Interaction of CETP rs708272 Polymorphism on Trans Fatty Acid Intake and Glucose Metabolism Markers
Abstract
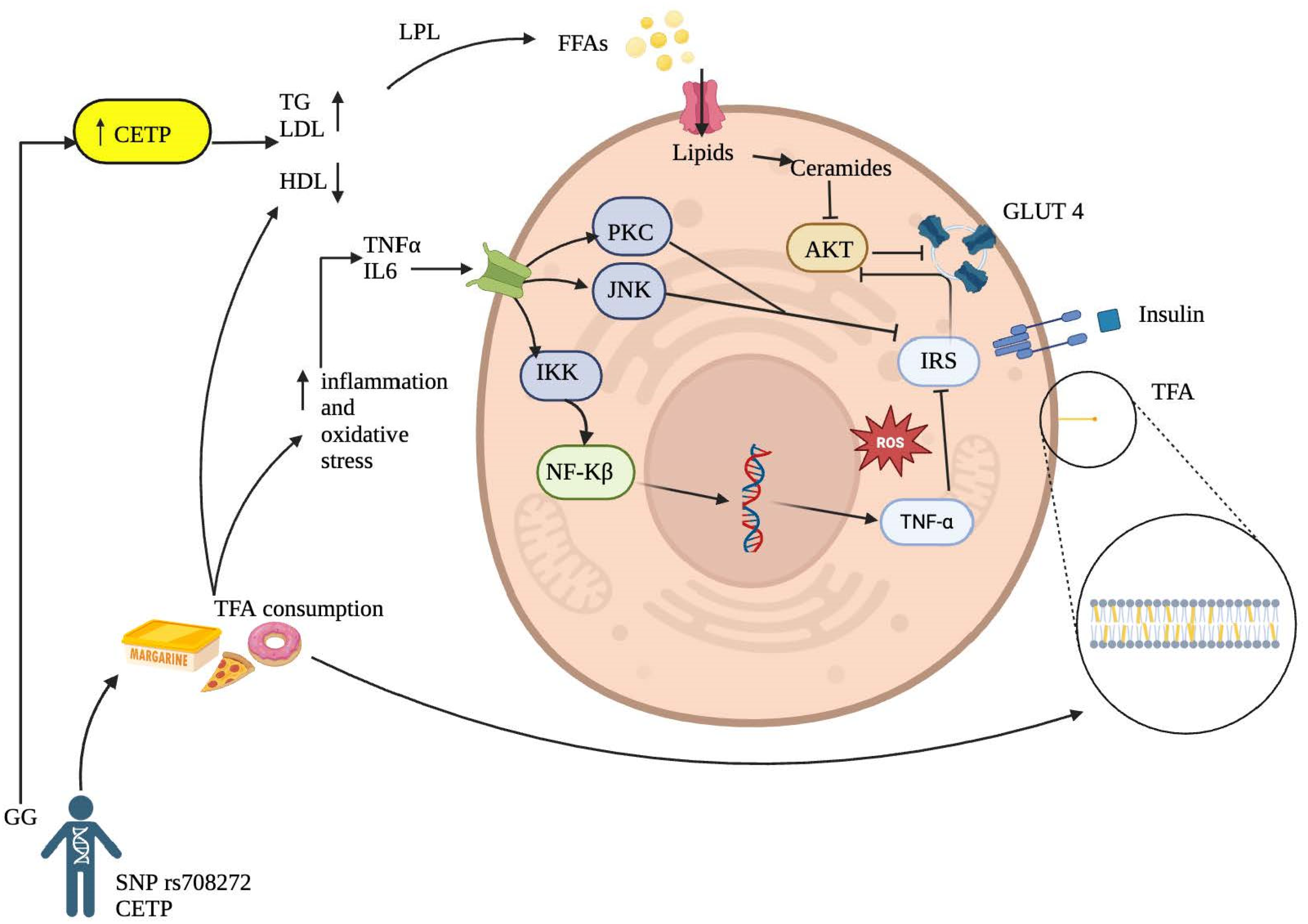
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Genotyping
2.3. Dietary Intake
2.4. Anthropometric and Biochemical Measurements
2.5. Statistical Analyses
3. Results
3.1. Demographic Characteristics of the Study Population
3.2. Dietary Intake
3.3. Anthropometric, Body Composition, and Biochemical Parameters
3.4. Interactions Between CETP rs708272 Genotypes and Baseline Trans-Fatty Acid Intake on Glucose, Insulin, and HOMA-IR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes Mellitus, the Fastest Growing Global Public Health Concern: Early Detection Should Be Focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of Diabetes Mellitus and Diabetes Complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Garces, T.S.; Damasceno, L.L.V.; Sousa, G.J.B.; Cestari, V.R.F.; Pereira, M.L.D.; Moreira, T.M.M. Relationship between Social Development Indicators and Mortality Due to Diabetes Mellitus in Brazil: A Space-Time Analysis. Rev. Lat. Am. Enferm. 2023, 31, e3971. [Google Scholar] [CrossRef]
- Kumar, A.; Gangwar, R.; Ahmad Zargar, A.; Kumar, R.; Sharma, A. Prevalence of Diabetes in India: A Review of IDF Diabetes Atlas 10th Edition. Curr. Diabetes Rev. 2024, 20, 105–114. [Google Scholar] [CrossRef]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.E.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the Diagnosis of Insulin Resistance: Focusing on the Role of HOMA-IR and Tryglyceride/Glucose Index. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102581. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Precision Nutrition for Prevention and Management of Type 2 Diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef]
- Semaev, S.; Shakhtshneider, E.; Orlov, P.; Ivanoshchuk, D.; Malyutina, S.; Gafarov, V.; Ragino, Y.; Voevoda, M. Association of RS708272 (CETP Gene Variant) with Lipid Profile Parameters and the Risk of Myocardial Infarction in the White Population of Western Siberia. Biomolecules 2019, 9, 739. [Google Scholar] [CrossRef]
- El-Lebedy, D. Interaction between Endothelial Nitric Oxide Synthase Rs1799983, Cholesteryl Ester-Transfer Protein Rs708272 and Angiopoietin-like Protein 8 Rs2278426 Gene Variants Highly Elevates the Risk of Type 2 Diabetes Mellitus and Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 97. [Google Scholar] [CrossRef]
- Cupido, A.J.; Reeskamp, L.F.; Hingorani, A.D.; Finan, C.; Asselbergs, F.W.; Hovingh, G.K.; Schmidt, A.F. Joint Genetic Inhibition of PCSK9 and CETP and the Association with Coronary Artery Disease: A Factorial Mendelian Randomization Study. JAMA Cardiol. 2022, 7, 955–964. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The Crucial Role and Mechanism of Insulin Resistance in Metabolic Disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Oteng, A.B.; Kersten, S. Mechanisms of Action of Trans Fatty Acids. Adv. Nutr. 2020, 11, 697–708. [Google Scholar] [CrossRef]
- Wilczek, M.M.; Olszewski, R.; Krupienicz, A. Trans-Fatty Acids and Cardiovascular Disease: Urgent Need for Legislation. Cardiology 2017, 138, 254–258. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans Fatty Acids and Lipid Profile: A Serious Risk Factor to Cardiovascular Disease, Cancer and Diabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary Total Fat, Fatty Acids Intake, and Risk of Cardiovascular Disease: A Dose-Response Meta-Analysis of Cohort Studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef]
- Niforou, A.; Magriplis, E.; Klinaki, E.; Niforou, K.; Naska, A. On Account of Trans Fatty Acids and Cardiovascular Disease Risk—There Is Still Need to Upgrade the Knowledge and Educate Consumers. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1811–1818. [Google Scholar] [CrossRef]
- Pérez-Beltrán, Y.E.; González-Becerra, K.; Rivera-Iñiguez, I.; Martínez-López, E.; Ramos-López, O.; Alcaraz-Mejía, M.; Rodríguez-Echevarría, R.; Sáyago-Ayerdi, S.G.; Mendivil, E.J. A Nutrigenetic Strategy for Reducing Blood Lipids and Low-Grade Inflammation in Adults with Obesity and Overweight. Nutrients 2023, 15, 4324. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- OMS. 2018 ENT: Manual STEPS Manual de Vigilancia STEPS de La OMS: El Método STEPwise de La OMS Para La Vigilancia de Los Factores de Riesgo de Las Enfermedades Crónicas; OMS: Geneva, Switzerland, 2018. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- OMS Body Mass Index—BMI. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 7 August 2024).
- Vargas-Alarcón, G.; Pérez-Méndez, O.; Posadas-Sánchez, R.; Peña-Duque, M.A.; Martínez-Ríos, M.A.; Delgadillo-Rodriguez, H.; Fragoso, J.M. The Rs4783961 and Rs708272 Genetic Variants of the CETP Gene Are Associated with Coronary Artery Disease, but Not with Restenosis after Coronary Stenting. Arch. Cardiol. Mex. 2022, 92, 334–341. [Google Scholar] [CrossRef]
- Occa, A.; Leip, A.; Merritt, A.S.; Stapleton, J.L. Prevalence and Correlates of Invitation to Participate in Clinical Trials among US Adults. Prev. Med. Rep. 2022, 26, 101742. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Galván-Valencia, Ó.; Hernández-Barrera, L.; Oviedo-Solís, C.; Barquera, S. Prevalencia de Obesidad y Factores de Riesgo Asociados En Adultos Mexicanos: Resultados de La Ensanut 2022. Salud Publica Mex. 2023, 65, s238–s247. [Google Scholar] [CrossRef]
- Daneshzad, E.; Rostami, S.; Aghamahdi, F.; Mahdavi-Gorabi, A.; Qorbani, M. Association of Cardiometabolic Risk Factors with Insulin Resistance in Overweight and Obese Children. BMC Endocr. Disord. 2022, 22, 320. [Google Scholar] [CrossRef]
- Krishnamoorthy, Y.; Rajaa, S.; Murali, S.; Sahoo, J.; Kar, S.S. Association Between Anthropometric Risk Factors and Metabolic Syndrome Among Adults in India: A Systematic Review and Meta-Analysis of Observational Studies. Prev. Chronic Dis. 2022, 19, E24. [Google Scholar] [CrossRef]
- Most, J.; Redman, L.M. Impact of Calorie Restriction on Energy Metabolism in Humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef]
- OMS. Proyectos de Recomendaciones Para La Prevención y El Tratamiento de La Obesidad a Lo Largo Del Curso de La Vida, Incluidas Las Posibles Metas- Documento de Debate de La OMS; OMS: Geneva, Switzerland, 2021. [Google Scholar]
- Pavía L, A.; Aguilar S, C.; Alexanderson R, E.; Ahumada A, M.; Alcocer G, M.; Arenas, J.L.; Arenas A, L.D.R.; Borges V, O.; Benavides, M.A.; Cardona, E.; et al. Consenso de La Sociedad Mexicana de Cardiología En El Diagnóstico y Tratamiento de Las Dislipidemias y Aterosclerosis. Med. Interna Mex. 2020, 36, 390–413. [Google Scholar] [CrossRef]
- Hannon, B.A.; Khan, N.A.; Teran-Garcia, M. Nutrigenetic Contributions to Dyslipidemia: A Focus on Physiologically Relevant Pathways of Lipid and Lipoprotein Metabolism. Nutrients 2018, 10, 1404. [Google Scholar] [CrossRef]
- Rashid, S.; Genest, J. Effect of Obesity on High-Density Lipoprotein Metabolism. Obesity 2007, 15, 2875–2888. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of Current Evidence and Clinical Recommendations on the Effects of Low-Carbohydrate and Very-Low-Carbohydrate (Including Ketogenic) Diets for the Management of Body Weight and Other Cardiometabolic Risk Factors: A Scientific Statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Dellis, D.; Tsilingiris, D.; Eleftheriadou, I.; Tentolouris, A.; Sfikakis, P.P.; Dellis, G.; Karanasiou, M.; Meimari, A.; Dimosthenopoulos, C.; Lazarou, S.; et al. Carbohydrate Restriction in the Morning Increases Weight Loss Effect of a Hypocaloric Mediterranean Type Diet: A Randomized, Parallel Group Dietary Intervention in Overweight and Obese Subjects. Nutrition 2020, 71, 110578. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Alfredo Martinez, J.; Kohlmeier, M. Principles of Nutrigenetics and Nutrigenomics: Fundamentals for Individualized Nutrition; De Caterina, R., Alfredo Martinez, J., Kohlmeier, M., Eds.; Elsevier: London, UK, 2019; ISBN 9780128045725. [Google Scholar]
- Castro-Martínez, M.G.; Bolado-García, V.E.; Landa-Anell, M.V.; Liceaga-Cravioto, M.G.; Soto-González, J.; López-Alvarenga, J.C. Ácidos Grasos Trans de La Dieta y Sus Implicaciones Metabólicas. Gac. Med. Mex. 2010, 146, 281–288. [Google Scholar] [PubMed]
- Ballesteros-Vásquez, M.N.; Valenzuela-Calvillo, L.S.; Artalejo-Ochoa, E.; Robles-Sardin, A.E. Ácidos Grasos Trans: Un Análisis Del Efecto de Su Consumo En La Salud Humana, Regulación Del Contenido En Alimentos y Alternativas Para Disminuirlos. Nutr. Hosp. 2012, 27, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Schulze, M.B.; Meigs, J.B.; Manson, J.E.; Rifai, N.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Consumption of Trans Fatty Acids Is Related to Plasma Biomarkers of Inflammation and Endothelial Dysfunction1. J. Nutr. 2005, 135, 562–566. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Hu, Y.-Q.; Dai, Z.-C.; Li, X.-J.; Zhang, J. Associations between Trans Fatty Acids and Systemic Immune-Inflammation Index: A Cross-Sectional Study. Lipids Health Dis. 2024, 23, 122. [Google Scholar] [CrossRef]
- Fernández Michel, S.G.; García Díaz, C.L.; Alanís Guzmán, M.G.; Ramos Clamont, M.G. Ácidos grasos trans: Consumo e implicaciones en la salud en niños. Cienc. Tecnol. Aliment. 2008, 6, 71–80. [Google Scholar] [CrossRef]

| Parameter | Total Population | Genotype (GG) | Genotype (GA+AA) | p |
|---|---|---|---|---|
| N= | 78 | 24 (31%) | 54 (69%) | - |
| Age | 34.6 ± 8.1 | 36.0 ± 7.9 | 33.9 ± 8.2 | 0.28 * |
| Men | 38 (48%) | 14 (58%) | 24 (44%) | 0.37 # |
| Women | 40 (52%) | 10 (42%) | 30 (56%) |
| Component | Baseline | Final | ∆ | p |
|---|---|---|---|---|
| Energy (kcal) | 1975.15 ± 663.78 | 1582.82 ± 580.43 | −392.32± 833.04 | <0.001 * |
| Protein (g) | 98.0 ± 49.30 | 96.08 ± 25.84 | −1.92 ± 54.05 | 0.941 |
| Carbohydrates (g) | 251.62 ± 164.12 | 177.78 ± 65.20 | −73.83 ± 166.22 | <0.001 * |
| Lipids (g) | 78.73 ± 37.18 | 58.88 ± 24.22 | −19.84 ± 45.18 | <0.001 * |
| SFA (g) | 24.63 ± 12.12 | 17.76 ± 9.32 | −6.86 ± 15.59 | <0.001 * |
| TFA (g) | 0.3228 ± 0.4782 | 0.1069 ± 0.536 | −0.106 ± 0.536 | 0.733 |
| PUFA (g) | 15.54 ± 11.23 | 12.33 ± 5.33 | −3.212 ± 12.49 | 0.149 |
| MUFA (g) | 24.68 ± 14.98 | 20.51 ± 9.70 | −4.17 ± 18.31 | 0.053 |
| Cholesterol (mg) | 397.258 ± 211.93 | 337.58 ± 165.27 | −59.674 ± 216.99 | 0.020 * |
| Parameter | Baseline | Final | ∆ | p |
|---|---|---|---|---|
| Anthropometric parameters | ||||
| Body weight (kg) | 86.35 ±15.13 | 82.86 ± 14.60 | −3.48 ± 3.06 | <0.001 * |
| BMI (kg/m2) | 30.60 ± 4.08 | 29.34± 4.08 | −1.26 ± 1.02 | <0.001 * |
| Waist circumference (cm) | 94.96 ± 12.14 | 90.85 ± 11.37 | −4.10 ± 4.46 | <0.001 * |
| Hip circumference (cm) | 110.30 ± 7.91 | 107.31± 7.87 | −2.98 ± 2.81 | <0.001 * |
| Total body fat mass (kg) | 32.50 ± 8.57 | 29.66 ± 8.60 | −2.84 ± 2.56 | <0.001 * |
| Muscle mass (kg) | 30.15 ± 6.44 | 29.75 ± 6.46 | −0.40 ± 0.77 | <0.001 * |
| Biochemical parameters | ||||
| Glucose (mg/dL) | 89.56 ± 11.46 | 85.91 ± 12.42 | −3.65 ± 9.10 | <0.001 * |
| Insulin (µUI/mL) | 18.75 ± 12.46 | 14.14 ± 9.24 | −4.61 ± 9.83 | <0.001 * |
| HOMA-IR | 4.24 ± 3.27 | 3.07 ± 2.32 | −1.16 ± 2.39 | <0.001 * |
| Total cholesterol (mg/dL) | 175.55 ± 30.88 | 160.01 ± 29.31 | −15.53 ± 20.48 | <0.001 * |
| HDL-cholesterol (mg/dL) | 36.30 ± 9.16 | 35.73 ± 8.80 | −0.577 ± 5.42 | 0.274 |
| VLDL-cholesterol (mg/dL) | 32.66 ± 15.70 | 27.82 ± 13.79 | −4.84 ± 12.76 | <0.001 * |
| LDL cholesterol (mg/dL) | 106.59± 26.78 | 96.39 ± 23.93 | −10.19 ± 19.33 | <0.001 * |
| Triglycerides (mg/dL) | 163.15 ± 78.49 | 139.19 ± 68.91 | −23.96 ± 63.86 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendivil, E.J.; Barcenas-Rivera, G.; Ramos-Lopez, O.; Hernández-Guerrero, C.; Rivera-Iñiguez, I.; Pérez-Beltrán, Y.E. Interaction of CETP rs708272 Polymorphism on Trans Fatty Acid Intake and Glucose Metabolism Markers. Nutrients 2024, 16, 3683. https://doi.org/10.3390/nu16213683
Mendivil EJ, Barcenas-Rivera G, Ramos-Lopez O, Hernández-Guerrero C, Rivera-Iñiguez I, Pérez-Beltrán YE. Interaction of CETP rs708272 Polymorphism on Trans Fatty Acid Intake and Glucose Metabolism Markers. Nutrients. 2024; 16(21):3683. https://doi.org/10.3390/nu16213683
Chicago/Turabian StyleMendivil, Edgar J., Gerardo Barcenas-Rivera, Omar Ramos-Lopez, Cesar Hernández-Guerrero, Ingrid Rivera-Iñiguez, and Yolanda E. Pérez-Beltrán. 2024. "Interaction of CETP rs708272 Polymorphism on Trans Fatty Acid Intake and Glucose Metabolism Markers" Nutrients 16, no. 21: 3683. https://doi.org/10.3390/nu16213683
APA StyleMendivil, E. J., Barcenas-Rivera, G., Ramos-Lopez, O., Hernández-Guerrero, C., Rivera-Iñiguez, I., & Pérez-Beltrán, Y. E. (2024). Interaction of CETP rs708272 Polymorphism on Trans Fatty Acid Intake and Glucose Metabolism Markers. Nutrients, 16(21), 3683. https://doi.org/10.3390/nu16213683





