Gluten Unraveled: Latest Insights on Terminology, Diagnosis, Pathophysiology, Dietary Strategies, and Intestinal Microbiota Modulations—A Decade in Review
Highlights
- Label inaccuracies, gluten contamination and cross-contamination remain a concern in gluten-free products.
- Long-term adherence to a strict gluten-free diet—the gold standard therapy for gluten-related disorders—has been associated with nutritional imbalances, a reduced quality of life, increased financial stress, and increased social isolation in some patients.
- It has been found that the modulation of the gut microbiota plays a role in the progression and management of gluten-related disorders.
Abstract
1. Introduction
1.1. Investigating the Culprit
1.2. Living Gluten-Free: Dos and Don’ts
1.3. Gluten Contamination and the Oat Controversy
1.4. Development of Gluten-Free Products
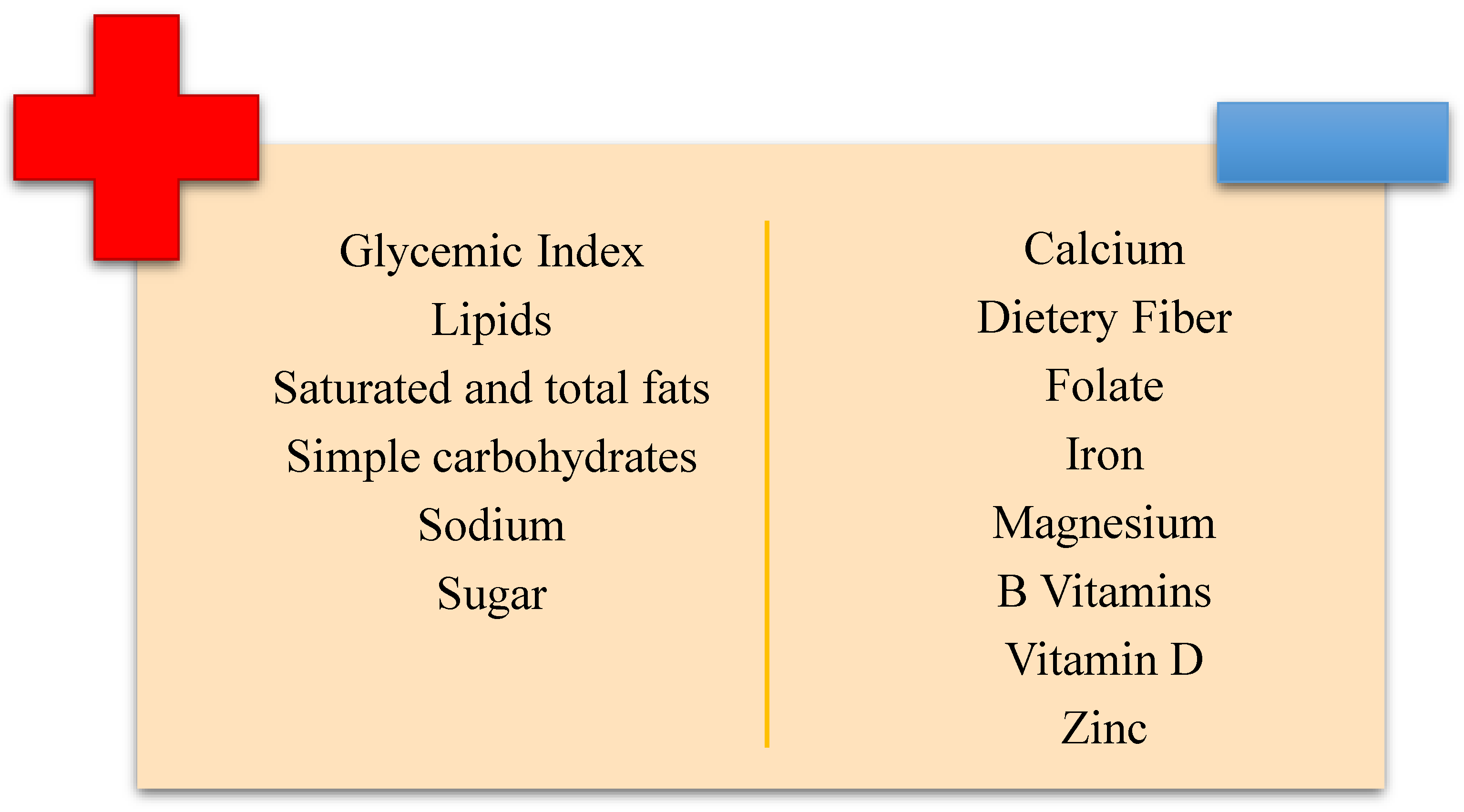
1.5. The Nutritional Deficiencies Linked to a GFD
2. Celiac Gluten Intolerance or “Celiac Disease”
2.1. Definition, Physiopathology, and General Presentation of the Disease
2.2. Symptomatology
2.3. Diagnosis
2.4. Nutritional Deficits Associated with CD
2.5. Therapy and Impact on the Quality of Life
2.6. Adherence to Therapy and Follow-Up
2.7. Reintroduction of Gluten—Is It Possible?
3. Non-Celiac Gluten Sensitivity
3.1. Definition, Diagnosis, Symptoms, and General Presentation
3.2. Pathophysiology
3.3. Nutritional Deficits Associated with NCGS
3.4. Therapy and Follow-Up
4. Intestinal Microbiota Modulations and Dysbiosis
Prebiotics, Probiotics, Synbiotics, Postbiotics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guandalini, S.; Polanco, I. Nonceliac Gluten Sensitivity or Wheat Intolerance Syndrome? J. Pediatr. 2015, 166, 805–811. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Therrien, A.; Kelly, C.P. Celiac Disease: Fallacies and Facts. Am. J. Gastroenterol. 2021, 116, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Valenti, S.; Corica, D.; Ricciardi, L.; Romano, C. Gluten-Related Disorders: Certainties, Questions and Doubts. Ann. Med. 2017, 49, 569–581. [Google Scholar] [CrossRef]
- Verdelli, A.; Corrà, A.; Mariotti, E.B.; Aimo, C.; Quintarelli, L.; Ruffo di Calabria, V.; Donati, M.E.; Bonciolini, V.; Antiga, E.; Caproni, M. Skin Gluten-Related Disorders: New and Old Cutaneous Manifestations to Be Considered. Front. Med. 2023, 10, 1155288. [Google Scholar] [CrossRef]
- Naik, R.D.; Seidner, D.L.; Adams, D.W. Nutritional Consideration in Celiac Disease and Nonceliac Gluten Sensitivity. Gastroenterol. Clin. 2018, 47, 139–154. [Google Scholar] [CrossRef]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Domigan, L.; Larsen, N.; Gerrard, J.; Demarais, N. Proteomic Modelling of Gluten Digestion from a Physiologically Relevant Food System: A Focus on the Digestion of Immunogenic Peptides from Wheat Implicated in Celiac Disease. Food Chem. 2020, 333, 127466. [Google Scholar] [CrossRef]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Gerrard, J.; Larsen, N.; Domigan, L. The Effect of Dough Mixing Speed and Work Input on the Structure, Digestibility and Celiac Immunogenicity of the Gluten Macropolymer within Bread. Food Chem. 2021, 359, 129841. [Google Scholar] [CrossRef]
- Sun, J.; Chen, M.; Hou, X.; Li, T.; Qian, H.; Zhang, H.; Li, Y.; Qi, X.; Wang, L. Effect of Phosphate Salts on the Gluten Network Structure and Quality of Wheat Noodles. Food Chem. 2021, 358, 129895. [Google Scholar] [CrossRef]
- Gilissen, L.; van der Meer, I.; Smulders, M. Why Oats Are Safe and Healthy for Celiac Disease Patients. Med. Sci. 2016, 4, 21. [Google Scholar] [CrossRef]
- Rallabhandi, P.; Sharma, G.M.; Pereira, M.; Williams, K.M. Immunological Characterization of the Gluten Fractions and Their Hydrolysates from Wheat, Rye and Barley. J. Agric. Food Chem. 2015, 63, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Sabença, C.; Ribeiro, M.; De Sousa, T.; Bagulho, A.S.; Igrejas, G. Wheat/Gluten-Related Disorders and Gluten-Free Diet Misconceptions: A Review. Foods 2021, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Sánchez, M.I.; Causada-Calo, N.; Bercik, P.; Ford, A.C.; Murray, J.A.; Armstrong, D.; Semrad, C.; Kupfer, S.S.; Alaedini, A.; Moayyedi, P.; et al. Safety of Adding Oats to a Gluten-Free Diet for Patients with Celiac Disease: Systematic Review and Meta-Analysis of Clinical and Observational Studies. Gastroenterology 2017, 153, 395–409.e3. [Google Scholar] [CrossRef] [PubMed]
- La Vieille, S.; Pulido, O.M.; Abbott, M.; Koerner, T.B.; Godefroy, S. Celiac Disease and Gluten-Free Oats: A Canadian Position Based on a Literature Review. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1870305. [Google Scholar] [CrossRef] [PubMed]
- DeGeorge, K.C.; Frye, J.W.; Stein, K.M.; Rollins, L.K.; McCarter, D.F. Celiac Disease and Gluten Sensitivity. Prim. Care-Clin. Off. Pract. 2017, 44, 693–707. [Google Scholar] [CrossRef]
- Ogilvie, O.; Roberts, S.; Sutton, K.; Gerrard, J.; Larsen, N.; Domigan, L. The Effect of Baking Time and Temperature on Gluten Protein Structure and Celiac Peptide Digestibility. Food Res. Int. 2021, 140, 109988. [Google Scholar] [CrossRef]
- Itzlinger, A.; Branchi, F.; Elli, L.; Schumann, M. Gluten-Free Diet in Celiac Disease—Forever and for All? Nutrients 2018, 10, 1796. [Google Scholar] [CrossRef]
- Rostami, K.; Bold, J.; Parr, A.; Johnson, M.W. Gluten-Free Diet Indications, Safety, Quality, Labels, and Challenges. Nutrients 2017, 9, 10–14. [Google Scholar] [CrossRef]
- Bascuñán, K.A.; Vespa, M.C.; Araya, M. Celiac Disease: Understanding the Gluten-Free Diet. Eur. J. Nutr. 2017, 56, 449–459. [Google Scholar] [CrossRef]
- Falcomer, A.L.; Santos Araújo, L.; Farage, P.; Santos Monteiro, J.; Yoshio Nakano, E.; Puppin Zandonadi, R. Gluten Contamination in Food Services and Industry: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 479–493. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Emerson, L. Gluten-Free Labeling: Are Growth Media Containing Wheat, Barley, and Rye Falling through the Cracks? J. Acad. Nutr. Diet. 2018, 118, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Gatti, S.; Galeazzi, T.; Monachesi, C.; Padella, L.; Del Baldo, G.; Annibali, R.; Lionetti, E.; Catassi, C. Gluten Contamination in Naturally or Labeled Gluten-Free Products Marketed in Italy. Nutrients 2017, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; McGough, N.; Urwin, H. Catering Gluten-Free When Simultaneously Using Wheat Flour. J. Food Prot. 2016, 79, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; El Khoury, D.; Joye, I.J. Gluten-Free Product Recalls and Their Impact on Consumer Trust. Nutrients 2023, 15, 4170. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.; Alonso-Garrido, M.; Lozano, M.; Peiró, J.; Manyes, L. Adherence to Dietary Treatment and Clinical Factors Associated with Anti-Transglutaminase Antibodies in Celiac Disease during the Follow-Up. Heliyon 2021, 7, e06642. [Google Scholar] [CrossRef]
- Defeudis, G.; Massari, M.C.; Terrana, G.; Coppola, L.; Napoli, N.; Migliaccio, S. Gluten-Free Diet and Metabolic Syndrome: Could Be a Not Benevolent Encounter? Nutrients 2023, 15, 627. [Google Scholar] [CrossRef]
- de-Magistris, T.; Belarbi, H.; Hellali, W. Examining Non-Celiac Consumers of Gluten-Free Products: An Empirical Evidence in Spain. In Celiac Disease and Non-Celiac Gluten Sensitivity; Rodrigo, L., Ed.; Headquarters IntechOpen Limited: London, UK, 2017. [Google Scholar]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac Disease: Overview and Considerations for Development of Gluten-Free Foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef]
- Calderón de la Barca, A.M.; Luna-Alcocer, V.; Valenzuela-Miranda, J.R.; Mejía-León, M.E. Gluten-Free Labeling Is Misused Frequently in Foods Marketed in Northwestern Mexico. Front. Nutr. 2021, 8, 687843. [Google Scholar] [CrossRef]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef]
- Savarese, M.; Wismer, W.; Graffigna, G. Conceptualizing “Free-from” Food Consumption Determinants: A Systematic Integrative Literature Review Focused on Gluten and Lactose. Food Qual. Prefer. 2021, 90, 104170. [Google Scholar] [CrossRef]
- Chellan, D.; Muktesh, G.; Vaiphei, K.; Berry, N.; Dhaka, N.; Sinha, S.K.; Thapa, B.R.; Kochhar, R. Effect of Gluten-Free Diet and Compliance on Quality of Life in Pediatric Celiac Disease Patients. JGH Open 2019, 3, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.M.; Maurella, C.; Gallina, S.; Gorrasi, I.S.R.; Caramelli, M.; Decastelli, L. Analysis of Gluten Content in Gluten-Free Pizza from Certified Take-Away Pizza Restaurants. Foods 2018, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Gluten-Free Certification. Available online: https://www.aoecs.org/working-with-food/gluten-free-certification/ (accessed on 12 July 2023).
- Gluten-Free Certification. Available online: https://www.beyondceliac.org/gluten-free-diet/gluten-free-certification/ (accessed on 12 July 2023).
- Fritz, R.D.; Chen, Y. Oat Safety for Celiac Disease Patients: Theoretical Analysis Correlates Adverse Symptoms in Clinical Studies to Contaminated Study Oats. Nutr. Res. 2018, 60, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Tanner, G.; Juhász, A.; Florides, C.G.; Nye-Wood, M.; Békés, F.; Colgrave, M.L.; Russell, A.K.; Hardy, M.Y.; Tye-Din, J.A. Preparation and Characterization of Avenin-Enriched Oat Protein by Chill Precipitation for Feeding Trials in Celiac Disease. Front. Nutr. 2019, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Gatti, S.; Galeazzi, T.; Caporelli, N.; Francavilla, R.; Cucchiara, S.; Roggero, P.; Malamisura, B.; Iacono, G.; Tomarchio, S.; et al. Safety of Oats in Children with Celiac Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Pediatr. 2018, 194, 116–122.e2. [Google Scholar] [CrossRef]
- Wieser, H.; Segura, V.; Ruiz-Carnicer, Á.; Sousa, C.; Comino, I. Food Safety and Cross-Contamination of Gluten-Free Products: A Narrative Review. Nutrients 2021, 13, 2244. [Google Scholar] [CrossRef]
- Cheng, F.W.; Handu, D. Nutrition Assessment, Interventions, and Monitoring for Patients with Celiac Disease: An Evidence Analysis Center Scoping Review. J. Acad. Nutr. Diet. 2020, 120, 1381–1406. [Google Scholar] [CrossRef]
- Alencar, N.M.M.; de Araújo, V.A.; Faggian, L.; da Silveira Araújo, M.B.; Capriles, V.D. What about Gluten-Free Products? An Insight on Celiac Consumers’ Opinions and Expectations. J. Sens. Stud. 2021, 36, e12664. [Google Scholar] [CrossRef]
- Demirkesen, I.; Ozkaya, B. Recent Strategies for Tackling the Problems in Gluten-Free Diet and Products. Crit. Rev. Food Sci. Nutr. 2022, 62, 571–597. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Soltanizadeh, N.; Mirmoghtadaee, P.; Banavand, P.; Mirmoghtadaie, L.; Shojaee-Aliabadi, S. Gluten-free Products in Celiac Disease: Nutritional and Technological Challenges and Solutions. J. Res. Med. Sci. 2018, 23, 109. [Google Scholar] [CrossRef]
- Carcea, M. Nutritional Value of Grain-Based Foods. Protein-Based Film. Coat. 2020, 9, 201–232. [Google Scholar] [CrossRef] [PubMed]
- Sahagún, M.; Gómez, M. Influence of Protein Source on Characteristics and Quality of Gluten-Free Cookies. J. Food Sci. Technol. 2018, 55, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, Y.; Zhang, W.; Wu, Y.; Messing, J. Towards Coeliac-Safe Bread. Plant Biotechnol. J. 2020, 18, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Marengo, M.; Bonomi, F.; Marti, A.; Pagani, M.A.; Elkhalifa, A.E.O.; Iametti, S. Molecular Features of Fermented and Sprouted Sorghum Flours Relate to Their Suitability as Components of Enriched Gluten-Free Pasta. LWT-Food Sci. Technol. 2015, 63, 511–518. [Google Scholar] [CrossRef]
- Aguiar, E.V.; Santos, F.G.; Centeno, A.C.L.S.; Capriles, V.D. Defining Amaranth, Buckwheat and Quinoa Flour Levels in Gluten-Free Bread: A Simultaneous Improvement on Physical Properties, Acceptability and Nutrient Composition through Mixture Design. Foods 2022, 11, 848. [Google Scholar] [CrossRef]
- Jamieson, J.A.; Neufeld, A. Food Sources of Energy and Nutrients among Canadian Adults Following a Gluten-Free Diet. PeerJ 2020, 27, e9590. [Google Scholar] [CrossRef]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative Analysis of Plant-Based High-Protein Ingredients and Their Impact on Quality of High-Protein Bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Hoehnel, A.; Bez, J.; Petersen, I.L.; Amarowicz, R.; Juśkiewicz, J.; Zannini, E.; Arendt, E.K. Combining High-Protein Ingredients from Pseudocereals and Legumes for the Development of Fresh High-Protein Hybrid Pasta: Enhanced Nutritional Profile. J. Sci. Food Agric. 2022, 102, 5000–5010. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Pitre, M.; Wanasundara, J.; Karboune, S.; L’Hocine, L. Investigation of the Nutritional Quality of Raw and Processed Canadian Faba Bean (Vicia Faba L.) Flours in Comparison to Pea and Soy Using a Human in Vitro Gastrointestinal Digestion Model. Food Res. Int. 2023, 173, 113264. [Google Scholar] [CrossRef]
- Laleg, K.; Cassan, D.; Barron, C.; Prabhasankar, P.; Micard, V. Structural, Culinary, Nutritional and Anti-Nutritional Properties of High Protein, Gluten Free, 100% Legume Pasta. PLoS ONE 2016, 11, e0160721. [Google Scholar] [CrossRef]
- Padalino, L.; Mastromatteo, M.; Lecce, L.; Spinelli, S.; Conte, A.; Alessandro Del Nobile, M. Optimization and Characterization of Gluten-Free Spaghetti Enriched with Chickpea Flour. Int. J. Food Sci. Nutr. 2015, 66, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Burešová, I.; Tokár, M.; Mareček, J.; Hřivna, L.; Faměra, O.; Šottníková, V. The Comparison of the Effect of Added Amaranth, Buckwheat, Chickpea, Corn, Millet and Quinoa Flour on Rice Dough Rheological Characteristics, Textural and Sensory Quality of Bread. J. Cereal Sci. 2017, 75, 158–164. [Google Scholar] [CrossRef]
- Chochkov, R.; Savova-Stoyanova, D.; Papageorgiou, M.; Rocha, J.M.; Gotcheva, V.; Angelov, A. Effects of Teff-Based Sourdoughs on Dough Rheology and Gluten-Free Bread Quality. Foods 2022, 11, 1012. [Google Scholar] [CrossRef] [PubMed]
- Kostecka, M.; Kostecka-Jarecka, J.; Iłowiecka, K.; Kostecka, J. An Evaluation of Nutritional Status and Problems with Dietary Compliance in Polish Patients with Celiac Disease. Nutrients 2022, 14, 2581. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Wolf, R.L.; Lebwohl, B.; Ciaccio, E.J.; Green, P.H.R. Persistent Economic Burden of the Gluten Free Diet. Nutrients 2019, 11, 399. [Google Scholar] [CrossRef]
- Falcomer, A.L.; Luchine, B.A.; Gadelha, H.R.; Szelmenczi, J.R.; Nakano, E.Y.; Farage, P.; Zandonadi, R.P. Worldwide Public Policies for Celiac Disease: Are Patients Well Assisted? Int. J. Public Health 2020, 65, 937–945. [Google Scholar] [CrossRef]
- Estévez, V.; Ayala, J.; Vespa, C.; Araya, M. The Gluten-Free Basic Food Basket: A Problem of Availability, Cost and Nutritional Composition. Eur. J. Clin. Nutr. 2016, 70, 1215–1217. [Google Scholar] [CrossRef]
- Aziz, I.; Karajeh, M.A.; Zilkha, J.; Tubman, E.; Fowles, C.; Sanders, D.S. Change in Awareness of Gluten-Related Disorders among Chefs and the General Public in the UK: A 10-Year Follow-up Study. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1228–1233. [Google Scholar] [CrossRef]
- Kim, H.S.; Patel, K.G.; Orosz, E.; Kothari, N.; Demyen, M.F.; Pyrsopoulos, N.; Ahlawat, S.K. Time Trends in the Prevalence of Celiac Disease and Gluten-Free Diet in the US Population: Results from the National Health and Nutrition Examination Surveys 2009–2014. JAMA Intern. Med. 2016, 176, 1716–1717. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Silvester, J.A.; Lebwohl, B.; Leffler, D.A.; Anderson, R.P.; Therrien, A.; Kelly, C.P.; Verdu, E.F. Society for the Study of Celiac Disease Position Statement on Gaps and Opportunities in Coeliac Disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 875–884. [Google Scholar] [CrossRef]
- Arslain, K.; Gustafson, C.R.; Baishya, P.; Rose, D.J. Determinants of Gluten-Free Diet Adoption among Individuals without Celiac Disease or Non-Celiac Gluten Sensitivity. Appetite 2021, 156, 104958. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Jeejeebhoy, K.N.; Duerksen, D.R. Malnutrition in Gastrointestinal Disorders: Detection and Nutritional Assessment. Gastroenterol. Clin. 2018, 47, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cyrkot, S.; Anders, S.; Kamprath, C.; Liu, A.; Mileski, H.; Dowhaniuk, J.; Nasser, R.; Marcon, M.; Brill, H.; Turner, J.M.; et al. Folate Content of Gluten-Free Food Purchases and Dietary Intake Are Low in Children with Coeliac Disease. Int. J. Food Sci. Nutr. 2020, 71, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten Free Diet and Nutrient Deficiencies: A Review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Kulai, T.; Rashid, M. Assessment of Nutritional Adequacy of Packaged Gluten-Free Food Products. Can. J. Diet. Pract. Res. 2014, 75, 186–190. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Gibson, P.R. Nutritional Inadequacies of the Gluten-Free Diet in Both Recently-Diagnosed and Long-Term Patients with Coeliac Disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef]
- Remes-Troche, J.M.; Cobos-Quevedo, O.D.J.; Rivera-Gutiérrez, X.; Hernández, G.; de la Cruz-Patiño, E.; Uscanga-Domínquez, L.F. Metabolic Effects in Patients with Celiac Disease, Patients with Nonceliac Gluten Sensitivity, and Asymptomatic Controls, after Six Months of a Gluten-Free Diet. Rev. Gastroenterol. México Engl. Ed. 2020, 85, 109–117. [Google Scholar] [CrossRef]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional Consequences of Celiac Disease and the Gluten-Free Diet. Expert. Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef]
- da Silva Lopes, K.; Takemoto, Y.; Garcia-Casal, M.N.; Ota, E. Nutrition-Specific Interventions for Preventing and Controlling Anaemia throughout the Life Cycle: An Overview of Systematic Reviews (Protocol). Cochrane Database Syst. Rev. 2018, 8, CD013092. [Google Scholar] [CrossRef]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional Deficiencies in Children with Celiac Disease Resulting from a Gluten-Free Diet: A Systematic Review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- De Moreno, M.L.; Rodríguez-Herrera, A.; Sousa, C.; Comino, I. Biomarkers to Monitor Gluten-Free Diet Compliance in Celiac Patients. Nutrients 2017, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Dahal-Koirala, S.; Ciacchi, L.; Petersen, J.; Risnes, L.F.; Neumann, R.S.; Christophersen, A.; Lundin, K.E.A.; Reid, H.H.; Qiao, S.W.; Rossjohn, J.; et al. Discriminative T-Cell Receptor Recognition of Highly Homologous HLA-DQ2– Bound Gluten Epitopes. J. Biol. Chem. 2019, 294, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.M.; Sapone, A.; Catassi, C.; Fasano, A. Celiac Disease and Nonceliac Gluten Sensitivity: A Review. JAMA-J. Am. Med. Assoc. 2017, 318, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.M.S.; te Lintelo, M.; Vriezinga, S.L.; Putter, H.; Hopman, E.G.; Mearin, M.L. Assessment of Dietary Compliance in Celiac Children Using a Standardized Dietary Interview. Clin. Nutr. 2018, 37, 1000–1004. [Google Scholar] [CrossRef]
- Dotsenko, V.; Oittinen, M.; Taavela, J.; Popp, A.; Peräaho, M.; Staff, S.; Sarin, J.; Leon, F.; Isola, J.; Mäki, M.; et al. Genome-Wide Transcriptomic Analysis of Intestinal Mucosa in Celiac Disease Patients on a Gluten-Free Diet and Postgluten Challenge. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 13–32. [Google Scholar] [CrossRef]
- Gnodi, E.; Meneveri, R.; Barisani, D. Celiac Disease: From Genetics to Epigenetics. World J. Gastroenterol. 2022, 28, 449–463. [Google Scholar] [CrossRef]
- Liu, E.; Dong, F.; Barón, A.E.; Taki, I.; Norris, J.M.; Frohnert, B.I.; Hoffenberg, E.J.; Rewers, M. High Incidence of Celiac Disease in a Long-Term Study of Adolescents with Susceptibility Genotypes. Gastroenterology 2017, 152, 1329–1336.e1. [Google Scholar] [CrossRef]
- Andrén Aronsson, C.; Lee, H.S.; Hård Af Segerstad, E.M.; Uusitalo, U.; Yang, J.; Koletzko, S.; Liu, E.; Kurppa, K.; Bingley, P.J.; Toppari, J.; et al. Association of Gluten Intake during the First 5 Years of Life with Incidence of Celiac Disease Autoimmunity and Celiac Disease among Children at Increased Risk. JAMA-J. Am. Med. Assoc. 2019, 322, 514–523. [Google Scholar] [CrossRef]
- Murray, J.A.; Frey, M.R.; Oliva-Hemker, M. Celiac Disease. Gastroenterology 2018, 154, 2005–2008. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; Debruyn, J.; Ronksley, P.E.; Shaheen, A.A.; et al. Incidence of Celiac Disease Is Increasing over Time: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac Disease: A Comprehensive Current Review. BMC Med. 2019, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, I.A.; Reilly, N.R.; Rubio-Tapia, A. Celiac Disease: Clinical Features and Diagnosis. Gastroenterol. Clin. 2019, 48, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, M.; Casey, L. Who Should Be Gluten-Free? A Review for the General Practitioner. Med. Clin. 2019, 103, 89–99. [Google Scholar] [CrossRef]
- Rubin, J.E.; Crowe, S.E. Celiac Disease. Ann. Intern. Med. 2020, 172, ITC1–ITC16. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Murray, J.A. Epidemiology of Celiac Disease. Gastroenterol. Clin. 2019, 48, 1–18. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, A.D.; Tan, I.L.; Gonera-De Jong, B.C.; Visschedijk, M.C.; Jonkers, I.; Withoff, S. Molecular Biomarkers for Celiac Disease: Past, Present and Future. Int. J. Mol. Sci. 2020, 21, 8528. [Google Scholar] [CrossRef]
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease—A Narrative Review. Nutrients 2023, 15, 2499. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac Wheat Sensitivity: An Immune-Mediated Condition with Systemic Manifestations. Gastroenterol. Clin. 2019, 48, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Significance, C.; Africa, N.; East, M.; Basics, C.D.; Abstracts, D. Oral Medicine Celiac Disease. Dent. Abstr. 2017, 62, 336–337. [Google Scholar] [CrossRef]
- Muthukumar, J.; Selvasekaran, P.; Lokanadham, M.; Chidambaram, R. Food and Food Products Associated with Food Allergy and Food Intolerance—An Overview. Food Res. Int. 2020, 138, 109780. [Google Scholar] [CrossRef] [PubMed]
- Jansson-Knodell, C.L.; Rubio-Tapia, A. Gluten-Related Disorders from Bench to Bedside. Clin. Gastroenterol. Hepatol. 2024, 22, 693–704.e1. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, V.; Kurppa, K.; Huhtala, H.; Laurila, K.; Mäki, M.; Collin, P.; Salmi, T.; Luostarinen, L.; Saavalainen, P.; Kaukinen, K. Serology-based Criteria for Adult Coeliac Disease Have Excellent Accuracy across the Range of Pre-test Probabilities. Aliment. Pharmacol. Ther. 2019, 49, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease: Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef]
- Ben Houmich, T.; Admou, B. Celiac Disease: Understandings in Diagnostic, Nutritional, and Medicinal Aspects. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008709. [Google Scholar] [CrossRef]
- Robert, M.E.; Crowe, S.E.; Burgart, L.; Yantiss, R.K.; Lebwohl, B.; Greenson, J.K.; Guandalini, S.; Murray, J.A. Statement on Best Practices in the Use of Pathology as a Diagnostic Tool for Celiac Disease: A Guide for Clinicians and Pathologists. Am. J. Surg. Pathol. 2018, 42, e44–e58. [Google Scholar] [CrossRef]
- Efthymakis, K.; Milano, A.; Laterza, F.; Serio, M.; Neri, M. Iron Deficiency Anemia despite Effective Gluten-Free Diet in Celiac Disease: Diagnostic Role of Small Bowel Capsule Endoscopy. Dig. Liver Dis. 2017, 49, 412–416. [Google Scholar] [CrossRef]
- Peña, A.S. What Is the Best Histopathological Classification for Celiac Disease? Does It Matter? Gastroenterol. Hepatol. Bed Bench 2015, 8, 239–243. [Google Scholar]
- Volta, U.; Caio, G.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Boschetti, E.; Stanghellini, V.; De Giorgio, R. Features and Progression of Potential Celiac Disease in Adults. Clin. Gastroenterol. Hepatol. 2016, 14, 686–693.e1. [Google Scholar] [CrossRef] [PubMed]
- Greenson, J.K. The Biopsy Pathology of Non-Coeliac Enteropathy. Histopathology 2015, 66, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Boschetti, E.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Paterini, P.; De Giorgio, R. Seronegative Celiac Disease: Shedding Light on an Obscure Clinical Entity. Dig. Liver Dis. 2016, 48, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.; Kelly, C.P.; Silvester, J.A. Celiac Disease: Extraintestinal Manifestations and Associated Conditions. J. Clin. Gastroenterol. 2020, 54, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef]
- Jivraj, A.; Hutchinson, J.M.; Ching, E.; Marwaha, A.; Verdu, E.F.; Armstrong, D.; Pinto-Sanchez, M.I. Micronutrient Deficiencies Are Frequent in Adult Patients with and without Celiac Disease on a Gluten-Free Diet, Regardless of Duration and Adherence to the Diet. Nutrition 2022, 103–104, 111809. [Google Scholar] [CrossRef]
- McGrogan, L.; Mackinder, M.; Stefanowicz, F.; Aroutiounova, M.; Catchpole, A.; Wadsworth, J.; Buchanan, E.; Cardigan, T.; Duncan, H.; Hansen, R.; et al. Micronutrient Deficiencies in Children with Coeliac Disease; a Double-Edged Sword of Both Untreated Disease and Treatment with Gluten-Free Diet. Clin. Nutr. 2021, 40, 2784–2790. [Google Scholar] [CrossRef]
- Moya, D.A.; Nugent, C.A.; Baker, R.D.; Baker, S.S. Celiac Disease Nutritional Status and Poor Adherence to Follow-Up. Clin. Pediatr. 2020, 59, 649–655. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Dietary Compliance in Celiac Disease. World J. Gastroenterol. 2017, 23, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Mangge, H.; Schenk, M.; Enko, D. Non-Responsive Celiac Disease May Coincide with Additional Food Intolerance/Malabsorption, Including Histamine Intolerance. Med. Hypotheses 2021, 146, 110404. [Google Scholar] [CrossRef] [PubMed]
- Dochat, C.; Afari, N.; Satherley, R.-M.; Coburn, S.; McBeth, J.F. Celiac Disease Symptom Profiles and Their Relationship to Gluten-Free Diet Adherence, Mental Health, and Quality of Life. BMC Gastroenterol. 2024, 24, 9. [Google Scholar] [CrossRef]
- Tye-Din, J.A. Evolution in Coeliac Disease Diagnosis and Management. JGH Open 2024, 8, e13107. [Google Scholar] [CrossRef]
- Tye-Din, J.A. Review Article: Follow-up of Coeliac Disease. Aliment. Pharmacol. Ther. 2022, 56, S49–S63. [Google Scholar] [CrossRef]
- Comino, I.; Fernández-bañares, F.; Esteve, M.; Ortigosa, L.; Castillejo, G. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am. J. Gastroenterol. 2016, 111, 1456–1465. [Google Scholar] [CrossRef]
- Rubio-tapia, A.; Schumann, M.; Tye-din, J.; Zeitz, J.; Al-toma, A. Follow-Up of Celiac Disease in Adults: “When, What, Who, and Where”. Nutrients 2023, 15, 2048. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; Van Heel, D.A.; et al. Diagnosis and Management of Adult Coeliac Disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Elias, E.D.; Silvester, J.A.; Bernstein, C.N.; Rigaux, L.N.; Graff, L.A.; Duerksen, D.R. Patient Perspectives on the Long-Term Management of Celiac Disease. J. Clin. Gastroenterol. 2022, 56, 869–874. [Google Scholar] [CrossRef]
- World Gastroenterology Organisation. Available online: https://www.worldgastroenterology.org/guidelines/celiac-disease/celiac-disease-english (accessed on 28 December 2023).
- ICMR Guideline on Diagnosis and Management of Celiac Disease. Available online: https://main.icmr.nic.in/sites/default/files/guidelines/ICMR%20-%20Diagnosis%20and%20Managmemnt.pdf (accessed on 21 July 2024).
- Coeliac Disease: Recognition, Assessment and Management | Guidance | NICE Guideline. Available online: https://www.nice.org.uk/guidance/ng20 (accessed on 31 July 2024).
- Comino, I.; Segura, V.; Ortigosa, L.; Espín, B.; Castillejo, G.; Antonio, J.; Carlos, G.; Antonio, S.; Ribes, C.; Enriqueta, K.; et al. Prospective Longitudinal Study: Use of Faecal Gluten Immunogenic Peptides to Monitor Children Diagnosed with Coeliac Disease during Transition to a Gluten - Free Diet. Aliment. Pharmacol. Ther. 2019, 49, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Meijer, C.R.; Bakker, J.; Boers, A.; Jansen, S.; Mengi, Z.; Mearin, M.L. Association in Clinical Practice Between Gluten Intake and Gluten Immunogenic Peptides in Celiac Children. Gastro Hep Adv. 2022, 1, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Mouslih, A.; Rhazi, K.; El Bahra, N.; Idrissi, M.L.; Hida, M. Gluten-Free Diet Compliance in Children with Celiac Disease and Its Effect on Clinical Symptoms: A Retrospective Cohort Study. Cureus 2023, 15, e50217. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.D.L.; Cebolla, Á.; Muñoz-suano, A.; Carrillo-carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-herrera, A.; Sousa, C. Detection of Gluten Immunogenic Peptides in the Urine of Patients with Coeliac Disease Reveals Transgressions in the Gluten-Free Diet and Incomplete Mucosal Healing Study Patients. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef]
- Costa, A.F.; Sugai, E.; De, M.; Temprano, P.; Niveloni, S.I.; Vázquez, H.; Moreno, M.L.; Domínguez-flores, M.R.; Muñoz-suano, A.; Smecuol, E.; et al. Gluten Immunogenic Peptide Excretion Detects Dietary Transgressions in Treated Celiac Disease Patients. World J. Gastroenterol. 2019, 25, 1409–1420. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Zafeiropoulou, K.; Mackinder, M.; Ijaz, U.Z.; Duncan, H.; Buchanan, E.; Cardigan, T. Comparison of Clinical Methods with the Faecal Gluten Immunogenic Peptide to Assess Gluten Intake in Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 356–360. [Google Scholar] [CrossRef]
- Bakhshipour, A.; Rafaiee, R. Spontaneous Latency in Adult Patients with Celiac Disease on a Normal Diet after Gluten-Free Diet: Case Series. Middle East. J. Dig. Dis. 2022, 14, 354–358. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; De Giorgio, R.; Henriksen, C.; Skodje, G.; Lundin, K.E. Non-Celiac Gluten Sensitivity: A Work-in-Progress Entity in the Spectrum of Wheat-Related Disorders. Best. Pract. Res. Clin. Gastroenterol. 2015, 29, 477–491. [Google Scholar] [CrossRef]
- Calabriso, N.; Scoditti, E.; Massaro, M.; Maffia, M.; Chieppa, M.; Laddomada, B.; Carluccio, M.A. Non-Celiac Gluten Sensitivity and Protective Role of Dietary Polyphenols. Nutrients 2022, 14, 2679. [Google Scholar] [CrossRef]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R.; Bagnato, C.; Belcari, C.; Bellantoni, A.; Caio, G.; Calella, F.; et al. An Italian Prospective Multicenter Survey on Patients Suspected of Having Non-Celiac Gluten Sensitivity. BMC Med. 2014, 12, 4–11. [Google Scholar] [CrossRef]
- Alkalay, M.J. Nutrition in Patients with Lactose Malabsorption, Celiac Disease, and Related Disorders. Nutrients 2022, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Cruchet, S.; Lucero, Y.; Cornejo, V. Truths, Myths and Needs of Special Diets: Attention-Deficit/Hyperactivity Disorder, Autism, Non-Celiac Gluten Sensitivity, and Vegetarianism. Ann. Nutr. Metab. 2016, 68, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of Gluten Related Disorders: Celiac Disease, Wheat Allergy and Non-Celiac Gluten Sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Tovoli, F.; De Giorgio, R. Effect of Gluten Free Diet on Immune Response to Gliadin in Patients with Non-Celiac Gluten Sensitivity. BMC Gastroenterol. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Adam Daulatzai, M. Non-Celiac Gluten Sensitivity Triggers Gut Dysbiosis, Neuroinflammation, Gut-Brain Axis Dysfunction, and Vulnerability for Dementia. CNS Neurol. Disord. Targets 2015, 14, 110–131. [Google Scholar] [CrossRef]
- Francavilla, R.; Cristofori, F.; Castellaneta, S.; Polloni, C.; Albano, V.; Dellatte, S.; Indrio, F.; Cavallo, L.; Catassi, C. Clinical, Serologic, and Histologic Features of Gluten Sensitivity in Children. J. Pediatr. 2014, 164, 463–467.e1. [Google Scholar] [CrossRef]
- Leccioli, V.; Oliveri, M.; Romeo, M.; Berretta, M.; Rossi, P. A New Proposal for the Pathogenic Mechanism of Non-Coeliac/Non-Allergic Gluten/Wheat Sensitivity: Piecing Together the Puzzle of Recent Scientific Evidence. Nutrients 2017, 9, 1203. [Google Scholar] [CrossRef]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac Gluten Sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Rivera-Gutierrez, X.; de Cobos-Quevedo, O.J.; Grube-Pagola, P.; Meixueiro-Daza, A.; Hernandez-Flores, K.; Cabrera-Jorge, F.J.; Vivanco-Cid, H.; Dowd, S.E.; Remes-Troche, J.M. First Insights into the Gut Microbiota of Mexican Patients with Celiac Disease and Non-Celiac Gluten Sensitivity. Nutrients 2018, 10, 1641. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Halmos, E.; Glance, S. The Role of FODMAPs in Irritable Bowel Syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 605–609. [Google Scholar] [CrossRef]
- Skodje, G.I.; Minelle, I.H.; Rolfsen, K.L.; Iacovou, M.; Lundin, K.E.A.; Veierød, M.B.; Henriksen, C. Dietary and Symptom Assessment in Adults with Self-Reported Non-Coeliac Gluten Sensitivity. Clin. Nutr. ESPEN 2019, 31, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Soresi, M.; Alcamo, A.D.; Sciumè, C.; Iacono, G.; Geraci, G.; Brusca, I.; Seidita, A.; Adragna, F.; Carta, M.; et al. Risk of Low Bone Mineral Density and Low Body Mass Index in Patients with Non-Celiac Wheat- Sensitivity: A Prospective Observation Study. BMC Med. 2014, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koumbi, L.; Giouleme, O.; Vassilopoulou, E. Non-Celiac Gluten Sensitivity and Irritable Bowel Disease: Looking for the Culprits. Curr. Dev. Nutr. 2020, 4, nzaa176. [Google Scholar] [CrossRef] [PubMed]
- Bozomitu, L.; Miron, I.; Adam Raileanu, A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117. [Google Scholar] [CrossRef] [PubMed]
- Wagh, S.K.; Lammers, K.M.; Padul, M.V.; Rodriguez-Herrera, A.; Dodero, V.I. Celiac Disease and Possible Dietary Interventions: From Enzymes and Probiotics to Postbiotics and Viruses. Int. J. Mol. Sci. 2022, 23, 11748. [Google Scholar] [CrossRef]
- Caio, G.; Lungaro, L.; Segata, N.; Guarino, M.; Zoli, G.; Volta, U.; De Giorgio, R. Effect of Gluten-Free Diet on Gut Microbiota Composition in Patients with Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2020, 12, 1832. [Google Scholar] [CrossRef]
- Transeth, E.L.; Dale, H.F.; Lied, G.A. Comparison of Gut Microbiota Profile in Celiac Disease, Non-Celiac Gluten Sensitivity and Irritable Bowel Syndrome: A Systematic Review. Turkish J. Gastroenterol. 2020, 31, 735–745. [Google Scholar] [CrossRef]
- Leonard, M.M.; Valitutti, F.; Karathia, H.; Pujolassos, M.; Kenyon, V.; Fanelli, B.; Troisi, J.; Subramanian, P.; Camhi, S.; Colucci, A.; et al. Microbiome Signatures of Progression toward Celiac Disease Onset in At-Risk Children in a Longitudinal Prospective Cohort Study. Proc. Natl. Acad. Sci. USA 2021, 118, e2020322118. [Google Scholar] [CrossRef]
- Arcila-Galvis, J.E.; Loria-Kohen, V.; Ramírez de Molina, A.; Carrillo de Santa Pau, E.; Marcos-Zambrano, L.J. A Comprehensive Map of Microbial Biomarkers along the Gastrointestinal Tract for Celiac Disease Patients. Front. Microbiol. 2022, 13, 956119. [Google Scholar] [CrossRef]
- Qasim, H.; Nasr, M.; Mohammad, A.; Hor, M.; Baradeiya, A.M. Dysbiosis and Migraine Headaches in Adults with Celiac Disease. Cureus 2022, 14, e28346. [Google Scholar] [CrossRef]
- Leonard, M.M.; Karathia, H.; Pujolassos, M.; Troisi, J.; Valitutti, F.; Subramanian, P.; Camhi, S.; Kenyon, V.; Colucci, A.; Serena, G.; et al. Multi-Omics Analysis Reveals the Influence of Genetic and Environmental Risk Factors on Developing Gut Microbiota in Infants at Risk of Celiac Disease. Microbiome 2020, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hov, J.R.; Zhong, H.; Qin, B.; Anmarkrud, J.A.; Holm, K.; Franke, A.; Lie, B.A.; Karlsen, T.H. The Influence of the Autoimmunity-Associated Ancestral HLA Haplotype AH8.1 on the Human Gut Microbiota: A Cross-Sectional Study. PLoS ONE 2015, 10, e0133804. [Google Scholar] [CrossRef] [PubMed]
- Olshan, K.L.; Leonard, M.M.; Serena, G.; Fasano, A.; Renzi, V.S. De Gut Microbiota in Celiac Disease: Microbes, Metabolites, Pathways and Therapeutics. Expert. Rev. Clin. Immunol. 2020, 16, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Miron, I.C.; Raileanu, A.A.; Starcea, I.M.; Lupu, A.; Tarca, E.; Mocanu, A.; Buga, A.M.L.; Lupu, V.; Fotea, S. Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth—A Narrative Review. Life 2023, 13, 300. [Google Scholar] [CrossRef]
- Shah, A.; Kang, S.; Talley, N.J.; Do, A.; Walker, M.M.; Shanahan, E.R.; Koloski, N.A.; Jones, M.P.; Keely, S.; Morrison, M.; et al. The Duodenal Mucosa Associated Microbiome, Visceral Sensory Function, Immune Activation and Psychological Comorbidities in Functional Gastrointestinal Disorders with and without Self-Reported Non-Celiac Wheat Sensitivity. Gut Microbes 2022, 14, 2132078. [Google Scholar] [CrossRef]
- Belei, O.; Jugănaru, I.; Basaca, D.-G.; Munteanu, A.I.; Mărginean, O. The Role of Intestinal Microbiota in Celiac Disease and Further Therapeutic Perspectives. Life 2023, 13, 2039. [Google Scholar] [CrossRef]
- Saviano, A.; Petruzziello, C.; Brigida, M.; Morabito Loprete, M.R.; Savioli, G.; Migneco, A.; Ojetti, V. Gut Microbiota Alteration and Its Modulation with Probiotics in Celiac Disease. Biomedicines 2023, 11, 2638. [Google Scholar] [CrossRef]
- Naseri, K.; Dabiri, H.; Olfatifar, M.; Shahrbaf, M.A.; Yadegar, A.; Soheilian-Khorzoghi, M.; Sadeghi, A.; Saadati, S.; Rostami-Nejad, M.; Verma, A.K.; et al. Evaluation of Gut Microbiota of Iranian Patients with Celiac Disease, Non-Celiac Wheat Sensitivity, and Irritable Bowel Syndrome: Are There Any Similarities? BMC Gastroenterol. 2023, 23, 15. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Rubio, R.; Jofré, A.; Martín, B.; Aymerich, T.; Garriga, M. Characterization of Lactic Acid Bacteria Isolated from Infant Faeces as Potential Probiotic Starter Cultures for Fermented Sausages. Food Microbiol. 2014, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Siragusa, S.; Vacca, M.; Di Cagno, R.; Cristofori, F.; Schwarm, M.; Pelzer, S.; Flügel, M.; Speckmann, B.; Francavilla, R.; et al. Selection of Gut-Resistant Bacteria and Construction of Microbial Consortia for Improving Gluten Digestion under Simulated Gastrointestinal Conditions. Nutrients 2021, 13, 992. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Markowiak, P.; Ślizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Freire, R.; Ingano, L.; Serena, G.; Cetinbas, M.; Anselmo, A.; Sapone, A.; Sadreyev, R.I.; Fasano, A.; Senger, S. Human Gut Derived-Organoids Provide Model to Study Gluten Response and Effects of Microbiota-Derived Molecules in Celiac Disease. Sci. Rep. 2019, 9, 7029. [Google Scholar] [CrossRef]





| Source | Flour Protein (g%) | Reference |
|---|---|---|
| Potato | 94.06 | [50] |
| Pea | 80.19 | [50] |
| Faba bean | 61.25 | [51] |
| Soy | 56.60 | [52] |
| Carob | 55.04 | [50] |
| Lentil | 28.10 | [53] |
| Chickpeas | 23.37 | [54] |
| Buckwheat | 22.52 | [51] |
| Amaranth | 19.40 | [55] |
| Quinoa | 19.00 | [55] |
| Teff | 13.30 | [56] |
| Sorghum | 13.08 | [47] |
| Millet | 11.90 | [55] |
| Rice | 7.90 | [55] |
| Maize | 7.45 | [54] |
| Grade | Villous Atrophy | Crypt Hyperplasia | Intraepithelial Lymphocytic Infiltration |
|---|---|---|---|
| 0 | − | − | − |
| 1 | − | − | + |
| 2 | − | + | + |
| 3a | + (mild) | + | + |
| 3b | + (moderate) | + | + |
| 3c | + (severe) | + | + |
| 4 | + (severe) | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanciu, D.; Staykov, H.; Dragomanova, S.; Tancheva, L.; Pop, R.S.; Ielciu, I.; Crișan, G. Gluten Unraveled: Latest Insights on Terminology, Diagnosis, Pathophysiology, Dietary Strategies, and Intestinal Microbiota Modulations—A Decade in Review. Nutrients 2024, 16, 3636. https://doi.org/10.3390/nu16213636
Stanciu D, Staykov H, Dragomanova S, Tancheva L, Pop RS, Ielciu I, Crișan G. Gluten Unraveled: Latest Insights on Terminology, Diagnosis, Pathophysiology, Dietary Strategies, and Intestinal Microbiota Modulations—A Decade in Review. Nutrients. 2024; 16(21):3636. https://doi.org/10.3390/nu16213636
Chicago/Turabian StyleStanciu, Dana, Hristian Staykov, Stela Dragomanova, Lyubka Tancheva, Radu Samuel Pop, Irina Ielciu, and Gianina Crișan. 2024. "Gluten Unraveled: Latest Insights on Terminology, Diagnosis, Pathophysiology, Dietary Strategies, and Intestinal Microbiota Modulations—A Decade in Review" Nutrients 16, no. 21: 3636. https://doi.org/10.3390/nu16213636
APA StyleStanciu, D., Staykov, H., Dragomanova, S., Tancheva, L., Pop, R. S., Ielciu, I., & Crișan, G. (2024). Gluten Unraveled: Latest Insights on Terminology, Diagnosis, Pathophysiology, Dietary Strategies, and Intestinal Microbiota Modulations—A Decade in Review. Nutrients, 16(21), 3636. https://doi.org/10.3390/nu16213636











