Improvement in Glycolipid Metabolism Parameters After Supplementing Fish Oil-Derived Omega-3 Fatty Acids Is Associated with Gut Microbiota and Lipid Metabolites in Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
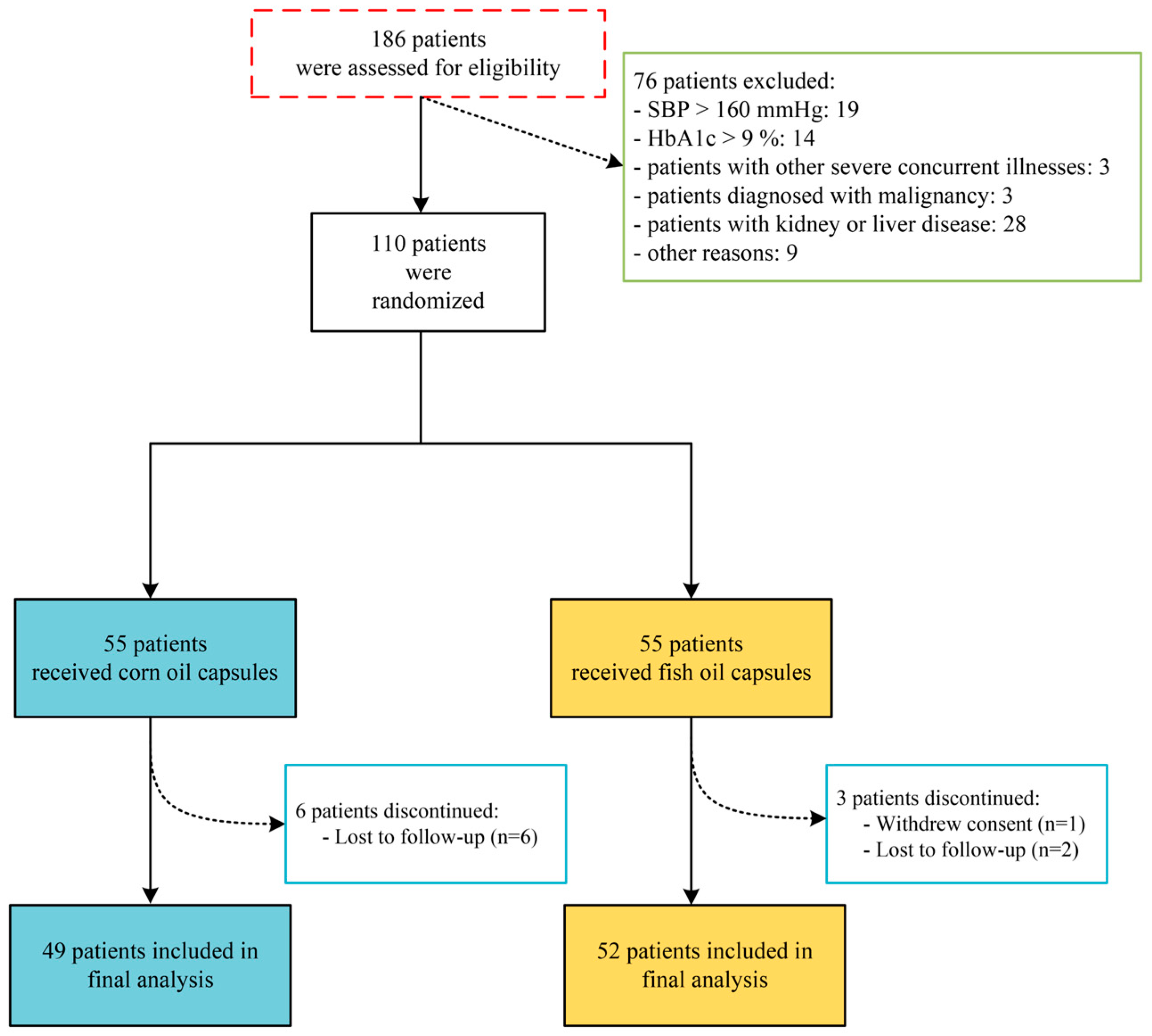
2.1. Patient Recruitment and Ethics Approval
2.2. Study Design and Intervention
2.3. Clinical Measures
2.4. Blood Sampling, Feces Sampling, and Analytical Methods
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Impact of Omega-3 PUFAs Intervention on Glucose Parameters and Lipid Profiles in Patients with T2DM
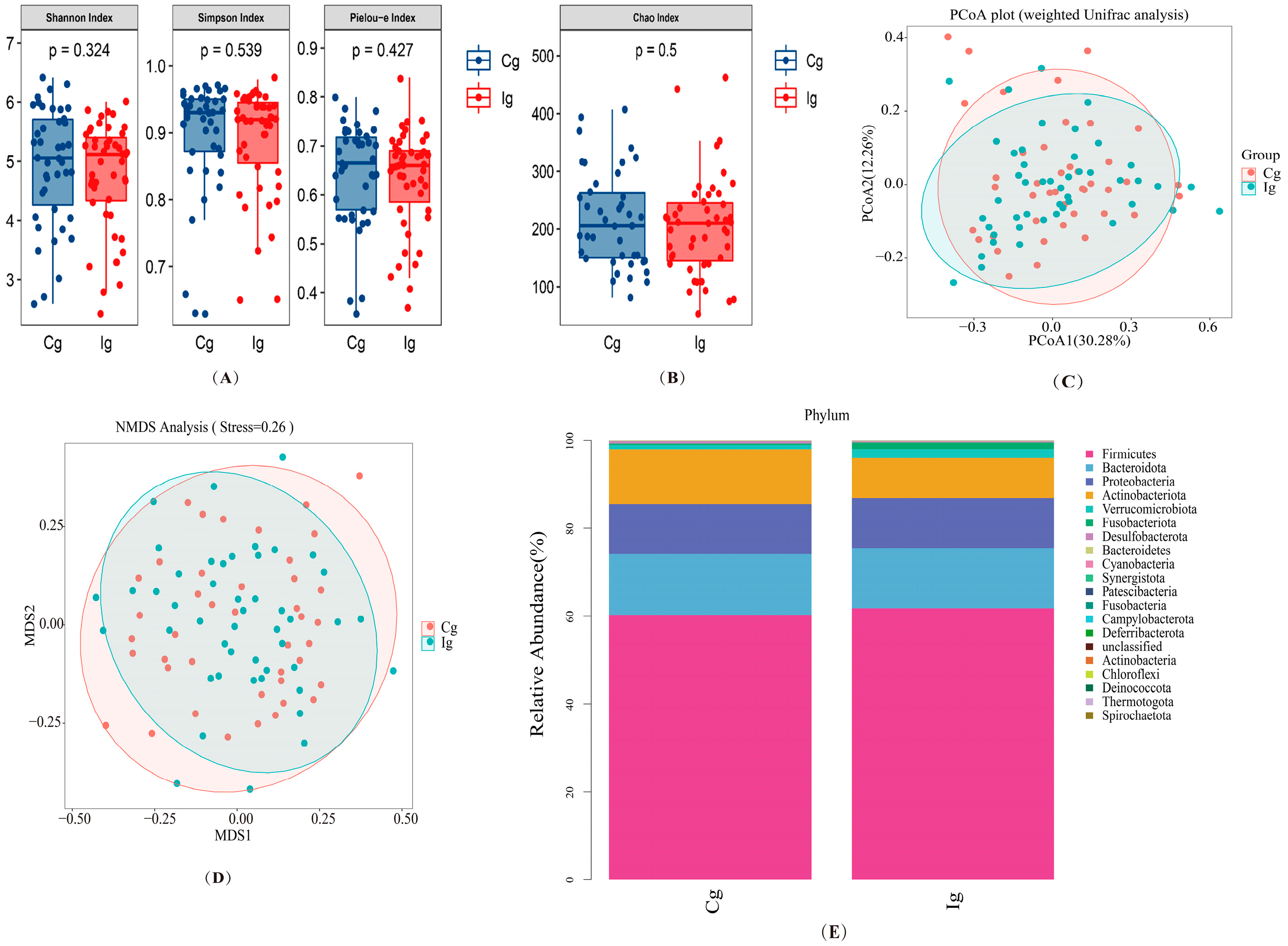
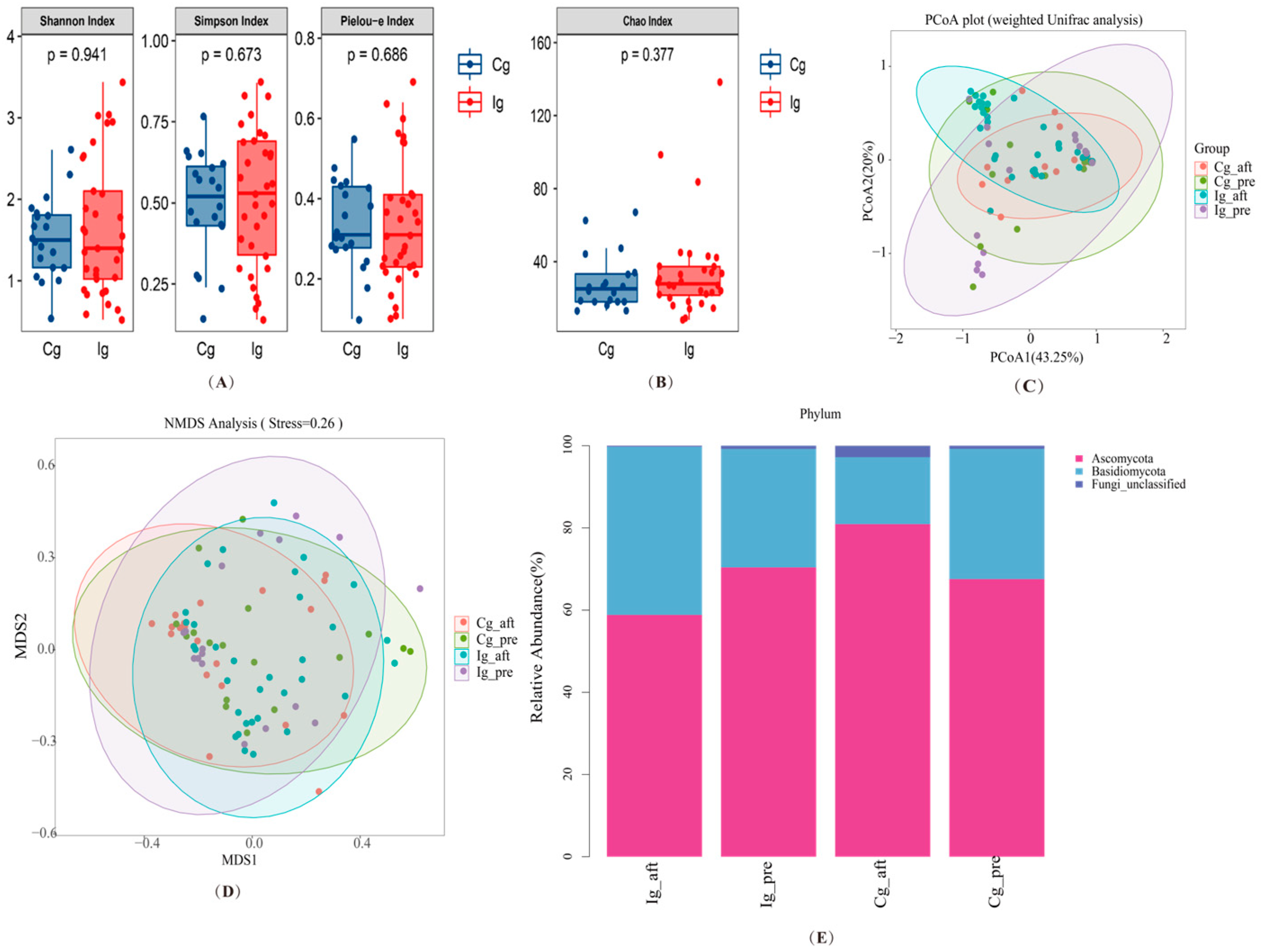
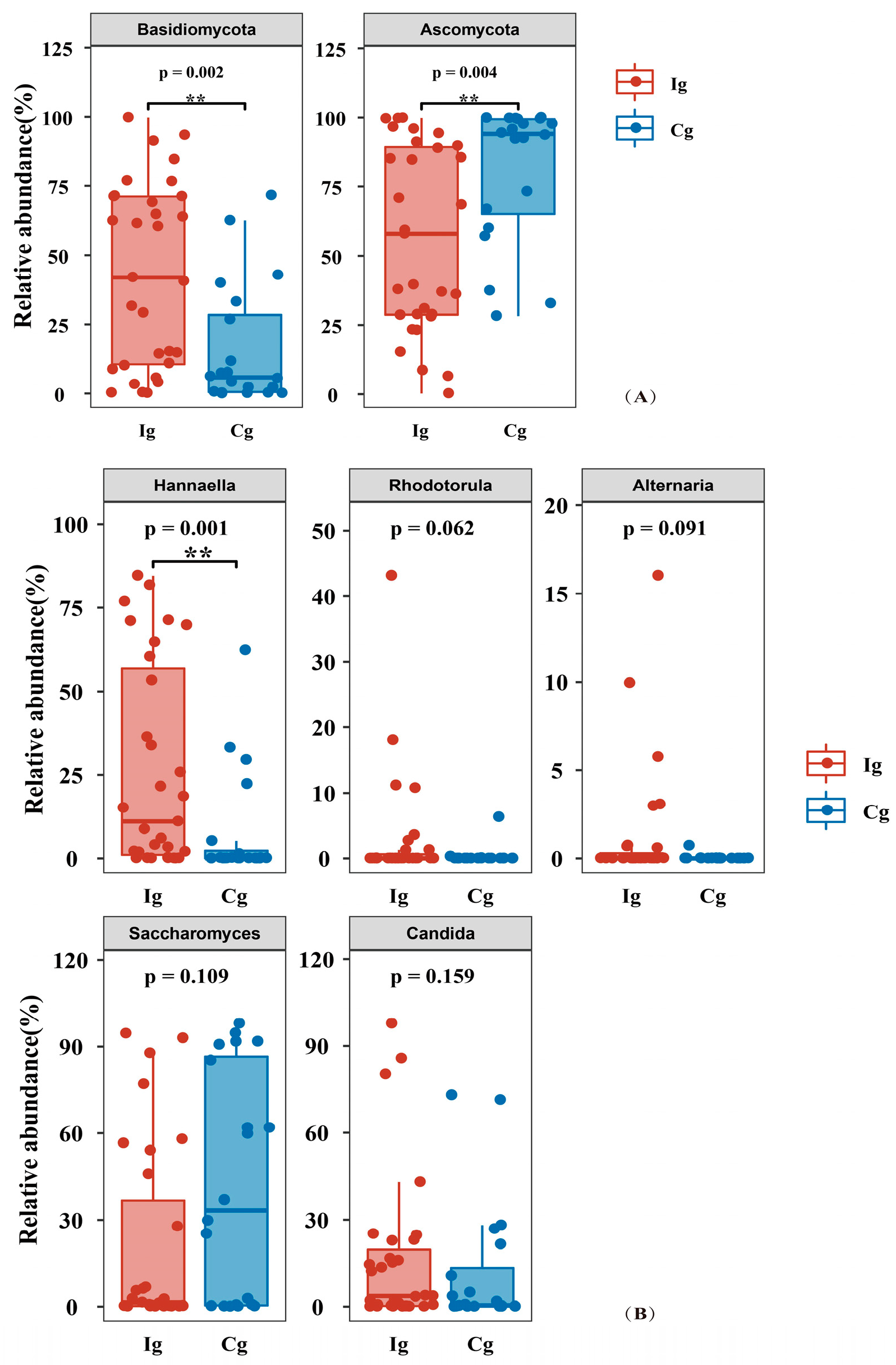
3.3. Impact of Omega-3 PUFAs Intervention on Gut Microbiota and Fungi Richness, Diversity, and Composition in Subjects with T2DM
3.4. Impact of Omega-3 PUFAs Intervention on Serum Lipid Metabolites
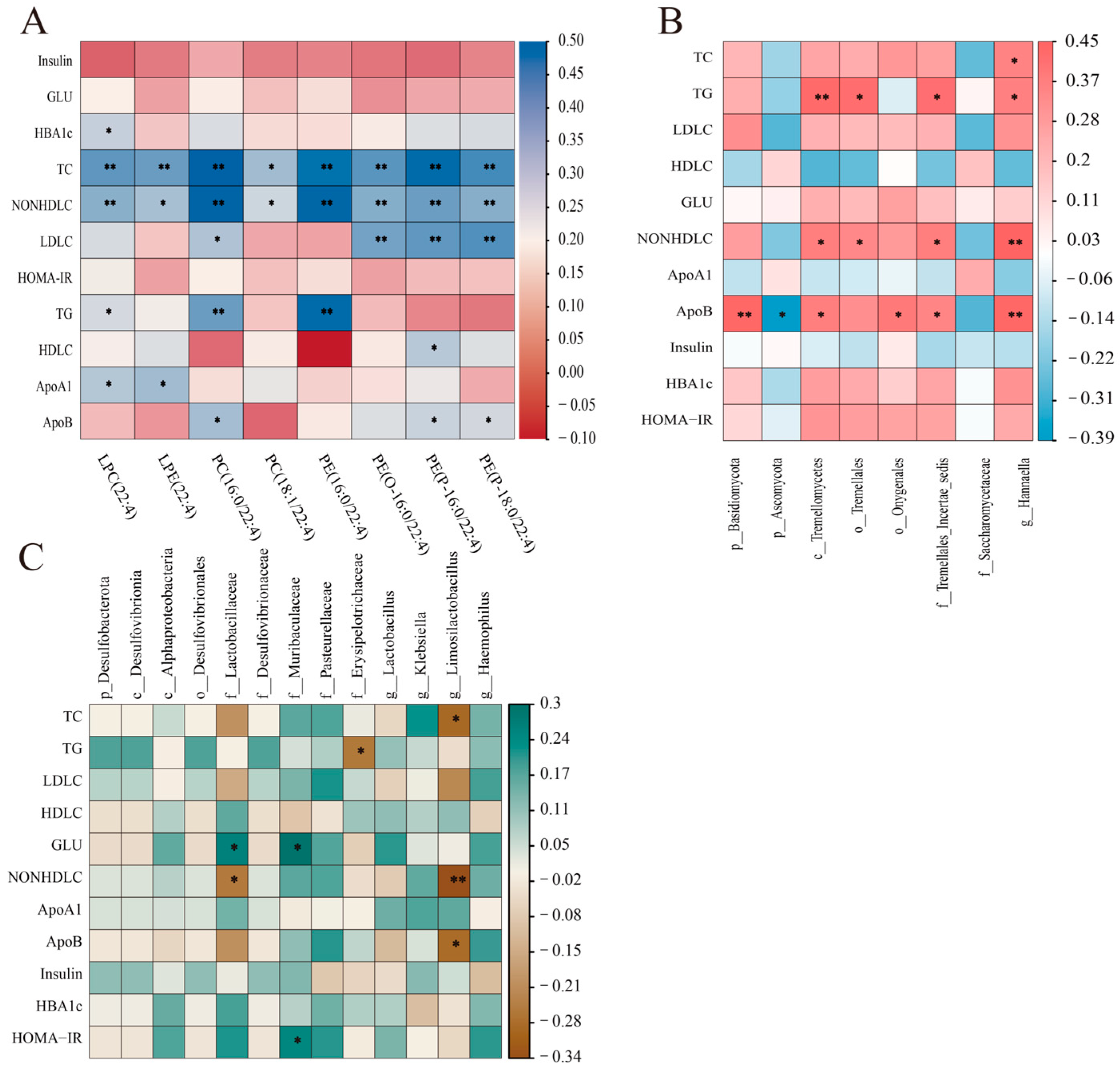
3.5. Correlation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Drenjančević, I.; Pitha, J. Omega-3 Polyunsaturated Fatty Acids-Vascular and Cardiac Effects on the Cellular and Molecular Level (Narrative Review). Int. J. Mol. Sci. 2022, 23, 2104. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Buoite Stella, A.; Gortan Cappellari, G.; Barazzoni, R.; Zanetti, M. Update on the Impact of Omega 3 Fatty Acids on Inflammation, Insulin Resistance and Sarcopenia: A Review. Int. J. Mol. Sci. 2018, 19, 218. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Zhao, B. Research progress in the relationship between type 2 diabetes mellitus and intestinal flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Weir, J.M.; Greeve, M.A.; MacIntosh, G.L.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Kowalczyk, A.; et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS ONE 2013, 8, e74341. [Google Scholar] [CrossRef]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferré, M.; et al. Plasma Lipidomic Profiling and Risk of Type 2 Diabetes in the PREDIMED Trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef]
- Hannich, J.T.; Loizides-Mangold, U.; Sinturel, F.; Harayama, T.; Vandereycken, B.; Saini, C.; Gosselin, P.; Brulhart-Meynet, M.C.; Robert, M.; Chanon, S.; et al. Ether lipids, sphingolipids and toxic 1-deoxyceramides as hallmarks for lean and obese type 2 diabetic patients. Acta Physiol. 2021, 232, e13610. [Google Scholar] [CrossRef] [PubMed]
- Rumora, A.E.; Guo, K.; Alakwaa, F.M.; Andersen, S.T.; Reynolds, E.L.; Jørgensen, M.E.; Witte, D.R.; Tankisi, H.; Charles, M.; Savelieff, M.G.; et al. Plasma lipid metabolites associate with diabetic polyneuropathy in a cohort with type 2 diabetes. Ann. Clin. Transl. Neurol. 2021, 8, 1292–1307. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Hu, M.; Wang, J.; Xia, H.; Yang, X.; Yang, L.; Sun, G. Perilla Oil Supplementation Improves Hypertriglyceridemia and Gut Dysbiosis in Diabetic KKAy Mice. Mol. Nutr. Food Res. 2018, 62, e1800299. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Shaw, J.E.; Zimmet, P.Z.; McCarty, D.; de Courten, M. Type 2 diabetes worldwide according to the new classification and criteria. Diabetes Care 2000, 23 (Suppl. S2), B5–B10. [Google Scholar]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Raubenheimer, P.J.; Nyirenda, M.J.; Walker, B.R. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes 2006, 55, 2015–2020. [Google Scholar] [CrossRef]
- Jacobs, R.L.; Zhao, Y.; Koonen, D.P.; Sletten, T.; Su, B.; Lingrell, S.; Cao, G.; Peake, D.A.; Kuo, M.S.; Proctor, S.D.; et al. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J. Biol. Chem. 2010, 285, 22403–22413. [Google Scholar] [CrossRef]
- Zhu, C.; Sawrey-Kubicek, L.; Beals, E.; Hughes, R.L.; Rhodes, C.H.; Sacchi, R.; Zivkovic, A.M. The HDL lipidome is widely remodeled by fast food versus Mediterranean diet in 4 days. Metabolomics 2019, 15, 114. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Giardina, S.; Cañueto, D.; Salas-Salvadó, J.; Cañellas, N.; Bulló, M. Changes in Plasma Metabolite Concentrations after a Low-Glycemic Index Diet Intervention. Mol. Nutr. Food Res. 2019, 63, e1700975. [Google Scholar] [CrossRef] [PubMed]
- Žáček, P.; Bukowski, M.; Johnson, L.; Raatz, S.K.; Picklo, M. Selective enrichment of n-3 fatty acids in human plasma lipid motifs following intake of marine fish. J. Nutr. Biochem. 2018, 54, 57–65. [Google Scholar] [CrossRef]
- Burdge, G.C.; Tricon, S.; Morgan, R.; Kliem, K.E.; Childs, C.; Jones, E.; Russell, J.J.; Grimble, R.F.; Williams, C.M.; Yaqoob, P.; et al. Incorporation of cis-9, trans-11 conjugated linoleic acid and vaccenic acid (trans-11 18:1) into plasma and leucocyte lipids in healthy men consuming dairy products naturally enriched in these fatty acids. Br. J. Nutr. 2005, 94, 237–243. [Google Scholar] [CrossRef]
- Chen, S.; Zong, G.; Wu, Q.; Yun, H.; Niu, Z.; Zheng, H.; Zeng, R.; Sun, L.; Lin, X. Associations of plasma glycerophospholipid profile with modifiable lifestyles and incident diabetes in middle-aged and older Chinese. Diabetologia 2022, 65, 315–328. [Google Scholar] [CrossRef]
- Lu, J.; Lam, S.M.; Wan, Q.; Shi, L.; Huo, Y.; Chen, L.; Tang, X.; Li, B.; Wu, X.; Peng, K.; et al. High-Coverage Targeted Lipidomics Reveals Novel Serum Lipid Predictors and Lipid Pathway Dysregulation Antecedent to Type 2 Diabetes Onset in Normoglycemic Chinese Adults. Diabetes Care 2019, 42, 2117–2126. [Google Scholar] [CrossRef]
- Rinella, M.E.; Elias, M.S.; Smolak, R.R.; Fu, T.; Borensztajn, J.; Green, R.M. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J. Lipid Res. 2008, 49, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; von Ruesten, A.; Drogan, D.; Schulze, M.B.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H. Variation of serum metabolites related to habitual diet: A targeted metabolomic approach in EPIC-Potsdam. Eur. J. Clin. Nutr. 2013, 67, 1100–1108. [Google Scholar] [CrossRef]
- Wittenbecher, C.; Mühlenbruch, K.; Kröger, J.; Jacobs, S.; Kuxhaus, O.; Floegel, A.; Fritsche, A.; Pischon, T.; Prehn, C.; Adamski, J.; et al. Amino acids, lipid metabolites, and ferritin as potential mediators linking red meat consumption to type 2 diabetes. Am. J. Clin. Nutr. 2015, 101, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Zhu, J.; Sun, L.; Ye, X.; Lu, L.; Jin, Q.; Zheng, H.; Yu, Z.; Zhu, Z.; Li, H.; et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am. J. Clin. Nutr. 2013, 98, 319–326. [Google Scholar] [CrossRef]
- Sieber, J.; Lindenmeyer, M.T.; Kampe, K.; Campbell, K.N.; Cohen, C.D.; Hopfer, H.; Mundel, P.; Jehle, A.W. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Renal Physiol. 2010, 299, F821–F829. [Google Scholar] [CrossRef] [PubMed]
- Weigert, C.; Brodbeck, K.; Staiger, H.; Kausch, C.; Machicao, F.; Häring, H.U.; Schleicher, E.D. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. 2004, 279, 23942–23952. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Orho-Melander, M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: From current human evidence to future possibilities. Diabetologia 2017, 60, 943–951. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
- Ulven, T.; Christiansen, E. Dietary Fatty Acids and Their Potential for Controlling Metabolic Diseases Through Activation of FFA4/GPR120. Annu. Rev. Nutr. 2015, 35, 239–263. [Google Scholar] [CrossRef]
- White, P.J.; Marette, A. Potential role of omega-3-derived resolution mediators in metabolic inflammation. Immunol. Cell Biol. 2014, 92, 324–330. [Google Scholar] [CrossRef]
- Pan, H.; Guo, R.; Zhu, J.; Wang, Q.; Ju, Y.; Xie, Y.; Zheng, Y.; Wang, Z.; Li, T.; Liu, Z.; et al. A gene catalogue of the Sprague-Dawley rat gut metagenome. Gigascience 2018, 7, giy055. [Google Scholar] [CrossRef]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Lee, S.S.; Mamuad, L.; Choi, Y.J.; Jeong, C.D.; Son, A.; Cho, K.K.; Kim, E.T.; Kim, S.B.; Lee, S.S. Enhancing Butyrate Production, Ruminal Fermentation and Microbial Population through Supplementation with Clostridium saccharobutylicum. J. Microbiol. Biotechnol. 2019, 29, 1083–1095. [Google Scholar] [CrossRef]
- Xu, Q.; Qiao, Q.; Gao, Y.; Hou, J.; Hu, M.; Du, Y.; Zhao, K.; Li, X. Gut Microbiota and Their Role in Health and Metabolic Disease of Dairy Cow. Front. Nutr. 2021, 8, 701511. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Osińska-Jaroszuk, M.; Jarosz-Wilkołazka, A.; Jaroszuk-Ściseł, J.; Szałapata, K.; Nowak, A.; Jaszek, M.; Ozimek, E.; Majewska, M. Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 2015, 31, 1823–1844. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Zeng, Z.; Yuan, Q.; Yu, R.; Zhang, J.; Ma, H.; Chen, S. Ameliorative Effects of Probiotic Lactobacillus paracasei NL41 on Insulin Sensitivity, Oxidative Stress, and Beta-Cell Function in a Type 2 Diabetes Mellitus Rat Model. Mol. Nutr. Food Res. 2019, 63, e1900457. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, H.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota-SCFA-hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Dong, L.; Chang, P. Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis. Adv. Nutr. 2019, 10, 133–147. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]

| All (n = 101) | Corn Oil (n = 49) | Fish Oil (n = 52) | p Value | |
|---|---|---|---|---|
| Age (years) | 53.81 ± 8.82 | 54.29 ± 8.45 | 53.37 ± 9.22 | 0.603 |
| Sex (male/female) | 66/39 | 35/14 | 31/21 | 0.212 |
| Duration of T2DM (years) | 6.0 (3.0, 11.0) | 6.0 (2.0, 11.0) | 7.0 (3.0, 12.0) | 0.758 |
| BMI (kg/m2) | 24.95 ± 2.94 | 24.86 ± 2.86 | 25.03 ± 3.04 | 0.767 |
| Waist-to-hip ratio | 0.91 ± 0.06 | 0.90 ± 0.06 | 0.91 ± 0.06 | 0.478 |
| Diurnal systolic BP (mm Hg) | 129.90 ± 17.52 | 129.53 ± 18.02 | 130.25 ± 17.20 | 0.838 |
| Diurnal diastolic BP (mm Hg) | 76.90 ± 10.46 | 76.37 ± 10.53 | 77.4 ± 10.47 | 0.621 |
| Fasting plasma glucose (mmol/L) | 6.38 ± 1.78 | 6.25 ± 2.00 | 6.49 ± 1.56 | 0.502 |
| Hemoglobin A1c (mmol/moL) | 51.56 ± 11.01 | 52.54 ± 11.98 | 50.63 ± 10.04 | 0.387 |
| Fasting plasma insulin (mU/L) | 10.76 ± 8.89 | 10.92 ± 11.17 | 10.61 ± 6.13 | 0.861 |
| HOMA-IR | 1.98 ± 0.76 | 1.98 ± 0.86 | 1.98 ± 0.65 | 0.961 |
| Total cholesterol (mmol/L) | 4.69 ± 0.90 | 4.56 ± 0.92 | 4.81 ± 0.87 | 0.160 |
| LDL-C (mmol/L) | 2.40 ± 0.81 | 2.49 ± 0.87 | 2.32 ± 0.75 | 0.302 |
| HDL-C (mmol/L) | 1.25 ± 0.30 | 1.30 ± 0.27 | 1.19 ± 0.33 | 0.109 |
| Triglycerides (mmol/L) | 2.28 ± 2.09 | 2.09 ± 1.74 | 2.46 ± 2.37 | 0.370 |
| ApoA1 (g/L) | 1.14 ± 0.23 | 1.17 ± 0.22 | 1.11 ± 0.24 | 0.159 |
| ApoB (g/L) | 0.80 ± 0.17 | 0.83 ± 0.18 | 0.77 ± 0.15 | 0.084 |
| Total physical activity (MET-minute/per week) | 2807.52 ± 509.28 | 2824.03 ± 552.39 | 2791.97 ± 469.95 | 0.754 |
| Diabetes medications | ||||
| Oral antidiabetic agent (s) | 40 | 19 | 21 | |
| Insulin injection | 19 | 9 | 10 | 0.658 |
| Both | 35 | 19 | 16 | |
| Dietary therapy alone | 7 | 2 | 5 |
| Variable | Corn Oil Group (n = 49) | Fish Oil Group (n = 52) | MD (95% CI), p |
|---|---|---|---|
| Energy (kcal/day) | |||
| Baseline | 1653.75 ± 325.61 | 1796.40 ± 537.18 | −142.65 (−323.35, 38.04), 0.120 |
| 3 months | 1695.73 ± 457.55 | 1808.11 ± 533.41 | −112.39 (−316.84, 92.06), 0.278 |
| MD (95% CI), p | 41.98 (−119.28, 203.23), 0.602 | 11.71 (−193.58, 217.01), 0.909 | |
| Fat (g) | |||
| Baseline | 51.22 ± 13.00 | 58.46 ± 24.12 | −7.24 (−15.28, 0.80), 0.077 |
| 3 months | 52.72 ± 10.11 | 59.95 ± 22.93 | −7.23 (−14.60, 0.14), 0.054 |
| MD (95% CI), p | 1.50 (−0.99, 3.99), 0.230 | 1.49 (−2.77, 5.76), 0.485 | |
| Protein (g) | |||
| Baseline | 51.78 ± 15.66 | 53.31 ± 17.43 | −1.54 (−8.35, 5.28), 0.655 |
| 3 months | 51.88 ± 16.69 | 52.61 ± 18.82 | −0.73 (−8.05, 6.58), 0.842 |
| MD (95% CI), p | 0.10 (−7.37, 7.57), 0.978 | −0.70 (−8.23, 6.83), 0.853 | |
| Carbohydrate (g) | |||
| Baseline | 246.42 ± 63.95 | 264.26 ± 89.32 | −17.85 (−49.92, 14.23), 0.272 |
| 3 months | 253.43 ± 98.89 | 264.53 ± 99.75 | −11.10 (−51.83, 29.64), 0.590 |
| MD (95% CI), p | 7.02 (−26.68, 40.71), 0.677 | 0.27 (−42.13, 42.66), 0.990 | |
| Cellulose (g) | |||
| Baseline | 7.50 ± 5.32 | 7.51 ± 4.52 | −0.01 (−2.03, 2.00), 0.990 |
| 3 months | 7.10 ± 3.40 | 7.04 ± 3.20 | 0.06 (−1.29, 1.41), 0.930 |
| MD (95% CI), p | −0.39 (−2.22, 1.43), 0.666 | −0.47 (−2.10, 1.17), 0.568 |
| Variable | Corn Oil Group (n = 49) | Fish Oil Group (n = 52) | MD (95% CI), p |
|---|---|---|---|
| FBS (mmol/L) | |||
| Baseline | 6.25 ± 2.00 | 6.49 ± 1.56 | −0.24 (−0.95, 0.47), 0.502 |
| 3 months | 7.66 ± 2.61 | 6.76 ± 1.55 | 0.90 (0.05, 1.75), 0.039 |
| MD (95% CI), p | 1.41 (0.78, 2.04), <0.001 | 0.27 (−0.27, 0.81), 0.328 | |
| FI (mU/L) | |||
| Baseline | 10.92 ± 11.17 | 10.61 ± 16.13 | 0.31 (−3.22, 3.84), 0.861 |
| 3 months | 10.64 ± 6.95 | 8.87 ± 2.81 | 1.77 (−0.36, 3.90), 0.102 |
| MD (95% CI), p | −0.29 (−2.58, 2.00), 0.801 | −1.74 (−3.20, −0.29), 0.019 | |
| HbA1c (%) | |||
| Baseline | 6.96 ± 1.10 | 6.78 ± 0.92 | 0.17 (−0.22, 0.57), 0.387 |
| 3 months | 7.03 ± 1.04 | 6.66 ± 0.81 | 0.37 (0.00, 0.74), 0.048 |
| MD (95% CI), p | 0.07 (−0.21, 0.36), 0.606 | −0.12 (−0.33, 0.09), 0.259 | |
| HOMA-IR | |||
| Baseline | 1.98 ± 0.86 | 1.98 ± 0.65 | 0.00 (−0.31, 0.29), 0.961 |
| 3 months | 2.48 ± 1.24 | 2.02 ± 0.62 | 0.46 (0.07, 0.85), 0.022 |
| MD (95% CI), p | 0.51 (0.23, 0.79), 0.001 | 0.04 (−0.17, 0.25), 0.705 | |
| TC (mmol/L) | |||
| Baseline | 4.56 ± 0.92 | 4.81 ± 0.87 | −0.25 (−0.61, 0.10), 0.160 |
| 3 months | 5.06 ± 1.02 | 4.32 ± 0.75 | 0.73 (0.38, 1.08), <0.001 |
| MD (95% CI), p | 0.50 (0.28, 0.71), <0.001 | −0.49 (−0.71, −0.27), <0.001 | |
| TG (mmol/L) | |||
| Baseline | 2.09 ± 1.74 | 2.46 ± 2.37 | −0.37 (−1.20, 0.45), 0.370 |
| 3 months | 2.06 ± 2.26 | 1.33 ± 0.63 | 0.73 (0.06, 1.40), 0.034 |
| MD (95% CI), p | −0.03 (−0.37, 0.31), 0.867 | −1.13 (−1.75, −0.51), 0.001 | |
| LDL-C (mmol/L) | |||
| Baseline | 2.49 ± 0.87 | 2.32 ± 0.75 | 0.17 (−0.15, 0.49), 0.302 |
| 3 months | 2.89 ± 0.89 | 2.58 ± 0.65 | 0.31 (0.00, 0.61), 0.048 |
| MD (95% CI), p | 0.40 (0.21, 0.59), <0.001 | 0.26 (0.05, 0.47), 0.018 | |
| HDL-C (mmol/L) | |||
| Baseline | 1.30 ± 0.27 | 1.19 ± 0.33 | 0.10 (−0.01, 0.22), 0.084 |
| 3 months | 1.55 ± 0.32 | 1.46 ± 0.36 | 0.09 (−0.05, 0.22), 0.210 |
| MD (95% CI), p | 0.25 (0.19, 0.30), <0.001 | 0.27 (0.21, 0.32), <0.001 | |
| Non-HDL (mmol/L) | |||
| Baseline | 3.26 ± 0.86 | 3.62 ± 0.92 | −0.36 (−0.71, 0.00), 0.046 |
| 3 months | 3.51 ± 1.10 | 2.86 ± 0.78 | −0.36 (−0.71, 0.00), 0.046 |
| MD (95% CI), p | 0.24 (0.01, 0.48), 0.041 | −0.76 (−0.99, −0.52), <0.001 | 0.65 (0.26, 1.03), 0.001 |
| Fish Oil Group (n = 38) | Corn Oil Group (n = 37) | |
|---|---|---|
| LPC(22:4) | 28.15 (23.42, 38.51) | 61.33 (49.05, 77.03) |
| LPE(22:4) | 1.46 (0.88, 2.20) | 3.08 (2.25, 4.43) |
| PC(16:0/22:4) | 1869.97 (1468.17, 2428.25) | 3926.66 (3054.74, 5474.43) |
| PC(18:1/22:4) | 163.13 (133.72, 186.76) | 335.58 (274.94, 455.75) |
| PE(16:0/22:4) | 8.31 (6.00, 15.26) | 28.48 (19.81, 42.54) |
| PE(O-16:0/22:4) | 13.64 (8.92, 20.33) | 33.35 (24.75, 42.23) |
| PE(P-16:0/22:4) | 78.23 (57.80, 88.45) | 156.51 (115.55, 194.56) |
| PE(P-18:0/22:4) | 84.54 (57.73, 107.74) | 176.93 (125.70, 211.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Yin, S.; Yu, J.; Wang, J.; Jin, X.; Wang, Y.; Liu, H.; Sun, G. Improvement in Glycolipid Metabolism Parameters After Supplementing Fish Oil-Derived Omega-3 Fatty Acids Is Associated with Gut Microbiota and Lipid Metabolites in Type 2 Diabetes Mellitus. Nutrients 2024, 16, 3755. https://doi.org/10.3390/nu16213755
Xia J, Yin S, Yu J, Wang J, Jin X, Wang Y, Liu H, Sun G. Improvement in Glycolipid Metabolism Parameters After Supplementing Fish Oil-Derived Omega-3 Fatty Acids Is Associated with Gut Microbiota and Lipid Metabolites in Type 2 Diabetes Mellitus. Nutrients. 2024; 16(21):3755. https://doi.org/10.3390/nu16213755
Chicago/Turabian StyleXia, Jiayue, Shiyu Yin, Junhui Yu, Jiongnan Wang, Xingyi Jin, Yuanyuan Wang, Hechun Liu, and Guiju Sun. 2024. "Improvement in Glycolipid Metabolism Parameters After Supplementing Fish Oil-Derived Omega-3 Fatty Acids Is Associated with Gut Microbiota and Lipid Metabolites in Type 2 Diabetes Mellitus" Nutrients 16, no. 21: 3755. https://doi.org/10.3390/nu16213755
APA StyleXia, J., Yin, S., Yu, J., Wang, J., Jin, X., Wang, Y., Liu, H., & Sun, G. (2024). Improvement in Glycolipid Metabolism Parameters After Supplementing Fish Oil-Derived Omega-3 Fatty Acids Is Associated with Gut Microbiota and Lipid Metabolites in Type 2 Diabetes Mellitus. Nutrients, 16(21), 3755. https://doi.org/10.3390/nu16213755








