The Potential Role of Dietary Polyphenols in the Prevention and Treatment of Acute Leukemia
Highlights
- The consumption of dietary polyphenols may be effective in the prevention of acute leukemia.
- Dietary polyphenols, when combined with standard treatments for acute leukemia, can mitigate the associated side effects.
- The encapsulation of dietary polyphenols, either in combination with standard treatments or independently, can facilitate their intake and serve as a dietary supplement for preventive purposes.
- Due to the progressive nature of acute leukemia, the therapeutic application of dietary polyphenols has been largely overlooked in clinical trials.
Abstract
:1. Introduction
2. Quantitative Content of Dietary Polyphenols
3. Antioxidant Properties of Polyphenols
3.1. Antioxidant Properties in AML
3.2. Antioxidant Properties in ALL
4. Anticancer Properties of Polyphenols
4.1. Anticancer Properties in AML
4.2. Anticancer Properties in ALL
5. Anti-Inflammatory Properties of Polyphenols
5.1. Anti-Inflammatory Properties in AML
5.2. Anti-Inflammatory Properties in ALL
6. Microencapsulated and Nano-Encapsulated Forms of Dietary Polyphenols’ Impact on AML and ALL
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tebbi, C.K. Etiology of acute leukemia: A review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Karunarathna, I.; De Alvis, K.; Gunasena, P.; Gunathilake, S.; Leukemia: Classification, Risk Factors, and Diagnostic Challenges. ResearchGate. 2024. Available online: https://www.researchgate.net/publication/381847043_Leukemia_Classification_Risk_Factors_and_Diagnostic_Challenges (accessed on 27 October 2024).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Wachter, F.; Pikman, Y. Pathophysiology of acute myeloid leukemia. Acta Haematol. 2024, 147, 229–246. [Google Scholar] [CrossRef]
- Aureli, A.; Marziani, B.; Venditti, A.; Sconocchia, T.; Sconocchia, G. Acute lymphoblastic leukemia immunotherapy treatment: Now, next, and beyond. Cancers 2023, 15, 3346. [Google Scholar] [CrossRef]
- Roman Diaz, J.L.; Martinez, M.V.; Khimani, F. New Approaches for the Treatment of AML beyond the 7+3 Regimen: Current Concepts and New Approaches. Cancers 2024, 16, 677. [Google Scholar] [CrossRef]
- Chergui, A.; Reagan, J.L. Immunotherapy in acute leukemias: Past success paves the way for future progress. Cancers 2023, 15, 4137. [Google Scholar] [CrossRef] [PubMed]
- Ekpa, Q.L.; Akahara, P.C.; Anderson, A.M.; Adekoya, O.O.; Ajayi, O.O.; Alabi, P.O.; Okobi, O.E.; Jaiyeola, O.; Ekanem, M.S. A Review of Acute Lymphocytic Leukemia (ALL) in the Pediatric Population: Evaluating Current Trends and Changes in Guidelines in the Past Decade. Cureus 2023, 15, e49930. [Google Scholar] [CrossRef]
- Rafiq, S.; Raza, M.H.; Younas, M.; Naeem, F.; Adeeb, R.; Iqbal, J.; Anwar, P.; Sajid, U.; Manzoor, H.M. Molecular targets of curcumin and future therapeutic role in leukemia. J. Biosci. Med. 2018, 6, 33. [Google Scholar] [CrossRef]
- Récher, C. Clinical implications of inflammation in acute myeloid leukemia. Front. Oncol. 2021, 11, 623952. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhang, N.-J.; Zhang, L.-J. Oxidative stress in leukemia and antioxidant treatment. Chin. Med. J. 2021, 134, 1897–1907. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Keramat, S.; Sharebiani, H.; Patel, M.; Fazeli, B.; Stanek, A. The potential role of antioxidants in the treatment of peripheral arterial disease: A systematic review. Antioxidants 2022, 11, 2126. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Cipolletti, M.; Fernandez, V.S.; Montalesi, E.; Marino, M.; Fiocchetti, M. Beyond the antioxidant activity of dietary polyphenols in cancer: The modulation of estrogen receptors (ERs) signaling. Int. J. Mol. Sci. 2018, 19, 2624. [Google Scholar] [CrossRef]
- Alaswad, H.A.; Mahbub, A.A.; Le Maitre, C.L.; Jordan-Mahy, N. Molecular action of polyphenols in leukaemia and their therapeutic potential. Int. J. Mol. Sci. 2021, 22, 3085. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Ang, B.S. Polyphenols as key players for the antileukaemic effects of propolis. Evid.-Based Complement. Altern. Med. 2014, 2014, 371730. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Aatif, M.; Muteeb, G.; Khan, K.; Siddiqui, F.A. Dietary Polyphenols, Plant Metabolites, and Allergic Disorders: A Comprehensive Review. Pharmaceuticals 2024, 17, 670. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in plants: Structure, biosynthesis, abiotic stress regulation, and practical applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Yan, H.; Han, C.; Wang, W.; Tian, Y.; Chen, X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264.7 macrophages. Int. J. Biol. Macromol. 2014, 69, 382–387. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Trinovani, E.; Prawira-Atmaja, M.I.; Kusmiyati, M.; Harianto, S.; Shabri; Maulana, H. Total polyphenols and antioxidant activities of green tea powder from GMB 7 and GMB 9 tea clones. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012113. [Google Scholar] [CrossRef]
- Rechner, A.; Wagner, E.; van Buren, L.; van de Put, F.; Wiseman, S.; Rice-Evans, C. Black tea represents a major source of dietary phenolics among regular tea drinkers. Free Radic. Res. 2002, 36, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lee, H.J.; Lee, C.Y. Antioxidant activity of black tea vs. green tea. J. Nutr. 2002, 132, 785. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Conigrave, K.M.; Mittleman, M.A.; Camargo, C.A., Jr.; Stampfer, M.J.; Willett, W.C.; Rimm, E.B. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N. Engl. J. Med. 2003, 348, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Sharebiani, H.; Keramat, S.; Chavoshan, A.; Fazeli, B.; Stanek, A. The influence of antioxidants on oxidative stress-induced vascular aging in obesity. Antioxidants 2023, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, K.; Fakhar, F.; Keramat, S.; Stanek, A. The Role of Antioxidants in the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease: A Systematic Review. Antioxidants 2024, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Huang, J.; de Paulis, T.; May, J.M. Antioxidant effects of dihydrocaffeic acid in human EA. hy926 endothelial cells. J. Nutr. Biochem. 2004, 15, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Laddomada, B.; Blando, F.; De Santis, S.; Verna, G.; Chieppa, M.; Santino, A. The chelating ability of plant polyphenols can affect iron homeostasis and gut microbiota. Antioxidants 2023, 12, 630. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Halake, K.; Birajdar, M.; Lee, J. Structural implications of polyphenolic antioxidants. J. Ind. Eng. Chem. 2016, 35, 1–7. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- de Graft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti-and pro-oxidant actions. Molecules 2016, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Bin Dukhyil, A.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.Y.; Zubair, H.; Ullah, M.F.; Ahmad, A.; Hadi, S.M. A prooxidant mechanism for the anticancer and chemopreventive properties of plant polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Szostak-Węgierek, D. Plant flavonoids in health, prevention, and treatment of chronic diseases. In Nutritional Antioxidant Therapies: Treatments and Perspectives; Springer: Cham, Switzerland, 2017; pp. 347–376. [Google Scholar]
- Zhou, F.; Pan, Y.; Wei, Y.; Zhang, R.; Bai, G.; Shen, Q.; Meng, S.; Le, X.F.; Andreeff, M.; Claret, F.X. Jab1/Csn5-Thioredoxin Signaling in Relapsed Acute Monocytic Leukemia under Oxidative Stress. Clin. Cancer Res. 2017, 23, 4450–4461. [Google Scholar] [CrossRef] [PubMed]
- Mosa, A.A.; Ali, G.S.; Selamoglu, Z. Survey on the Oxidative Stress Status and the Role of Antioxidants in Acute Myeloid Leukemia Therapy. J. Pharm. Care 2023, 11, 165–172. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, Z.; Găman, M.-A.; Xu, L.; Li, J. NADPH oxidase mediated oxidative stress signaling in FLT3-ITD acute myeloid leukemia. Cell Death Discov. 2023, 9, 208. [Google Scholar] [CrossRef]
- Dávalos, A.; de la Peña, G.; Sánchez-Martín, C.C.; Guerra, M.T.; Bartolomé, B.; Lasunción, M.A. Effects of red grape juice polyphenols in NADPH oxidase subunit expression in human neutrophils and mononuclear blood cells. Br. J. Nutr. 2009, 102, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Zhang, J. Studies on the prevention of cancer and cardiometabolic diseases by tea: Issues on mechanisms, effective doses, and toxicities. J. Agric. Food Chem. 2018, 67, 5446–5456. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Miyakawa, Y.; Kinjo, K.; Yamada, T.; Hozumi, N.; Ikeda, Y.; Kizaki, M. Catechin, a green tea component, rapidly induces apoptosis of myeloid leukemic cells via modulation of reactive oxygen species production in vitro and inhibits tumor growth in vivo. Haematologica 2005, 90, 317–325. [Google Scholar] [PubMed]
- Torello, C.O.; Shiraishi, R.N.; Della Via, F.I.; Castro, T.C.L.; Longhini, A.L.; Santos, I.; Bombeiro, A.L.; Silva, C.L.A.; Queiroz, M.L.S.; Rego, E.M.; et al. Reactive oxygen species production triggers green tea-induced anti-leukaemic effects on acute promyelocytic leukaemia model. Cancer Lett. 2018, 414, 116–126. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.S.; Xu, P.P.; Qian, Y.; Wang, A.H.; Xiao, D.; Zhao, Y.; Sheng, Y.; Wen, X.Q.; Zhao, W.L. Catechins induced acute promyelocytic leukemia cell apoptosis and triggered PML-RARα oncoprotein degradation. J. Hematol. Oncol. 2014, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.; Germon, Z.P.; Chamberlain, J.; Sillar, J.R.; Nixon, B.; Dun, M.D. Reactive oxygen species in acute lymphoblastic leukaemia: Reducing radicals to refine responses. Antioxidants 2021, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.B.; Carrilho, E.; Nixdorf, S.L. Evaluation of oxidative stress in patients with acute lymphoblastic leukemia: Experimental evidence of the efficacy of MDA as cancer biomarker in young patients. J. Braz. Chem. Soc. 2018, 29, 615–621. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. A novel function of red wine polyphenols in humans: Prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Hayakawa, S.; Miyoshi, N. Involvement of microRNA modifications in anticancer effects of major polyphenols from green tea, coffee, wine, and curry. Crit. Rev. Food Sci. Nutr. 2023, 63, 7148–7179. [Google Scholar] [CrossRef]
- Lee, K.W.; Hur, H.J.; Lee, H.J.; Lee, C.Y. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J. Agric. Food Chem. 2005, 53, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant effects of epigallocatechin-3-gallate in health benefits and potential adverse effect. Oxidative Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W.; Lee, C.Y. Phenolic compounds and their changes in apples during maturation and cold storage. J. Agric. Food Chem. 1990, 38, 945–948. [Google Scholar] [CrossRef]
- Mayr, U.; Santos-Buelga, C.; Treutter, D.; Santos-Buelga, C.; Bauer, H.; Feucht, W. Developmental changes in the phenol concentrations of ‘Golden Delicious’ apple fruits and leaves. Phytochemistry 1995, 38, 1151–1155. [Google Scholar] [CrossRef]
- Szeto, Y.-T.; Benzie, I.F. Effects of dietary antioxidants on human DNA ex vivo. Free Radic. Res. 2002, 36, 113–118. [Google Scholar] [CrossRef]
- Oleaga, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Coffee Polyphenols Change the Expression of STAT5B and ATF-2 Modifying Cyclin D1 Levels in Cancer Cells. Oxidative Med. Cell. Longev. 2012, 2012, 390385. [Google Scholar] [CrossRef]
- Milne, E.; Royle, J.A.; Bennett, L.C.; de Klerk, N.H.; Bailey, H.D.; Bower, C.; Miller, M.; Attia, J.; Scott, R.J.; Kirby, M.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: Results from an Australian case–control study. Cancer Causes Control 2011, 22, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Dessypris, N.; Clavel, J.; Metayer, C.; Erdmann, F.; Orsi, L.; Kang, A.Y.; Schüz, J.; Bonaventure, A.; Greenop, K.R.; et al. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: A Childhood Leukemia International Consortium (CLIC) study. Cancer Epidemiol. 2019, 62, 101581. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, M.; Bilotto, S.; Tedesco, I.; Laratta, B.; Russo, G.L. Dietary polyphenols in cancer prevention: The example of the flavonoid quercetin in leukemia. Ann. N. Y. Acad. Sci. 2012, 1259, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sharif, T.; Auger, C.; Alhosin, M.; Ebel, C.; Achour, M.; Etienne-Selloum, N.; Fuhrmann, G.; Bronner, C.; Schini-Kerth, V.B. Red wine polyphenols cause growth inhibition and apoptosis in acute lymphoblastic leukaemia cells by inducing a redox-sensitive up-regulation of p73 and down-regulation of UHRF1. Eur. J. Cancer 2010, 46, 983–994. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Sharif, T.; Auger, C.; Abbas, M.; Fuhrmann, G.; Schini-Kerth, V.B. Anthocyanin-rich bilberry extract induces apoptosis in acute lymphoblastic leukemia cells via redox-sensitive epigenetic modifications. J. Funct. Foods 2018, 44, 227–234. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20 (Suppl. S2), 1700–1741. [Google Scholar] [CrossRef]
- Pan, X.A.; Chang, Y.; Ruan, G.; Zhou, S.; Jiang, H.; Jiang, Q.; Zhao, X.S. TET2 mutations contribute to adverse prognosis in acute myeloid leukemia (AML): Results from a comprehensive analysis of 502 AML cases and the Beat AML public database. Clin. Exp. Med. 2024, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Smith-Díaz, C.C.; Magon, N.J.; McKenzie, J.L.; Hampton, M.B.; Vissers, M.C.M.; Das, A.B. Ascorbate Inhibits Proliferation and Promotes Myeloid Differentiation in TP53-Mutant Leukemia. Front. Oncol. 2021, 11, 709543. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Imbert, V.; Peyron, J.F. NF-κB in Hematological Malignancies. Biomedicines 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor. Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Bi, C.; Janakakumara, J.V.; Liu, S.-C.; Chng, W.-J.; Tay, K.-G.; Poon, L.-F.; Xie, Z.; Palaniyandi, S.; Yu, H.; et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood 2009, 113, 4052–4062. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002, 180, 91–101. [Google Scholar] [CrossRef]
- Takahashi, S.; Harigae, H.; Ishii, K.K.; Inomata, M.; Fujiwara, T.; Yokoyama, H.; Kaku, M. Over-expression of Flt3 induces NF-kappaB pathway and increases the expression of IL-6. Leuk. Res. 2005, 29, 893–899. [Google Scholar] [CrossRef]
- Ly, B.T.K.; Chi, H.T.; Yamagishi, M.; Kano, Y.; Hara, Y.; Nakano, K.; Sato, Y.; Watanabe, T. Inhibition of FLT3 expression by green tea catechins in FLT3 mutated-AML cells. PLoS ONE 2013, 8, e66378. [Google Scholar] [CrossRef]
- Dahlawi, H.; Jordan-Mahy, N.; Clench, M.R.; Le Maitre, C.L. Bioactive actions of pomegranate fruit extracts on leukemia cell lines in vitro hold promise for new therapeutic agents for leukemia. Nutr. Cancer 2012, 64, 100–110. [Google Scholar] [CrossRef]
- Fabiani, R.; De Bartolomeo, A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; Di Saverio, C.; Morozzi, G. Virgin olive oil phenols inhibit proliferation of human promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J. Nutr. 2006, 136, 614–619. [Google Scholar] [CrossRef]
- Lanoue, L.; Green, K.K.; Kwik-Uribe, C.; Keen, C.L. Dietary factors and the risk for acute infant leukemia: Evaluating the effects of cocoa-derived flavanols on DNA topoisomerase activity. Exp. Biol. Med. 2010, 235, 77–89. [Google Scholar] [CrossRef] [PubMed]
- McGill, C.M.; Brown, T.J.; Cheng, Y.-Y.; Fisher, L.N.; Shanmugavelandy, S.S.; Gustafson, S.J.; Dunlap, K.L.; Lila, M.A.; Kester, M.; Toran, P.T.; et al. Therapeutic Effect of Blueberry Extracts for Acute Myeloid Leukemia. Int. J. Biopharm. Sci. 2018, 1, 102. [Google Scholar]
- Garcia-Martinez, D.J.; Calzada Funes, J.; Martin Saborido, C.; Santos, C. Grape Polyphenols to Arrest in Vitro Proliferation of Human Leukemia Cells: A Systematic Review and Meta-analysis. Food Rev. Int. 2022, 38, 402–419. [Google Scholar] [CrossRef]
- Zhu, G.; Shen, Q.; Jiang, H.; Ji, O.; Zhu, L.; Zhang, L. Curcumin inhibited the growth and invasion of human monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP signalling. Pharm. Biol. 2020, 58, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ning, Y.; Zeng, G.; Zhou, C.; Ding, X. Curcumin promotes cell cycle arrest and apoptosis of acute myeloid leukemia cells by inactivating AKT. Oncol. Rep. 2021, 45, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1485. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; Detakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Shi, H.; Li, X.Y.; Chen, Y.; Zhang, X.; Wu, Y.; Wang, Z.X.; Chen, P.H.; Dai, H.Q.; Feng, J.; Chatterjee, S.; et al. Corrigendum: Quercetin Induces Apoptosis Via Downregulation of VEGF/Akt Signaling Pathway in Acute Myeloid Leukemia Cells. Front. Pharmacol. 2020, 11, 640750. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Yamagishi, K.; Iso, H.; Tamakoshi, A. Green tea consumption and risk of hematologic neoplasms: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Cancer Causes Control 2019, 30, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Uckun, F.M.; Cogle, C.R.; Lin, T.L.; Qazi, S.; Trieu, V.N.; Schiller, G.; Watts, J.M. A Phase 1B Clinical Study of Combretastatin A1 Diphosphate (OXi4503) and Cytarabine (ARA-C) in Combination (OXA) for Patients with Relapsed or Refractory Acute Myeloid Leukemia. Cancers 2019, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Zhang, Y.; Seeram, N.P.; Storms, D.H. Berry fruit extracts inhibit growth and induce apoptosis of high-risk acute lymphoblastic leukemia cells in vitro. J. Funct. Foods 2010, 2, 187–195. [Google Scholar] [CrossRef]
- Shanafelt, T.; Lee, Y.; Call, T.; Nowakowski, G.; Dingli, D.; Zent, C.; Kay, N. Clinical effects of oral green tea extracts in four patients with low grade B-cell malignancies. Leuk. Res. 2006, 30, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Campo, C.; Gangemi, S.; Pioggia, G.; Allegra, A. Beneficial Effect of Olive Oil and Its Derivates: Focus on Hematological Neoplasm. Life 2024, 14, 583. [Google Scholar] [CrossRef]
- Parra-Perez, A.M.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Bonel-Pérez, G.C.; Carrasco-Díaz, L.M.; Mokhtari, K.; García-Salguero, L.; Lupiáñez, J.A.; Rufino-Palomares, E.E. Involvement of the PI3K/AKT Intracellular Signaling Pathway in the AntiCancer Activity of Hydroxytyrosol, a Polyphenol from Olea europaea, in Hematological Cells and Implication of HSP60 Levels in Its Anti-Inflammatory Activity. Int. J. Mol. Sci. 2022, 23, 7053. [Google Scholar] [CrossRef]
- Alkhouli, M.; Laflouf, M.; Alhaddad, M. Evaluation of the effectiveness of olive oil to prevent chemotherapy induced oral mucositis: A randomized controlled clinical trial. Pediatr. Dent. J. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Ebeid, F.; El Gendy, Y.; Mehanna, N.; Aly, N.; Elaziz, S.A.; Emam, E. ALL-066 Safety and Efficacy of Turmeric in Children With Acute Lymphoblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2022, 22, S192. [Google Scholar] [CrossRef]
- Karasawa, K.; Uzuhashi, Y.; Hirota, M.; Otani, H. A matured fruit extract of date palm tree (Phoenix dactylifera L.) stimulates the cellular immune system in mice. J. Agric. Food Chem. 2011, 59, 11287–11293. [Google Scholar] [CrossRef]
- John, C.M.; Sandrasaigaran, P.; Tong, C.K.; Adam, A.; Ramasamy, R. Immunomodulatory activity of polyphenols derived from Cassia auriculata flowers in aged rats. Cell. Immunol. 2011, 271, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Keramat, S.; Karahan, O.; Patel, M.; Fazeli, B. Acute phase reactant proteins in Buerger’s disease: Is it a systemic disease? Vascular 2023, 31, 473–476. [Google Scholar] [CrossRef]
- Camacho-Barquero, L.; Villegas, I.; Sánchez-Calvo, J.M.; Talero, E.; Sánchez-Fidalgo, S.; Motilva, V.; de la Lastra, C.A. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mohar, D.S.; Malik, S. The sirtuin system: The holy grail of resveratrol? J. Clin. Exp. Cardiol. 2012, 3, 216. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.; Haque, T.; Gritsman, K. Inflammatory signaling pathways in preleukemic and leukemic stem cells. Front. Oncol. 2017, 7, 265. [Google Scholar] [CrossRef]
- Hassan, F.U.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an alternative epigenetic modulator: Mechanism of action and potential effects. Front. Genet. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Kloesch, B.; Becker, T.; Dietersdorfer, E.; Kiener, H.; Steiner, G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013, 15, 400–405. [Google Scholar] [CrossRef]
- Reikvam, H.; Aasebø, E.; Brenner, A.K.; Bartaula-Brevik, S.; Grønningsæter, I.S.; Forthun, R.B.; Hovland, R.; Bruserud, Ø. High constitutive cytokine release by primary human acute myeloid leukemia cells is associated with a specific intercellular communication phenotype. J. Clin. Med. 2019, 8, 970. [Google Scholar] [CrossRef] [PubMed]
- Balandrán, J.C.; Lasry, A.; Aifantis, I. The role of inflammation in the initiation and progression of myeloid neoplasms. Blood Cancer Discov. 2023, 4, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural polyphenols as targeted modulators in colon cancer: Molecular mechanisms and applications. Front. Immunol. 2021, 12, 635484. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Scavelli, C.; Roccaro, A.M.; Crivellato, E.; Nico, B.; Vacca, A. Hematopoietic cancer and angiogenesis. Stem Cells Dev. 2004, 13, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Annabi, B.; Currie, J.-C.; Moghrabi, A.; Béliveau, R. Inhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCg. Leuk. Res. 2007, 31, 1277–1284. [Google Scholar] [CrossRef]
- Reikvam, H. Inhibition of NF-κB signaling alters acute myelogenous leukemia cell transcriptomics. Cells 2020, 9, 1677. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Redhu, D.; Sánchez-Hidalgo, M.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Worm, M.; Babina, M. Olive-Oil-Derived Polyphenols Effectively Attenuate Inflammatory Responses of Human Keratinocytes by Interfering with the NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, e1900019. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.-P.; Koehn, F.E.; Abraham, R.T. The antibody-drug conjugate: An enabling modality for natural product-based cancer therapeutics. Nat. Prod. Rep. 2013, 30, 625–639. [Google Scholar] [CrossRef]
- Newman, D.J.; Giddings, L.-A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014, 13, 123–137. [Google Scholar] [CrossRef]
- Gustafson, S.J.; Barth, B.M.; McGill, C.M.; Clausen, T.P.; Kuhn, T.B. Wild alaskan blueberry extracts inhibit a magnesium-dependent neutral sphingomyelinase activity in neurons exposed to TNFα. Curr. Top. Nutraceutical Res. 2007, 5, 183. [Google Scholar]
- Huang, W.; Yao, L.; He, X.; Wang, L.; Li, M.; Yang, Y.; Wan, C. Hypoglycemic activity and constituents analysis of blueberry (Vaccinium corymbosum) fruit extracts. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 357–366. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Jensen, G.S.; Wu, X. Phenolic acids of the two major blueberry species in the US Market and their antioxidant and anti-inflammatory activities. Plant Foods Hum. Nutr. 2015, 70, 56–62. [Google Scholar] [CrossRef]
- Calgarotto, A.K.; Longhini, A.L.; de Souza, F.V.P.; Duarte, A.S.S.; Ferro, K.P.; Santos, I.; Maso, V.; Saad, S.T.O.; Torello, C.O. Immunomodulatory Effect of Green Tea Treatment in Combination with Low-dose Chemotherapy in Elderly Acute Myeloid Leukemia Patients with Myelodysplasia-related Changes. Integr. Cancer Ther. 2021, 20, 15347354211002647. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice-In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed]
- Kordes, U.; Krappmann, D.; Heissmeyer, V.; Ludwig, W.D.; Scheidereit, C. Transcription factor NF-kappaB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia 2000, 14, 399–402. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Rubio, L.; Macia, A.; Motilva, M.J. Impact of Various Factors on Pharmacokinetics of Bioactive Polyphenols: An Overview. Curr. Drug Metab. 2014, 15, 62–76. [Google Scholar] [CrossRef]
- Trindade, L.R.; da Silva, D.V.T.; Baião, D.D.S.; Paschoalin, V.M.F. Increasing the power of polyphenols through nanoencapsulation for adjuvant therapy against cardiovascular diseases. Molecules 2021, 26, 4621. [Google Scholar] [CrossRef] [PubMed]
- Kaushalya, K.; Rupasinghe, H.V. Health benefits of microencapsulated dietary polyphenols: A review. Food Rev. Int. 2024, 40, 2079–2102. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and nanoencapsulation: A review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Renault–Mahieux, M.; Mignet, N.; Seguin, J.; Alhareth, K.; Paul, M.; Andrieux, K. Co–encapsulation of flavonoids with anti–cancer drugs: A challenge ahead. Int. J. Pharm. 2022, 623, 121942. [Google Scholar] [CrossRef] [PubMed]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for drug delivery: An updated review of the last decade. Recent. Pat. Drug Deliv. Formul. 2018, 12, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation techniques for food bioactive components: A review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. J. Food Sci. 2019, 84, 111–120. [Google Scholar] [CrossRef]
- Kim, K.H.; Ki, M.-R.; Min, K.H.; Pack, S.P. Advanced delivery system of polyphenols for effective cancer prevention and therapy. Antioxidants 2023, 12, 1048. [Google Scholar] [CrossRef]
- Beconcini, D.; Felice, F.; Zambito, Y.; Fabiano, A.; Piras, A.M.; Macedo, M.H.; Sarmento, B.; Di Stefano, R. Anti-inflammatory effect of cherry extract loaded in polymeric nanoparticles: Relevance of particle internalization in endothelial cells. Pharmaceutics 2019, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 971–981. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Jang, D.; Lee, Y.M.; Lee, J.; Doh, J.; Kim, W.J. Remission of lymphoblastic leukaemia in an intravascular fluidic environment by pliable drug carrier with a sliding target ligand. Sci. Rep. 2017, 7, 40739. [Google Scholar] [CrossRef]
- Fang, W.; Peng, Z.L.; Dai, Y.J.; Wang, D.L.; Huang, P.; Huang, H.P. (-)-Epigallocatechin-3-gallate encapsulated realgar nanoparticles exhibit enhanced anticancer therapeutic efficacy against acute promyelocytic leukemia. Drug Deliv. 2019, 26, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bhatnagar, P.; Srivastava, A.; Kumar, P.; Shukla, Y.; Gupta, K. Enhancement of cancer chemosensitization potential of cisplatin by tea polyphenols poly(lactide-co-glycolide) nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Lai, F.; Mong, J.; Niibori-Nambu, A.; Chan, K.H.; Her, Z.; Osato, M.; Tan, M.-H.; Chen, Q.; Kurisawa, M. Bone marrow-targetable Green Tea Catechin-Based Micellar Nanocomplex for synergistic therapy of Acute myeloid leukemia. J. Nanobiotechnol. 2022, 20, 481. [Google Scholar] [CrossRef]
- Zou, T.; Percival, S.S.; Cheng, Q.; Li, Z.; Rowe, C.A.; Gu, L. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins-gelatin-chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2012, 82, 36–42. [Google Scholar] [CrossRef] [PubMed]

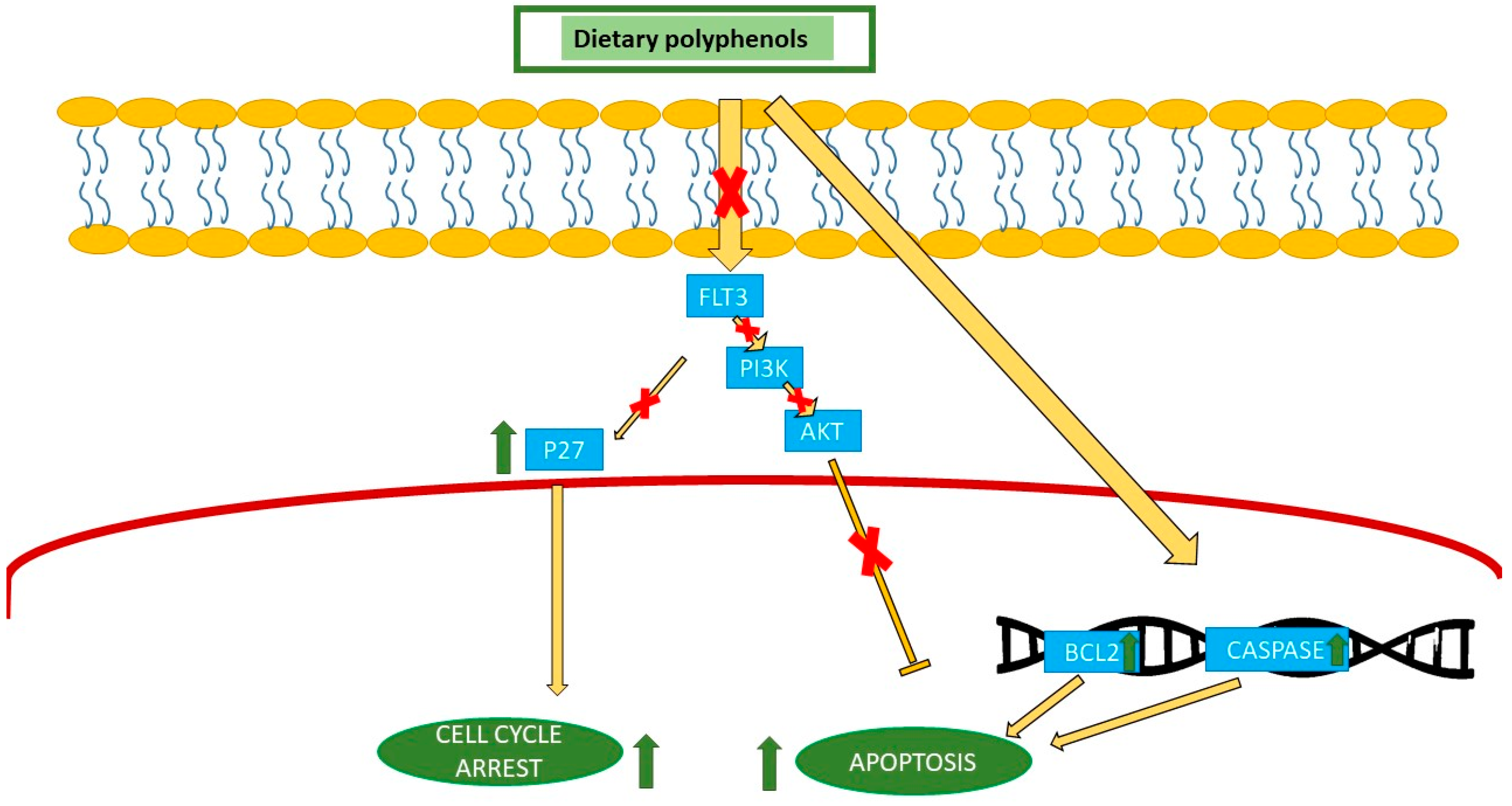
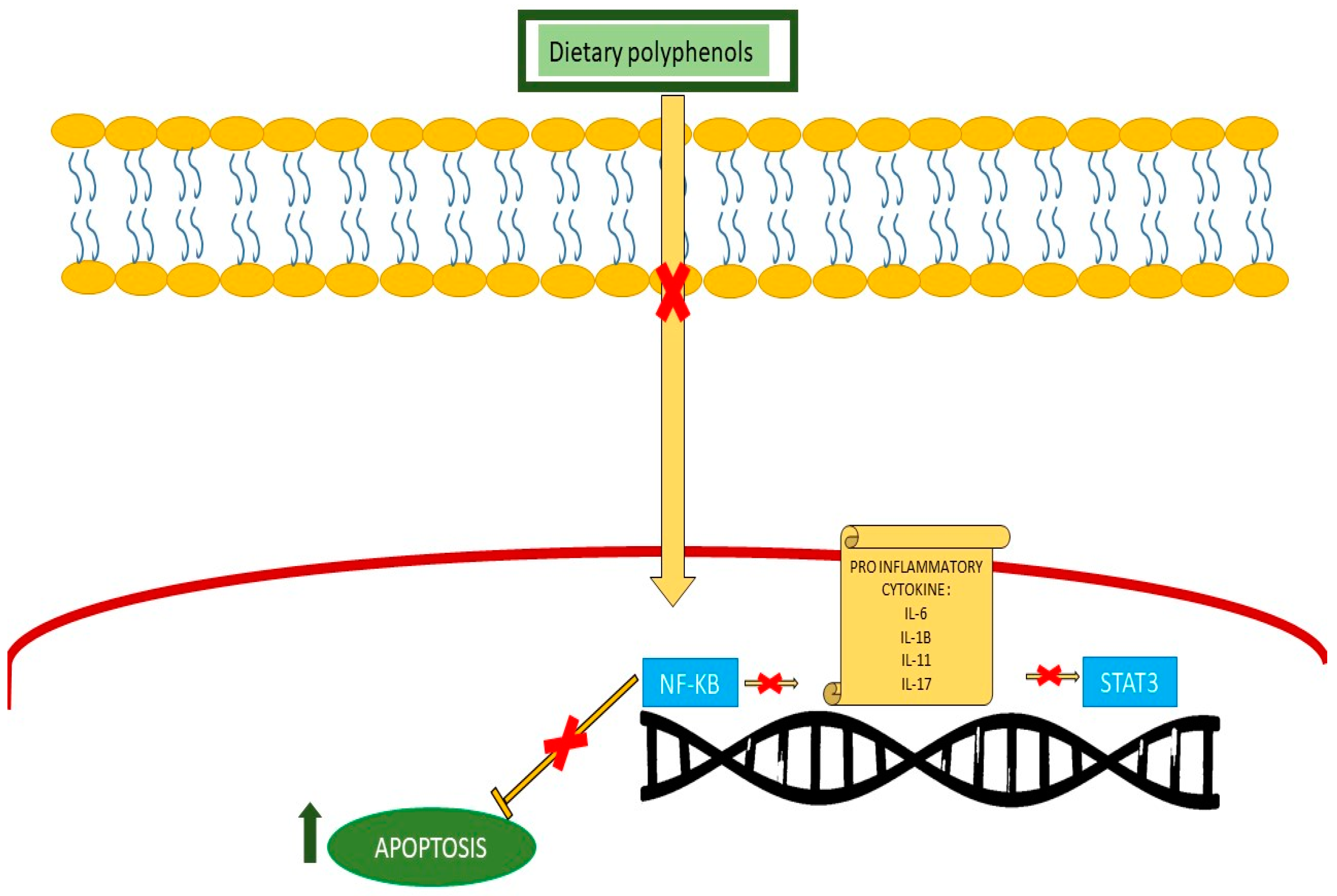
| Properties | Polyphenols | Type of Leukemia | Mechanism |
|---|---|---|---|
| Antioxidant | Green tea, olive oil, grapes | ALL | Cell growth inhibition and epigenetic modification. |
| Dietary polyphenol | AML/ALL | Iron-chelating, increase in pro-apoptotic genes, and increase in caspase. | |
| Anticancer | Polyphenols combined with chemotherapy treatment | AML/ALL | Increase in chemotherapy sensitivity and modulating molecular pathways. |
| Green tea | AML | Cell cycle arrest by P27, FLT3 inhibition, and apoptosis by PI3K/AKT. | |
| Green tea, grapes, virgin olive oil, strawberry | ALL | Cell growth inhibition, epigenetic modification, and induce apoptosis. | |
| Anti-inflammatory | Green tea, olive, blueberry | ALL/AML | Suppress NF-κB pathway, induce apoptosis, and reduce inflammatory cytokine. |
| Olive oil, hydroxytyrosol | ALL | Reducing thromboxane B2 and prostaglandin E2 and pro-inflammatory mediators. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhar, F.; Mohammadian, K.; Keramat, S.; Stanek, A. The Potential Role of Dietary Polyphenols in the Prevention and Treatment of Acute Leukemia. Nutrients 2024, 16, 4100. https://doi.org/10.3390/nu16234100
Fakhar F, Mohammadian K, Keramat S, Stanek A. The Potential Role of Dietary Polyphenols in the Prevention and Treatment of Acute Leukemia. Nutrients. 2024; 16(23):4100. https://doi.org/10.3390/nu16234100
Chicago/Turabian StyleFakhar, Fatemeh, Kiana Mohammadian, Shayan Keramat, and Agata Stanek. 2024. "The Potential Role of Dietary Polyphenols in the Prevention and Treatment of Acute Leukemia" Nutrients 16, no. 23: 4100. https://doi.org/10.3390/nu16234100
APA StyleFakhar, F., Mohammadian, K., Keramat, S., & Stanek, A. (2024). The Potential Role of Dietary Polyphenols in the Prevention and Treatment of Acute Leukemia. Nutrients, 16(23), 4100. https://doi.org/10.3390/nu16234100








