Ketogenic Diet and Neuroinflammation: Implications for Neuroimmunometabolism and Therapeutic Approaches to Refractory Epilepsy
Abstract
1. Introduction
2. Epilepsy
3. The Role of Immune System in Epilepsy
3.1. Neuroinflammation and Epilepsy
3.2. Immunometabolism and Epilepsy
4. Ketogenic Diet: Modulating Inflammatory Responses in Epilepsy
4.1. KD Variants
4.2. KD: Metabolism and Anticonvulsant Mechanisms
4.3. Impact of KD on Th17/Treg Homeostasis Disruption
5. Potential Adverse Effects of the KD to Be Considered
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verrotti, A.; Iapadre, G.; Di Francesco, L.; Zagaroli, L.; Farello, G. Diet in the Treatment of Epilepsy: What We Know So Far. Nutrients 2020, 12, 2645. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.P. Ketogenic diet for epilepsy treatment. Arq. Neuropsiquiatr. 2016, 74, 842–848. [Google Scholar] [CrossRef]
- Theodore, W.H. Epilepsy in the Hippocratic collection: Seizures and syndromes. Epilepsy Behav. 2021, 115, 107704. [Google Scholar] [CrossRef]
- Höhn, S.; Dozières-Puyravel, B.; Auvin, S. History of dietary treatment from Wilder’s hypothesis to the first open studies in the 1920s. Epilepsy Behav. 2019, 101, 106588. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R. Ketonemia and seizures: Metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Queathem, E.D.; Puchalska, P.; Crawford, P.A. Metabolic Messengers: Ketone bodies. Nat. Metab. 2023, 5, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Cross, H. Epilepsy: Behavioural, psychological, and ketogenic diet treatments. BMJ Clin. Evid. 2015, 2015, 1214. [Google Scholar] [PubMed]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef]
- Ramalho, R.; Rao, M.; Zhang, C.; Agrati, C.; Ippolito, G.; Wang, F.S.; Zumla, A.; Maeurer, M. Immunometabolism: New insights and lessons from antigen-directed cellular immune responses. Semin. Immunopathol. 2020, 42, 279–313. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Zeng, Y.; Zheng, W. Neuroinflammation in epileptogenesis: From pathophysiology to therapeutic strategies. Front. Immunol. 2023, 14, 1269241. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, S.T.; Abdullahi, A.M.; Jahan, N. Current Classification of Seizures and Epilepsies: Scope, Limitations and Recommendations for Future Action. Cureus 2020, 12, e10549. [Google Scholar] [CrossRef]
- Boleti, A.P.A.; Cardoso, P.H.O.; Frihling, B.E.F.; de Moraes, L.; Nunes, E.A.C.; Mukoyama, L.T.H.; Carvalho, C.M.E.; Macedo, M.L.R.; Migliolo, L. Pathophysiology to Risk Factor and Therapeutics to Treatment Strategies on Epilepsy. Brain Sci. 2024, 14, 71. [Google Scholar] [CrossRef]
- Mrabet, S.; Belguith, S.; Kacem, I.; Zgueb, Y.; Jenhani, R.; Ouali, U.; Nasri, A.; Abdelkefi, I.; Ben Djebara, M.; Jomli, R.; et al. Psychiatric disturbances in idiopathic epilepsy. Tunis. Med. 2023, 101, 839–844. [Google Scholar] [PubMed]
- Antaya, T.C.; Espino-Alvarado, P.H.; Oiamo, T.; Wilk, P.; Speechley, K.N.; Burneo, J.G. Association of outdoor air and noise pollution with unprovoked seizures and new onset epilepsy: A systematic review and meta-analysis. Epilepsia 2024, 65, 1847–1867. [Google Scholar] [CrossRef]
- Menon, B.; Harinarayan, C.V.; Raj, M.N.; Vemuri, S.; Himabindu, G.; Afsana, T.K. Prevalence of low dietary calcium intake in patients with epilepsy: A study from South India. Neurol. India 2010, 58, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Cui, Y.; Gao, F.; Zheng, W.; Li, J.; Xian, Z. Keap1/Nrf2 signaling pathway participating in the progression of epilepsy via regulation of oxidative stress and ferroptosis in neurons. Clinics 2024, 79, 100372. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Ghotbeddin, Z.; Rahimi, K.; Tabandeh, M.R. The effects of MEPaL on oxidative stress and motor function in the rats affected by prenatal hypoxia. Brain Behav. 2024, 14, e3539. [Google Scholar] [CrossRef]
- Vishnoi, S.; Raisuddin, S.; Parvez, S. Glutamate Excitotoxicity and Oxidative Stress in Epilepsy: Modulatory Role of Melatonin. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 365–374. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Pluta, R.; Bogucka-Kocka, A.; Czuczwar, S.J. To treat or not to treat drug-refractory epilepsy by the ketogenic diet? That is the question. Ann. Agric. Environ. Med. 2016, 23, 533–536. [Google Scholar] [CrossRef]
- Tanaka, T.; Ihara, M.; Fukuma, K.; Mishra, N.K.; Koepp, M.J.; Guekht, A.; Ikeda, A. Pathophysiology, Diagnosis, Prognosis, and Prevention of Poststroke Epilepsy: Clinical and Research Implications. Neurology 2024, 102, e209450. [Google Scholar] [CrossRef] [PubMed]
- AboElfarh, H.E.; Abdelhady, M.; Shehata, M.; Alkhawaldeh, I.; AbdelMeseh, M.; Ezz, M.H. Efficiency and Safety of Vagus Nerve Stimulation in Intractable Epilepsy: An Updated Systematic Review and Meta-Analysis (P7-1.014). Neurology 2024, 103, e209579. [Google Scholar] [CrossRef]
- Rugg-Gunn, F.; Miserocchi, A.; McEvoy, A. Epilepsy surgery. Pract. Neurol. 2020, 20, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Joris, V.; Weil, A.G.; Fallah, A. Brain Surgery for Medically Intractable Epilepsy. Adv. Pediatr. 2022, 69, 59–74. [Google Scholar] [CrossRef]
- West, S.; Nolan, S.J.; Newton, R. Surgery for epilepsy: A systematic review of current evidence. Epileptic Disord. 2016, 18, 113–121. [Google Scholar] [CrossRef]
- Millán, A.P.; van Straaten, E.C.W.; Stam, C.J.; Nissen, I.A.; Idema, S.; Baayen, J.C.; Van Mieghem, P.; Hillebrand, A. Epidemic models characterize seizure propagation and the effects of epilepsy surgery in individualized brain networks based on MEG and invasive EEG recordings. Sci. Rep. 2022, 12, 4086. [Google Scholar] [CrossRef]
- Kolkhir, P.; Akdis, C.A.; Akdis, M.; Bachert, C.; Bieber, T.; Canonica, G.W.; Guttman-Yassky, E.; Metz, M.; Mullol, J.; Palomares, O.; et al. Type 2 chronic inflammatory diseases: Targets, therapies and unmet needs. Nat. Rev. Drug Discov. 2023, 22, 743–767. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Zhao, X.; Cui, H.; Han, M.; Ren, X.; Gang, X.; Wang, G. Obesity and chronic kidney disease. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E24–E41. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Bowman, W.S.; Schmidt, R.J.; Sanghar, G.K.; Thompson, G.R., III; Ji, H.; Zeki, A.A.; Haczku, A. “Air That Once Was Breath” Part 1: Wildfire-Smoke-Induced Mechanisms of Airway Inflammation—“Climate Change, Allergy and Immunology” Special IAAI Article Collection: Collegium Internationale Allergologicum Update 2023. Int. Arch. Allergy Immunol. 2024, 185, 600–616. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link Between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef] [PubMed]
- Bernier, L.P.; York, E.M.; MacVicar, B.A. Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends Neurosci. 2020, 43, 854–869. [Google Scholar] [CrossRef]
- Di Liberto, V.; Mudò, G. Role of Bioactive Molecules on Neuroprotection, Oxidative Stress, and Neuroinflammation Modulation. Int. J. Mol. Sci. 2022, 23, 15925. [Google Scholar] [CrossRef] [PubMed]
- Spiljar, M.; Kuchroo, V.K. Metabolic regulation and function of T helper cells in neuroinflammation. Semin. Immunopathol. 2022, 44, 581–598. [Google Scholar] [CrossRef]
- Foiadelli, T.; Santangelo, A.; Costagliola, G.; Costa, E.; Scacciati, M.; Riva, A.; Volpedo, G.; Smaldone, M.; Bonuccelli, A.; Clemente, A.M.; et al. Neuroinflammation and status epilepticus: A narrative review unraveling a complex interplay. Front. Pediatr. 2023, 11, 1251914. [Google Scholar] [CrossRef]
- Liu, W.; Fan, M.; Lu, W.; Zhu, W.; Meng, L.; Lu, S. Emerging Roles of T Helper Cells in Non-Infectious Neuroinflammation: Savior or Sinner. Front. Immunol. 2022, 13, 872167. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Banik, A.; Saurabh, S.; Maulik, M.; Khatri, S.N. Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders. J. Neurosci. 2022, 42, 1888–1907. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, B.; Chen, Z. Cholinergic Signaling, Neural Excitability, and Epilepsy. Molecules 2021, 26, 2258. [Google Scholar] [CrossRef]
- Sakimoto, Y.; Oo, P.M.; Goshima, M.; Kanehisa, I.; Tsukada, Y.; Mitsushima, D. Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology. Int. J. Mol. Sci. 2021, 22, 12456. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, H.; Zhang, W.; Hou, S.; Yang, X.; Lin, J.; Ma, X.; Meng, H. Exploring correlations between immune cell phenotypes and the risk of epilepsy: A bidirectional Mendelian randomization study. Epilepsy Behav. 2024, 157, 109896. [Google Scholar] [CrossRef] [PubMed]
- Artyomov, M.N.; Van den Bossche, J. Immunometabolism in the Single-Cell Era. Cell Metab. 2020, 32, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.R.; Wang, J.; Wang, Z.J.; Xi, M.J.; Xia, B.H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Voss, K.; Hong, H.S.; Bader, J.E.; Sugiura, A.; Lyssiotis, C.A.; Rathmell, J.C. A guide to interrogating immunometabolism. Nat. Rev. Immunol. 2021, 21, 637–652. [Google Scholar] [CrossRef]
- Luís, C.; Maduro, A.T.; Pereira, P.; Mendes, J.J.; Soares, R.; Ramalho, R. Nutritional senolytics and senomorphics: Implications to immune cells metabolism and aging—From theory to practice. Front. Nutr. 2022, 9, 958563. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A. Can the emerging field of immunometabolism provide insights into neuroinflammation? Prog. Neurobiol. 2020, 184, 101719. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Martínez, F.; Salazar, K.; Cifuentes, M.; Nualart, F. Brain Glucose-Sensing Mechanism and Energy Homeostasis. Mol. Neurobiol. 2019, 56, 769–796. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Yang, H.; Wu, J.; Guo, R.; Peng, Y.; Zheng, W.; Liu, D.; Song, Z. Glycolysis in energy metabolism during seizures. Neural Regen. Res. 2013, 8, 1316–1326. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, Q.; Zhang, S.; Liu, W.; Meng, H.; Xu, M.; Huang, X.; Lin, X.; Lin, M.; Herman, P.; et al. Aerobic glycolysis imaging of epileptic foci during the inter-ictal period. EBioMedicine 2022, 79, 104004. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Gruenbaum, S.E.; Dhaher, R.; Lee, T.W.; Zhou, Y.; Danbolt, N.C. The Glutamate-Glutamine Cycle in Epilepsy. Adv. Neurobiol. 2016, 13, 351–400. [Google Scholar] [CrossRef] [PubMed]
- Eid, T.; Lee, T.W.; Patrylo, P.; Zaveri, H.P. Astrocytes and Glutamine Synthetase in Epileptogenesis. J. Neurosci. Res. 2019, 97, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Nathan, C.L.; Tate, M.C.; Horbinski, C.M.; Templer, J.W.; Rosenow, J.M.; Sita, T.L.; James, C.D.; Deneen, B.; Miller, S.D.; et al. The immune system and metabolic products in epilepsy and glioma-associated epilepsy: Emerging therapeutic directions. JCI Insight 2024, 9, e174753. [Google Scholar] [CrossRef]
- Douglas, A.; Stevens, B.; Lynch, L. Interleukin-17 as a key player in neuroimmunometabolism. Nat. Metab. 2023, 5, 1088–1100. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, P.; Xu, F.; Zheng, Y.; Zhao, H. Advances in the study of IL-17 in neurological diseases and mental disorders. Front. Neurol. 2023, 14, 1284304. [Google Scholar] [CrossRef]
- Barzegar, M.; Afghan, M.; Tarmahi, V.; Behtari, M.; Rahimi Khamaneh, S.; Raeisi, S. Ketogenic diet: Overview, types, and possible anti-seizure mechanisms. Nutr. Neurosci. 2021, 24, 307–316. [Google Scholar] [CrossRef]
- Zarnowska, I.M. Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients 2020, 12, 2616. [Google Scholar] [CrossRef]
- Ruan, Y.; Chen, L.; She, D.; Chung, Y.; Ge, L.; Han, L. Ketogenic diet for epilepsy: An overview of systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 1234–1244. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K.; Krawczyk, M.; Czernia, J. Ketogenic Diet in the Treatment of Epilepsy. Nutrients 2024, 16, 1258. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e1216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M. Ketogenic diet: Old treatment, new beginning. Clin. Neurophysiol. Pract. 2017, 2, 161–162. [Google Scholar] [CrossRef]
- El-Rashidy, O.F.; Nassar, M.F.; Abdel-Hamid, I.A.; Shatla, R.H.; Abdel-Hamid, M.H.; Gabr, S.S.; Mohamed, S.G.; El-Sayed, W.S.; Shaaban, S.Y. Modified Atkins diet vs classic ketogenic formula in intractable epilepsy. Acta Neurol. Scand. 2013, 128, 402–408. [Google Scholar] [CrossRef]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic Diet: An Effective Treatment Approach for Neurodegenerative Diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef]
- Dressler, A.; Trimmel-Schwahofer, P.; Reithofer, E.; Gröppel, G.; Mühlebner, A.; Samueli, S.; Grabner, V.; Abraham, K.; Benninger, F.; Feucht, M. The ketogenic diet in infants—Advantages of early use. Epilepsy Res. 2015, 116, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Neal, E.G.; Chaffe, H.; Schwartz, R.H.; Lawson, M.S.; Edwards, N.; Fitzsimmons, G.; Whitney, A.; Cross, J.H. The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 2008, 7, 500–506. [Google Scholar] [CrossRef]
- Liu, Y.M. Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 2008, 49 (Suppl. S8), 33–36. [Google Scholar] [CrossRef]
- Lin, T.Y.; Liu, H.W.; Hung, T.M. The Ketogenic Effect of Medium-Chain Triacylglycerides. Front. Nutr. 2021, 8, 747284. [Google Scholar] [CrossRef]
- Sondhi, V.; Agarwala, A.; Pandey, R.M.; Chakrabarty, B.; Jauhari, P.; Lodha, R.; Toteja, G.S.; Sharma, S.; Paul, V.K.; Kossoff, E.; et al. Efficacy of Ketogenic Diet, Modified Atkins Diet, and Low Glycemic Index Therapy Diet Among Children with Drug-Resistant Epilepsy: A Randomized Clinical Trial. JAMA Pediatr. 2020, 174, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Masino, S.A.; Rho, J.M. Metabolism and epilepsy: Ketogenic diets as a homeostatic link. Brain Res. 2019, 1703, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Calderón, N.; Betancourt, L.; Hernández, L.; Rada, P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci. Lett. 2017, 642, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kosonen, R.; Barua, S.; Kim, J.Y.; Lee, J.E. Role of agmatine in the application of neural progenitor cell in central nervous system diseases: Therapeutic potentials and effects. Anat. Cell Biol. 2021, 54, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, N.; Fujiwara, S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids 2020, 52, 181–197. [Google Scholar] [CrossRef]
- Neis, V.B.; Rosa, P.B.; Olescowicz, G.; Rodrigues, A.L.S. Therapeutic potential of agmatine for CNS disorders. Neurochem. Int. 2017, 108, 318–331. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Czernecki, R.; Wojtal, K.; Borowicz, K.K.; Czuczwar, S.J. Agmatine enhances the anticonvulsant action of phenobarbital and valproate in the mouse maximal electroshock seizure model. J. Neural Transm. 2008, 115, 1485–1494. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhang, K.; Yang, W.; Li, B. The Anticonvulsant Effects of Ketogenic Diet on Epileptic Seizures and Potential Mechanisms. Curr. Neuropharmacol. 2018, 16, 66–70. [Google Scholar] [CrossRef]
- Xu, D.; Robinson, A.P.; Ishii, T.; Duncan, D.S.; Alden, T.D.; Goings, G.E.; Ifergan, I.; Podojil, J.R.; Penaloza-MacMaster, P.; Kearney, J.A.; et al. Peripherally derived T regulatory and γδ T cells have opposing roles in the pathogenesis of intractable pediatric epilepsy. J. Exp. Med. 2018, 215, 1169–1186. [Google Scholar] [CrossRef]
- He, J.J.; Sun, F.J.; Wang, Y.; Luo, X.Q.; Lei, P.; Zhou, J.; Zhu, D.; Li, Z.Y.; Yang, H. Increased expression of interleukin 17 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy. J. Neuroimmunol. 2016, 298, 153–159. [Google Scholar] [CrossRef]
- Kumar, P.; Shih, D.C.W.; Lim, A.; Paleja, B.; Ling, S.; Li Yun, L.; Li Poh, S.; Ngoh, A.; Arkachaisri, T.; Yeo, J.G.; et al. Pro-inflammatory, IL-17 pathways dominate the architecture of the immunome in pediatric refractory epilepsy. JCI Insight 2019, 5, e126337. [Google Scholar] [CrossRef] [PubMed]
- Mazdeh, M.; Omrani, M.D.; Sayad, A.; Komaki, A.; Arsang-Jang, S.; Taheri, M.; Ghafouri-Fard, S. Expression analysis of cytokine coding genes in epileptic patients. Cytokine 2018, 110, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, W.; Wang, Q.; Zheng, X.; Zhang, R.; Zhang, N. A High MCT-Based Ketogenic Diet Suppresses Th1 and Th17 Responses to Ameliorate Experimental Autoimmune Encephalomyelitis in Mice by Inhibiting GSDMD and JAK2-STAT3/4 Pathways. Mol. Nutr. Food Res. 2024, 68, e2300602. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.F.; Li, C.R.; Liao, J.X.; Wang, G.B.; Lin, S.F.; Xia, Y.; Wen, J.L. The effects of ketogenic diet on the Th17/Treg cells imbalance in patients with intractable childhood epilepsy. Seizure 2016, 38, 17–22. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.E.; van den Munckhof, B.; Braun, K.P.; van Royen-Kerkhof, A.; de Jager, W.; Jansen, F.E. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 177–190. [Google Scholar] [CrossRef]
- Silverberg, J.; Ginsburg, D.; Orman, R.; Amassian, V.; Durkin, H.G.; Stewart, M. Lymphocyte infiltration of neocortex and hippocampus after a single brief seizure in mice. Brain Behav. Immun. 2010, 24, 263–272. [Google Scholar] [CrossRef]
- Marsh, E.B.; Freeman, J.M.; Kossoff, E.H.; Vining, E.P.; Rubenstein, J.E.; Pyzik, P.L.; Hemingway, C. The outcome of children with intractable seizures: A 3- to 6-year follow-up of 67 children who remained on the ketogenic diet less than one year. Epilepsia 2006, 47, 425–430. [Google Scholar] [CrossRef]
- Patel, A.; Pyzik, P.L.; Turner, Z.; Rubenstein, J.E.; Kossoff, E.H. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia 2010, 51, 1277–1282. [Google Scholar] [CrossRef]
- Kwiterovich, P.O., Jr.; Vining, E.P.; Pyzik, P.; Skolasky, R., Jr.; Freeman, J.M. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 2003, 290, 912–920. [Google Scholar] [CrossRef]
- Nizamuddin, J.; Turner, Z.; Rubenstein, J.E.; Pyzik, P.L.; Kossoff, E.H. Management and risk factors for dyslipidemia with the ketogenic diet. J. Child. Neurol. 2008, 23, 758–761. [Google Scholar] [CrossRef]
- Lin, A.; Turner, Z.; Doerrer, S.C.; Stanfield, A.; Kossoff, E.H. Complications During Ketogenic Diet Initiation: Prevalence, Treatment, and Influence on Seizure Outcomes. Pediatr. Neurol. 2017, 68, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Vining, E.P.; Pyzik, P.; McGrogan, J.; Hladky, H.; Anand, A.; Kriegler, S.; Freeman, J.M. Growth of children on the ketogenic diet. Dev. Med. Child. Neurol. 2002, 44, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Basualdo-Hammond, C.; Curtis, R.; Schuller, R. Growth retardation in children with epilepsy on the ketogenic diet: A retrospective chart review. J. Am. Diet. Assoc. 2002, 102, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Arslan, N.; Kose, E.; Guzel, O. The Effect of Ketogenic Diet on Serum Selenium Levels in Patients with Intractable Epilepsy. Biol. Trace Elem. Res. 2017, 178, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Pyzik, P.L.; Rubenstein, J.E.; Hamdy, R.F.; Kossoff, E.H. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics 2009, 124, e300–e304. [Google Scholar] [CrossRef]
- Schoeler, N.E.; Simpson, Z.; Whiteley, V.J.; Nguyen, P.; Meskell, R.; Lightfoot, K.; Martin-McGill, K.J.; Olpin, S.; Ivison, F. Biochemical assessment of patients following ketogenic diets for epilepsy: Current practice in the UK and Ireland. Epilepsia Open 2020, 5, 73–79. [Google Scholar] [CrossRef]
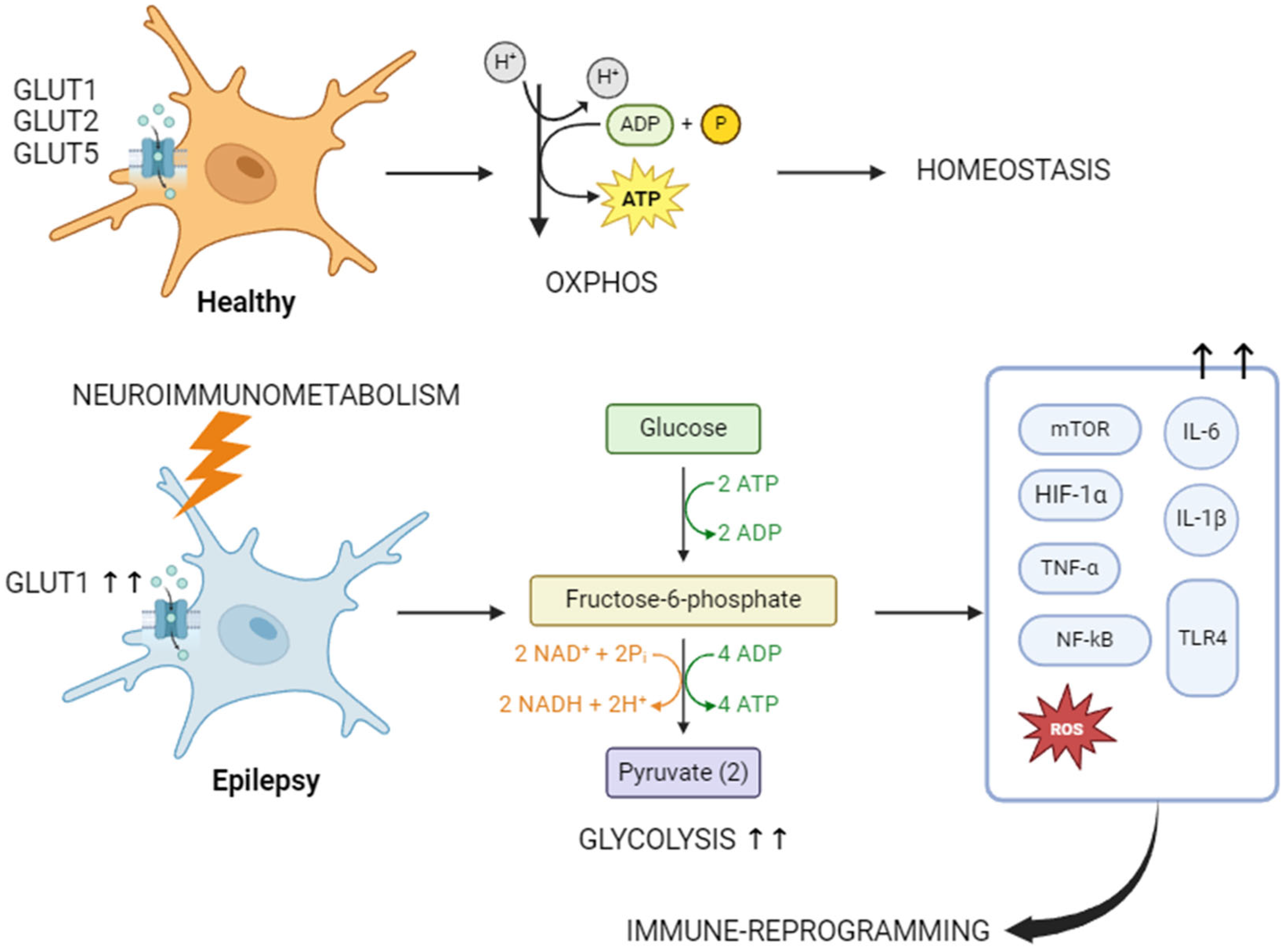
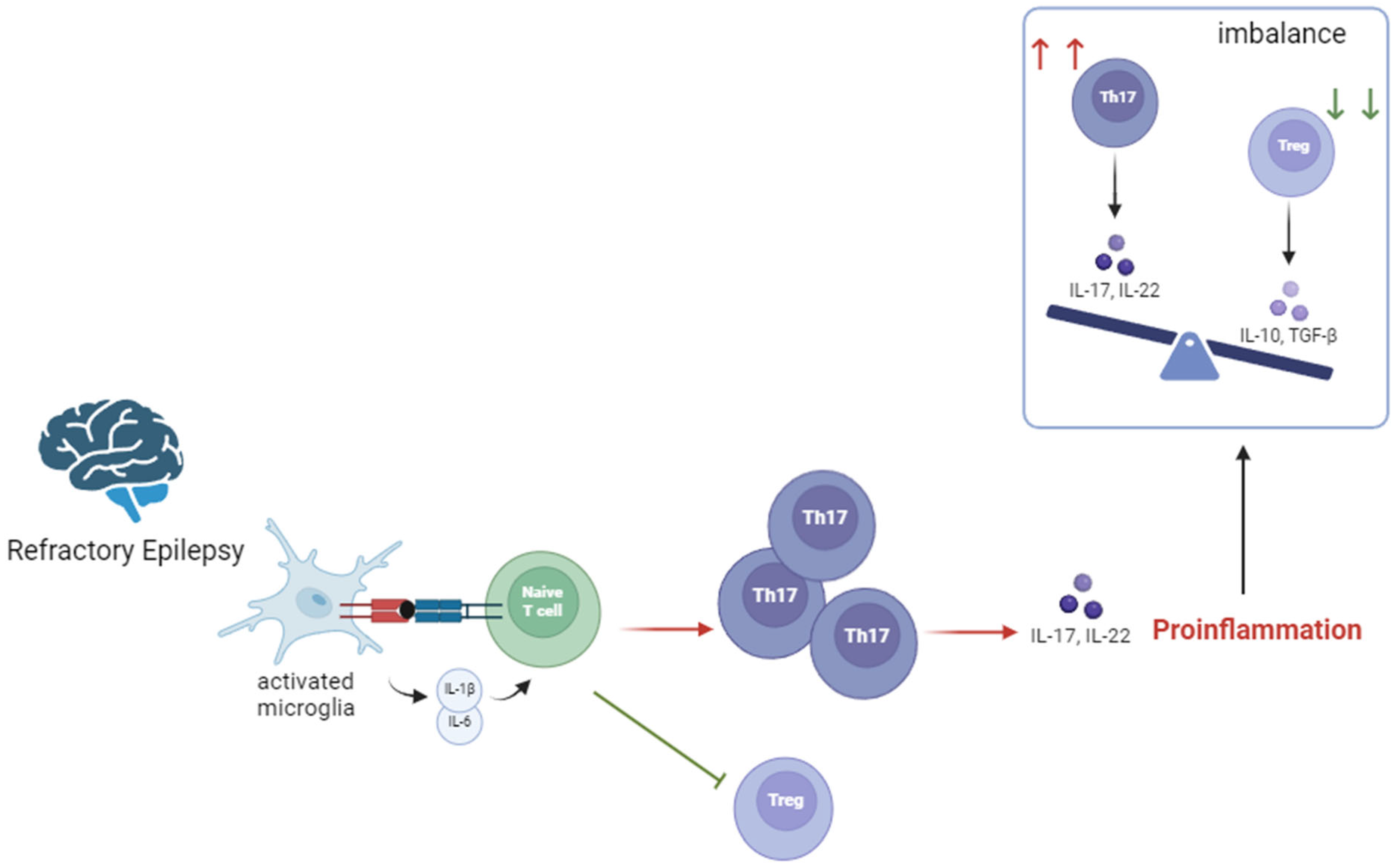
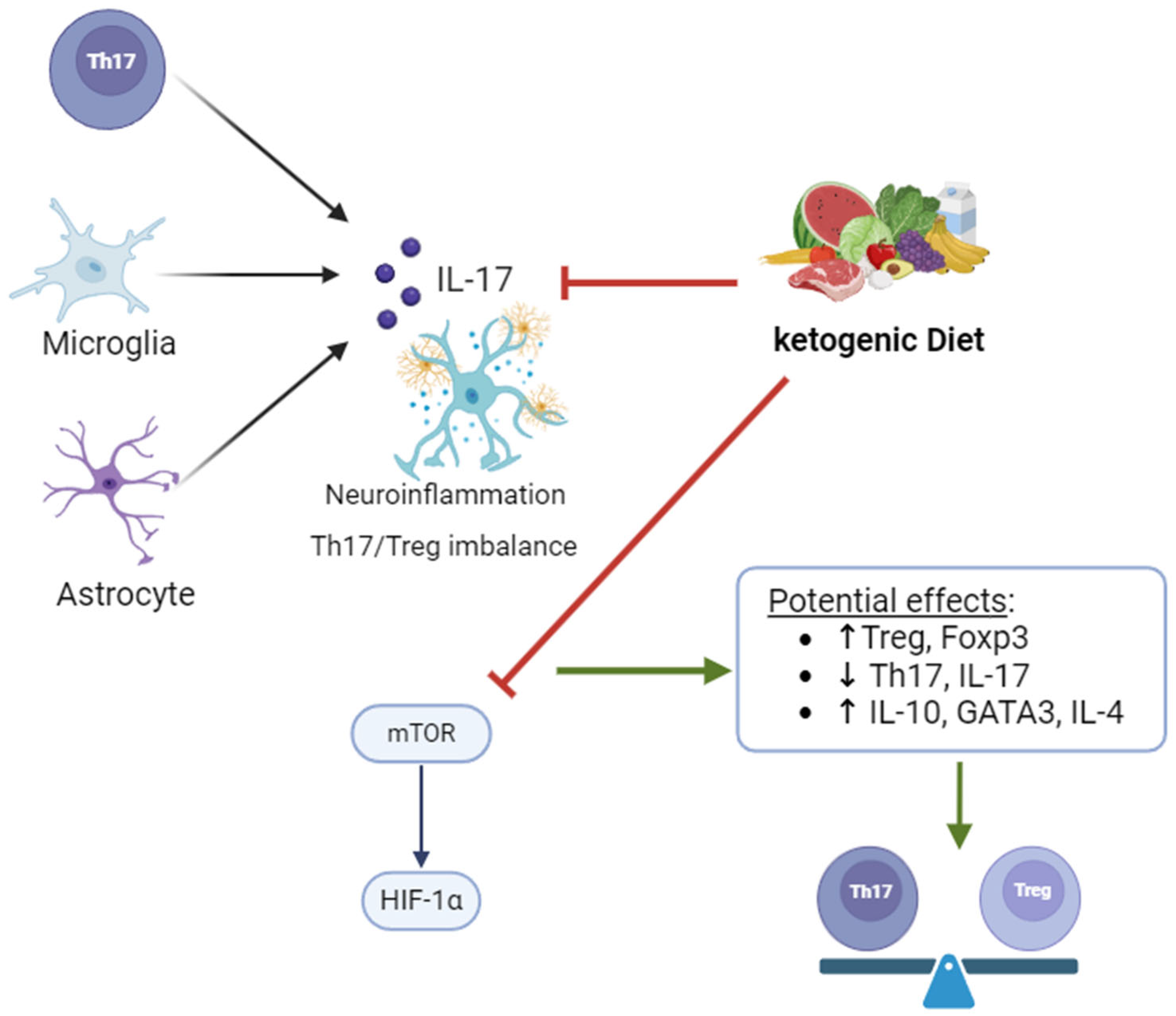
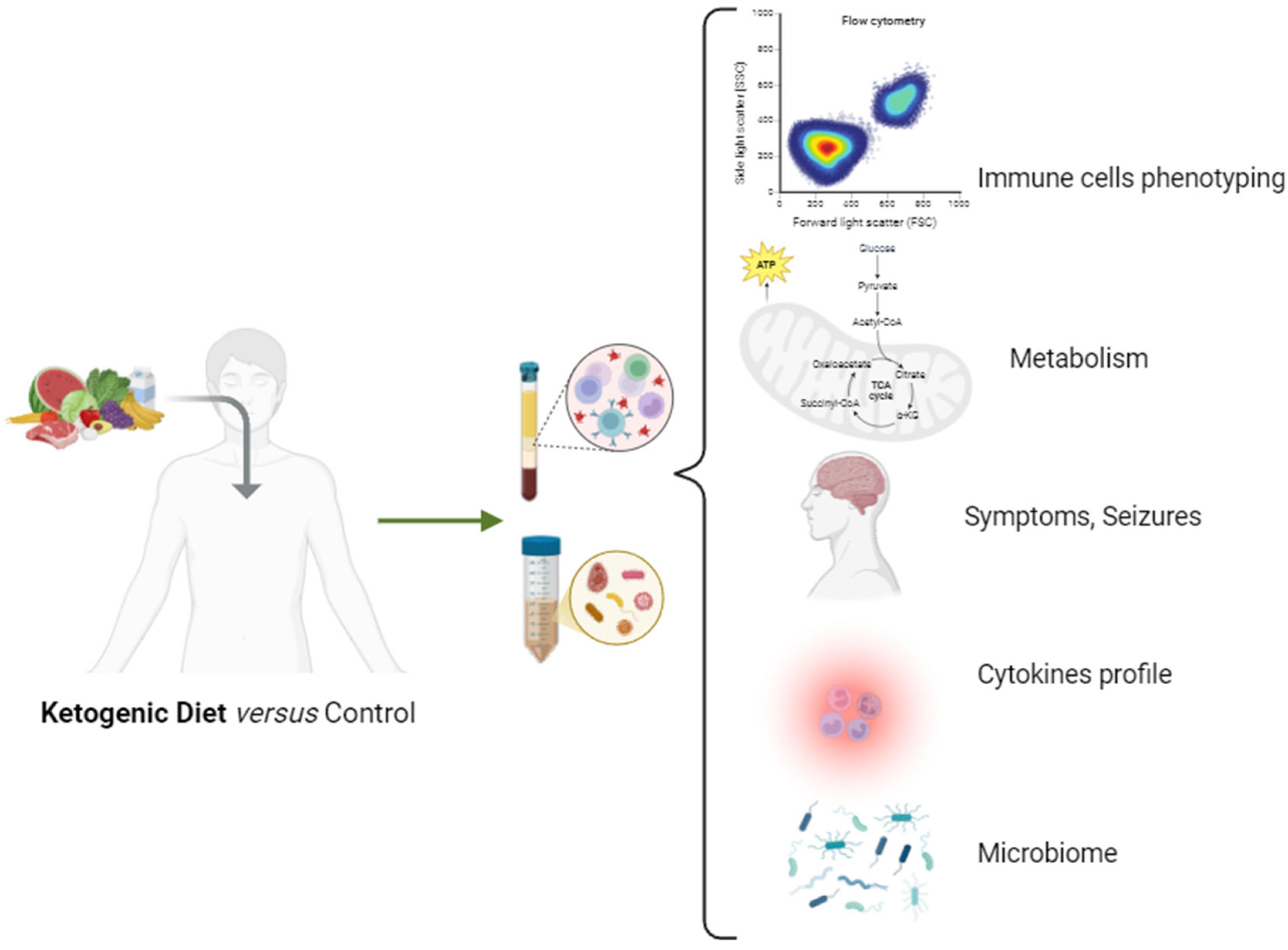
| Mediator | Secreted by | Mechanism |
|---|---|---|
| IL-1β | Microglia, Astrocytes, Neurons | Induce glutamate release Decrease glutamate reuptake Decrease GABAA flows |
| IL-6 | Microglia, Astrocytes | Induce glutamate release |
| IL-17 | Microglia, Astrocytes, T lymphocytes | Promote infiltration of peripheral immune cells Inhibit GABA-induced inhibitory synaptic transmission |
| TNF-α | Microglia, Astrocytes | Promote infiltration of peripheral T-Lymphocytes Induce glutamate release Induce GABA receptor endocytosis |
| TGF-β | Astrocytes | Mediate albumin uptake Downregulate Kir4.1 channel Impair AQP4 |
| HMGB1 | Microglia, Astrocytes, Neurons | Interact with TLR4, increasing Ca2+ influx and activating the NMDAR by phosphorylating NR2B subunit Promote pro-inflammatory cytokines release |
| CCL2, CCL3, CCL4, CX3CL1, CXCL13 | Microglia Astrocytes, Endothelial cells | Induce microglia activation Induce monocyte infiltration Promote neuron death through STAT3 and IL-1β signals |
| Signal | Mechanism |
|---|---|
| TLRs | Induction of innate and adaptive immune responses, followed by neuronal hyperexcitability and epileptogenesis Activation of microglia Induction TNF-α and IFN-β |
| NLRP3 | Induction of caspase-1 proteolysis Secretion of pro-inflammatory cytokines Epileptic neuron loss Seizures progression Induction of IL-1β and IL-18 |
| COX-2/mPGES-1 | Promotion of glutamate releasing by astrocytes, resulting in excitotoxicity Induction of PGE2 secretion by astrocytes and microglia |
| mTOR | Generation of monocytes and macrophages in marrow cavity Conversion of monocytes into macrophages by downregulation of CD115 expression Activation of T lymphocytes Activation of microglia BBB disruption Infiltration of peripheral immune cells into CNS Regulation of Th17 cells differentiation Mediation of IL-1β, IL-17 and TNF-α expression |
| Type of Sensors | Examples and Expression |
|---|---|
| Sugar sensors | Glucose GLUT 1 brain vasculature oligodendrocytes microglia astrocytes GLUT3 neurons GLUT5 oligodendrocytes microglia astrocytes |
| Lipid sensors | Cholesterol, aminophospholipids, gangliosides, sphingolipids CD36, LDLR, VLDR, LRP1, TREM2 Microglia |
| Amino-acid sensors | Tryptophan Quinolinic acid, IDO and kynurenic acid microglia astrocytes Glutamate EAAT1, EAAT2 and astrocytic glutaminase microglia astrocytes Arginine Nitric oxid Microglia |
| Study | Phase | Participants | Intervention | Outcomes | Status | Completion Date |
|---|---|---|---|---|---|---|
| NCT04063007, EpiMICRO | NA | 2 to 17 y, n = 60 | KD (Single Group Assignment) | -Gut microbiota -Changes in the -DNA methylation in WBC -QoL -AE | Recruiting | 2022—overdue, not completed |
| NCT05958160, TOPAMAD | Phase 2 Phase 3 | 9 mo to 3 y, n = 70 | MAD vs. Topiramate | -Reduction in clinical spasms | Recruiting | 2024 |
| NCT02216500 | NA | up to 50 y, n = 400 | KD (Single Group Assignment) | -Epilepsy control response rate | Recruiting | 2031 |
| NCT06310954 | NA | 6 mo to 12 y, n = 59 | KD vs. standard diet without any ketogenic restrictions | -Epilepsy control response rate -Serum levels of inflammatory markers -Relationship between KD and inflammation | Recruiting | 2024 |
| NCT05152771 | NA | 2 to 15 y, n = 26 | KD (Single Group Assignment) | -Epilepsy control response rate -Cognitive changes -Behavioral changes -Motor developmental changes | Not yet recruiting | 2025 |
| NCT04274179 | Phase 3 | 3 to 12 y, n = 40 | MAD vs. standard diet without any ketogenic restrictions | -Epilepsy control response rate -Tolerability | Recruiting | 2025 |
| NCT06369571 | Phase 1 Phase 2 | >18 y, n = 22 | MAD vs. replacement of 10% of saturated fat intake with polyunsaturated fat | -LDL changes -Epilepsy control response rate -AE | Not yet recruiting | 2027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerreiro, D.; Almeida, A.; Ramalho, R. Ketogenic Diet and Neuroinflammation: Implications for Neuroimmunometabolism and Therapeutic Approaches to Refractory Epilepsy. Nutrients 2024, 16, 3994. https://doi.org/10.3390/nu16233994
Guerreiro D, Almeida A, Ramalho R. Ketogenic Diet and Neuroinflammation: Implications for Neuroimmunometabolism and Therapeutic Approaches to Refractory Epilepsy. Nutrients. 2024; 16(23):3994. https://doi.org/10.3390/nu16233994
Chicago/Turabian StyleGuerreiro, Daniela, Anabela Almeida, and Renata Ramalho. 2024. "Ketogenic Diet and Neuroinflammation: Implications for Neuroimmunometabolism and Therapeutic Approaches to Refractory Epilepsy" Nutrients 16, no. 23: 3994. https://doi.org/10.3390/nu16233994
APA StyleGuerreiro, D., Almeida, A., & Ramalho, R. (2024). Ketogenic Diet and Neuroinflammation: Implications for Neuroimmunometabolism and Therapeutic Approaches to Refractory Epilepsy. Nutrients, 16(23), 3994. https://doi.org/10.3390/nu16233994








