃ prevents activation of hepatic stellate cells in vitro and ameliorates inflammatory liver damage but not fibrosis in the Abcb4−/− model. Biochem. Biophys. Res. Commun. 2015, 459, 227–233. [Google Scholar] [CrossRef]
- Chen, B.; Jin, L. Low serum level of 25-OH vitamin D relates to Th17 and treg changes in colorectal cancer patients. Immun. Inflamm. Dis. 2022, 10, e723. [Google Scholar] [CrossRef]
- Wu, X.; Hu, W.; Lu, L.; Zhao, Y.; Zhou, Y.; Xiao, Z.; Zhang, L.; Zhang, H.; Li, X.; Li, W.; et al. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm. Sin. B 2019, 9, 203–219. [Google Scholar] [CrossRef]
- Yu, M.; Wu, H.; Wang, J.; Chen, X.; Pan, J.; Liu, P.; Zhang, J.; Chen, Y.; Zhu, W.; Tang, C.; et al. Vitamin D receptor inhibits EMT via regulation of the epithelial mitochondrial function in intestinal fibrosis. J. Biol. Chem. 2021, 296, 100531. [Google Scholar] [CrossRef]
- Quigley, M.; Rieger, S.; Capobianco, E.; Wang, Z.; Zhao, H.; Hewison, M.; Lisse, T.S. Vitamin D Modulation of Mitochondrial Oxidative Metabolism and mTOR Enforces Stress Adaptations and Anticancer Responses. JBMR Plus 2022, 6, e10572. [Google Scholar] [CrossRef] [PubMed]
- Moz, S.; Contran, N.; Facco, M.; Trimarco, V.; Plebani, M.; Basso, D. Vitamin D Prevents Pancreatic Cancer-Induced Apoptosis Signaling of Inflammatory Cells. Biomolecules 2020, 10, E1055. [Google Scholar] [CrossRef] [PubMed]
- Wensheng, L.; Bo, Z.; Qiangsheng, H.; Wenyan, X.; Shunrong, J.; Jin, X.; Quanxing, N.; Xianjun, Y.; Xiaowu, X. MBD1 promotes the malignant behavior of gallbladder cancer cells and induces chemotherapeutic resistance to gemcitabine. Cancer Cell Int. 2019, 19, 232. [Google Scholar] [CrossRef]
- Chen, K.; Tang, H.; Zhu, P.; Ye, J.; Liu, D.; Pu, Y.; Zhang, L.; Zhai, W. Interleukin 17A promotes gallbladder cancer invasiveness via ERK/NF-κB signal pathway mediated epithelial-to-mesenchymal transition. J. Cancer 2020, 11, 4406–4412. [Google Scholar] [CrossRef]
- Qianmei, Y.; Zehong, S.; Guang, W.; Hui, L.; Lian, G. Recent advances in the role of Th17/Treg cells in tumor immunity and tumor therapy. Immunol. Res. 2021, 69, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Gentile, A.; de Angelis, C.; Montò, T.; Patalano, R.; Colao, A.; Pivonello, R.; Pivonello, C. Vitamin D-Induced Molecular Mechanisms to Potentiate Cancer Therapy and to Reverse Drug-Resistance in Cancer Cells. Nutrients 2020, 12, 1798. [Google Scholar] [CrossRef]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: A crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun. Signal. 2024, 22, 99. [Google Scholar] [CrossRef]
- Beckermann, K.E.; Dudzinski, S.O.; Rathmell, J.C. Dysfunctional T cell metabolism in the tumor microenvironment. Cytokine Growth Factor Rev. 2017, 35, 7–14. [Google Scholar] [CrossRef]
- Raychaudhuri, D.; Bhattacharya, R.; Sinha, B.P.; Liu, C.S.C.; Ghosh, A.R.; Rahaman, O.; Bandopadhyay, P.; Sarif, J.; D’Rozario, R.; Paul, S.; et al. Lactate Induces Pro-tumor Reprogramming in Intratumoral Plasmacytoid Dendritic Cells. Front. Immunol. 2019, 10, 1878. [Google Scholar] [CrossRef]
- Angelin, A.; Gil-de-Gómez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J.; Kopinski, P.K.; Wang, L.; et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017, 25, 1282–1293.e7. [Google Scholar] [CrossRef]
- Rao, D.; Verburg, F.; Renner, K.; Peeper, D.S.; Lacroix, R.; Blank, C.U. Metabolic profiles of regulatory T cells in the tumour microenvironment. Cancer Immunol. Immunother. CII 2021, 70, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Yin, N.; Peng, M.; Stamatiades, E.G.; Chhangawala, S.; Shyu, A.; Li, P.; Zhang, X.; Do, M.H.; Capistrano, K.J.; et al. Glycolytic ATP fuels phosphoinositide 3-kinase signaling to support effector T helper 17 cell responses. Immunity 2021, 54, 976–987.e7. [Google Scholar]
- Macciò, A.; Madeddu, C. Blocking inflammation to improve immunotherapy of advanced cancer. Immunology 2020, 159, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Mimura, K.; Ashizawa, M.; Okayama, H.; Endo, E.; Saito, K.; Sakamoto, W.; Fujita, S.; Endo, H.; Saito, M.; et al. Characterization of tumor-infiltrating immune cells in relation to microbiota in colorectal cancers. Cancer Immunol. Immunother. 2020, 69, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Wang, X.; Zhang, C.; Hou, M.; Liu, Y. Integration of single-cell RNA sequencing and bulk RNA transcriptome sequencing reveals a heterogeneous immune landscape and pivotal cell subpopulations associated with colorectal cancer prognosis. Front. Immunol. 2023, 14, 1184167. [Google Scholar] [CrossRef]
- Kedmi, R.; Najar, T.A.; Mesa, K.R.; Grayson, A.; Kroehling, L.; Hao, Y.; Hao, S.; Pokrovskii, M.; Xu, M.; Talbot, J.; et al. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature 2022, 610, 737–743. [Google Scholar] [CrossRef]
- Sui, H.; Deng, W.; Chai, Q.; Han, B.; Zhang, Y.; Wei, Z.; Li, Z.; Wang, T.; Feng, J.; Yuan, M.; et al. YTE-17 inhibits colonic carcinogenesis by resetting antitumor immune response via Wnt5a/JNK mediated metabolic signaling. J. Pharm. Anal. 2024, 14, 100901. [Google Scholar] [CrossRef]
- Gilad, Y.; Shimon, O.; Han, S.J.; Lonard, D.M.; O’Malley, B.W. Steroid receptor coactivators in Treg and Th17 cell biology and function. Front. Immunol. 2024, 15, 1389041. [Google Scholar] [CrossRef]
- Si, F.; Liu, X.; Tao, Y.; Zhang, Y.; Ma, F.; Hsueh, E.C.; Puram, S.V.; Peng, G. Blocking senescence and tolerogenic function of dendritic cells induced by γδ Treg cells enhances tumor-specific immunity for cancer immunotherapy. J. Immunother. Cancer 2024, 12, e008219. [Google Scholar] [CrossRef]
- Rocha Martins, P.; Luciano Pereira Morais, K.; de Lima Galdino, N.A.; Jacauna, A.; Paula, S.O.C.; Magalhães, W.C.S.; Zuccherato, L.W.; Campos, L.S.; Salles, P.G.O.; Gollob, K.J. Linking tumor immune infiltrate and systemic immune mediators to treatment response and prognosis in advanced cervical cancer. Sci. Rep. 2023, 13, 22634. [Google Scholar] [CrossRef]
- Pan, H.; Wei, W.; Fu, G.; Pan, J.; Jin, B. LINC00941 Promotes Cell Malignant Behavior and Is One of Five Costimulatory Molecule-Related lncRNAs That Predict Prognosis in Renal Clear Cell Carcinoma. Medicina 2023, 59, 187. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Huang, Y.; Ke, S.; Xie, F.; Li, D.; Li, B.; Lu, H. Increased infiltration of CD4+IL-17A+FOXP3+ T cells in Helicobacter pylori-induced gastritis. Eur. J. Immunol. 2024, 54, 2350662. [Google Scholar] [CrossRef] [PubMed]
- Minohara, K.; Imai, M.; Matoba, T.; Wing, J.B.; Shime, H.; Odanaka, M.; Uraki, R.; Kawakita, D.; Toyama, T.; Takahashi, S.; et al. Mature dendritic cells enriched in regulatory molecules may control regulatory T cells and the prognosis of head and neck cancer. Cancer Sci. 2023, 114, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Feng, P.; Pang, J.; Zou, D.; Li, X.; Geng, C.; Li, L.; Min, J.; Shi, J. A Novel HCC Prognosis Predictor EEF1E1 Is Related to Immune Infiltration and May Be Involved in EEF1E1/ATM/p53 Signaling. Front. Oncol. 2021, 11, 700972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zuo, C.; Li, Y.; Liu, L.; Yang, B.; Xia, J.; Cui, J.; Xu, K.; Wu, X.; Gong, W.; et al. Single-cell characterization of infiltrating T cells identifies novel targets for gallbladder cancer immunotherapy. Cancer Lett. 2024, 586, 216675. [Google Scholar] [CrossRef]
- Albrecht, T.; Brinkmann, F.; Albrecht, M.; Lonsdorf, A.S.; Mehrabi, A.; Hoffmann, K.; Kulu, Y.; Charbel, A.; Vogel, M.N.; Rupp, C.; et al. Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer. Cancers 2021, 13, 1682. [Google Scholar] [CrossRef]
- Kim, H.-D.; Kim, J.H.; Ryu, Y.-M.; Kim, D.; Lee, S.; Shin, J.; Hong, S.-M.; Kim, K.-H.; Jung, D.-H.; Song, G.-W.; et al. Spatial Distribution and Prognostic Implications of Tumor-Infiltrating FoxP3− CD4+ T Cells in Biliary Tract Cancer. Cancer Res. Treat. 2021, 53, 162–171. [Google Scholar] [CrossRef]
- Oguro, S.; Ino, Y.; Shimada, K.; Hatanaka, Y.; Matsuno, Y.; Esaki, M.; Nara, S.; Kishi, Y.; Kosuge, T.; Hiraoka, N. Clinical significance of tumor-infiltrating immune cells focusing on BTLA and Cbl-b in patients with gallbladder cancer. Cancer Sci. 2015, 106, 1750–1760. [Google Scholar] [CrossRef]
- Patil, P.A.; Lombardo, K.; Cao, W. Immune Microenvironment in Gallbladder Adenocarcinomas. Appl. Immunohistochem. Mol. Morphol. AIMM 2021, 29, 557–563. [Google Scholar] [CrossRef]
- Fluxá, P.; Rojas-Sepúlveda, D.; Gleisner, M.A.; Tittarelli, A.; Villegas, P.; Tapia, L.; Rivera, M.T.; López, M.N.; Catán, F.; Uribe, M.; et al. High CD8+ and absence of Foxp3+ T lymphocytes infiltration in gallbladder tumors correlate with prolonged patients survival. BMC Cancer 2018, 18, 243. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.; Li, J.; Bo, X.; Wang, J.; Nan, L.; Wang, C.; Ba, Q.; Liu, H.; Wang, H. Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin. Transl. Med. 2021, 11, e462. [Google Scholar] [CrossRef]
- Zhang, Y.; You, W.-H.; Li, X.; Wang, P.; Sha, B.; Liang, Y.; Qiu, J.; Zhou, J.; Hu, H.; Lu, L. Single-cell RNA-seq reveals transcriptional landscape and intratumor heterogenicity in gallbladder cancer liver metastasis microenvironment. Ann. Transl. Med. 2021, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Kobayashi, S.; Gotoh, K.; Kubo, M.; Hayashi, K.; Iwagami, Y.; Yamada, D.; Akita, H.; Noda, T.; Asaoka, T.; et al. Heterogeneity of Treg/Th17 According to Cancer Progression and Modification in Biliary Tract Cancers via Self-Producing Cytokines. Dig. Dis. Sci. 2020, 65, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Abaurrea, A.; Araujo, A.M.; Caffarel, M.M. The Role of the IL-6 Cytokine Family in Epithelial-Mesenchymal Plasticity in Cancer Progression. Int. J. Mol. Sci. 2021, 22, 8334. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Wang, Z.; Fu, X. Effect of diabetes mellitus on survival in patients with gallbladder Cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 689. [Google Scholar] [CrossRef]
- Patil, R.S.; Shah, S.U.; Shrikhande, S.V.; Goel, M.; Dikshit, R.P.; Chiplunkar, S.V. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int. J. Cancer 2016, 139, 869–881. [Google Scholar] [CrossRef]
- Wang, M.; Deng, C.; Yang, C.; Yan, M.; Lu, H.; Zhang, Y.; Liu, H.; Tong, Z.; Ma, J.; Wang, J.; et al. Unraveling temporal and spatial biomarkers of epithelial-mesenchymal transition in colorectal cancer: Insights into the crucial role of immunosuppressive cells. J. Transl. Med. 2023, 21, 794. [Google Scholar] [CrossRef]
- Oh, E.; Hong, J.; Yun, C.-O. Regulatory T Cells Induce Metastasis by Increasing Tgf-β and Enhancing the Epithelial–Mesenchymal Transition. Cells 2019, 8, 1387. [Google Scholar] [CrossRef]
- Huang, A.-H.; Wang, H.-B.; Wu, Z.-F.; Wang, Y.-H.; Hu, B.; Jiang, Z.-N.; Jin, M.; Wang, L.-B.; Gao, Y.-B. Infiltrating regulatory T cells promote invasiveness of liver cancer cells via inducing epithelial-mesenchymal transition. Transl. Cancer Res. 2019, 8, 2405–2415. [Google Scholar] [CrossRef]
- Wu, G.; Yang, Y.; Zhu, Y.; Li, Y.; Zhai, Z.; An, L.; Liu, M.; Zheng, Y.; Wang, Y.; Zhou, Y.; et al. Comprehensive Analysis to Identify the Epithelial-Mesenchymal Transition-Related Immune Signatures as a Prognostic and Therapeutic Biomarkers in Hepatocellular Carcinoma. Front. Surg. 2021, 8, 742443. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Y.; Tang, H.; Wang, H.; Jiang, E.; Shao, Z.; Liu, K.; Zhou, X.; Shang, Z. Melatonin inhibits EMT and PD-L1 expression through the ERK1/2/FOSL1 pathway and regulates anti-tumor immunity in HNSCC. Cancer Sci. 2022, 113, 2232–2245. [Google Scholar] [CrossRef]
- Brouwer, T.; Ijsselsteijn, M.; Oosting, J.; Ruano, D.; van der Ploeg, M.; Dijk, F.; Bonsing, B.; Fariña, A.; Morreau, H.; Vahrmeijer, A.; et al. A Paradoxical Role for Regulatory T Cells in the Tumor Microenvironment of Pancreatic Cancer. Cancers 2022, 14, 3862. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Sarkar, J.; Wu, R.; Tokumaru, Y.; Yan, L.; Nakagawa, K.; Ishibe, A.; Matsuyama, R.; Endo, I.; Takabe, K. Intratumoral density of regulatory T cells is a predictor of host immune response and chemotherapy response in colorectal cancer. Am. J. Cancer Res. 2022, 12, 490–503. [Google Scholar] [PubMed]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A.; Cook, N.R.; Chen, L.-J.; Cheng, T.-Y.D.; Hantunen, S.; Lee, I.-M.; et al. Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, M.M.; Bindels, L.B. Crosstalk between bile acid-activated receptors and microbiome in entero-hepatic inflammation. Trends Mol. Med. 2022, 28, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, D.; Kong, B.; Taylor, R.E.; Brinker, A.; Goedken, M.; Buckley, B.; Guo, G.L. Bile acid homeostasis in female mice deficient in Cyp7a1 and Cyp27a1. Acta Pharm. Sin. B 2021, 11, 3847–3856. [Google Scholar] [CrossRef]
- Sharma, V.; Chauhan, V.S.; Nath, G.; Kumar, A.; Shukla, V.K. Role of bile bacteria in gallbladder carcinoma. Hepatogastroenterology 2007, 54, 1622–1625. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartes-Velásquez, R.; Vera, A.; Torres-Quevedo, R.; Medrano-Díaz, J.; Pérez, A.; Muñoz, C.; Carrillo-Bestagno, H.; Nova-Lamperti, E. The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer. Nutrients 2024, 16, 4134. https://doi.org/10.3390/nu16234134
Cartes-Velásquez R, Vera A, Torres-Quevedo R, Medrano-Díaz J, Pérez A, Muñoz C, Carrillo-Bestagno H, Nova-Lamperti E. The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer. Nutrients. 2024; 16(23):4134. https://doi.org/10.3390/nu16234134
Chicago/Turabian StyleCartes-Velásquez, Ricardo, Agustín Vera, Rodrigo Torres-Quevedo, Jorge Medrano-Díaz, Andy Pérez, Camila Muñoz, Hernán Carrillo-Bestagno, and Estefanía Nova-Lamperti. 2024. "The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer" Nutrients 16, no. 23: 4134. https://doi.org/10.3390/nu16234134
APA StyleCartes-Velásquez, R., Vera, A., Torres-Quevedo, R., Medrano-Díaz, J., Pérez, A., Muñoz, C., Carrillo-Bestagno, H., & Nova-Lamperti, E. (2024). The Immunomodulatory Role of Vitamin D in Regulating the Th17/Treg Balance and Epithelial–Mesenchymal Transition: A Hypothesis for Gallbladder Cancer. Nutrients, 16(23), 4134. https://doi.org/10.3390/nu16234134






