Effect of Consuming Salmon Products on Vitamin D Status of Young Caucasian Women in Autumn—A Randomized 8-Week Dietary VISA 2 (Vitamin D in Salmon Part 2) Intervention Study
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
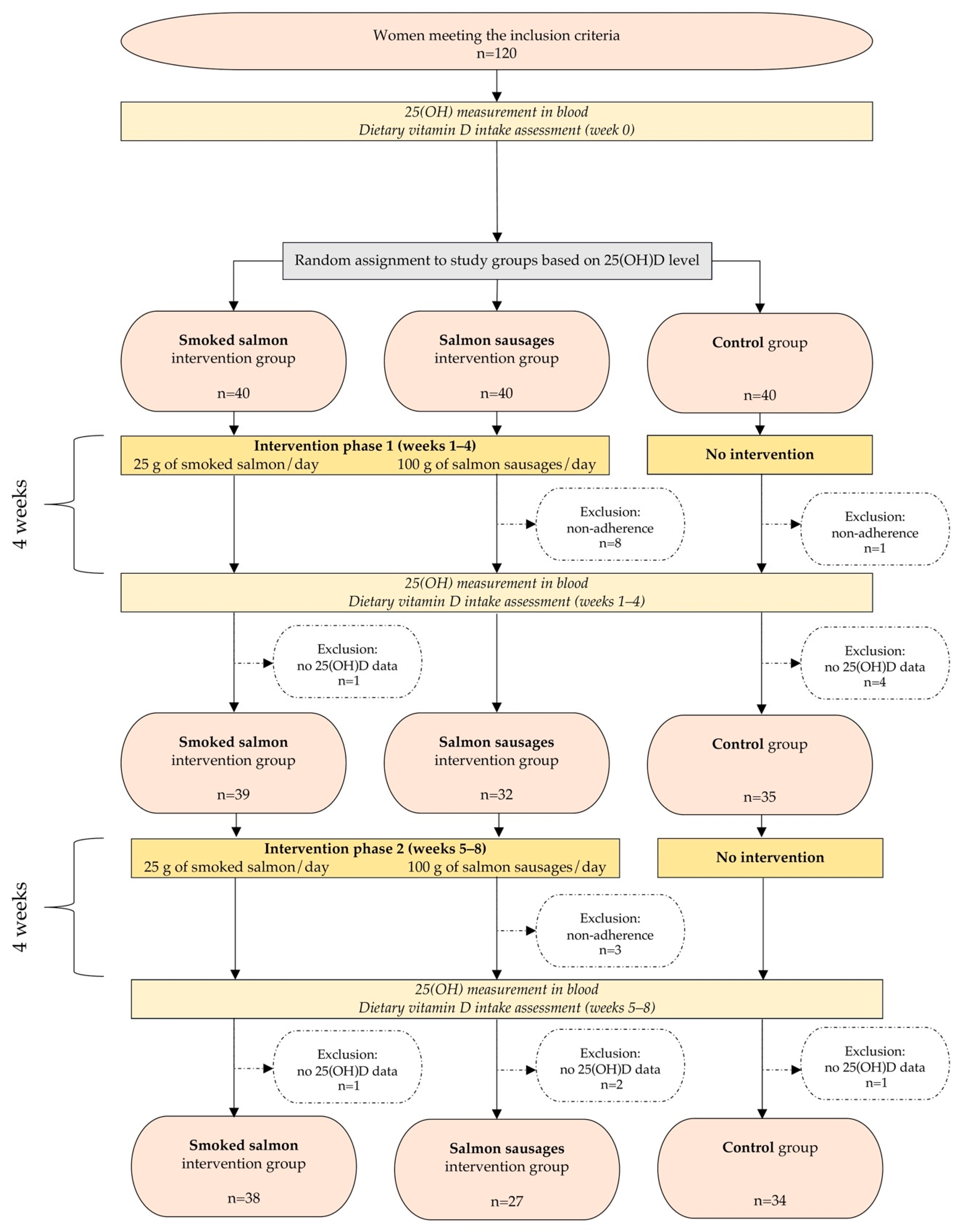
2.2. Studied Group
2.3. Dietary Intervention
2.4. Measurements
2.4.1. Vitamin D Status
- <75 nmol/L—inadequate, 75–250 nmol/L—adequate, >250 nmol/L—potentially toxic [17].
2.4.2. Anthropometric Measurements
2.5. Questionnaire
2.6. Study Course
2.6.1. Wash-Out Before Study Beginning
2.6.2. Study Time
2.7. Statistical Analyses
3. Results
3.1. Anthropometric Characteristics of the Participants
3.2. Fish Intake Throughout the Study
3.3. Vitamin D Intake Throughout the Study
3.4. Vitamin D Status (Total 25(OH)D Serum Concentration) Throughout the Study
3.5. Influence of Vitamin D Status at Baseline on the Intervention Outcomes
3.6. Intervention Efficacy
3.7. Additional Observations
4. Discussion
4.1. Serum Concentration Changes of 25(OH)D After 8 Weeks of Intervention
4.2. Possible Explanations
4.2.1. Fat Content
4.2.2. Production Process
4.2.3. Frequency of Consuming the Intervention Product
4.2.4. Dietary Habits Before Intervention
4.2.5. Physiological Explanation Connected to Vitamin D Metabolism in the Body
4.3. Baseline 25(OH)D Serum Concentration as an Important Factor for the Intervention Efficacy
4.4. Strengths and Limitations of the Study
4.5. Recommendations for Maintaining Adequate Vitamin D Status
4.6. Proposed Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rebelos, E.; Tentolouris, N.; Jude, E. The role of vitamin D in health and disease: A narrative review on the mechanisms linking vitamin D with disease and the effects of supplementation. Drugs 2023, 83, 665–685. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Segheto, K.J.; Pereira, M.; Da Silva, D.C.G.; De Carvalho, C.J.; Massardi, F.R.; Kakehasi, A.M.; Ju-vanhol, L.L.; Longo, G.Z. Vitamin D and bone health in adults: A systematic review and meta-analysis. Cien. Saude Colet. 2021, 26, 3221–3244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: A systematic review and meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 11784–11801. [Google Scholar] [CrossRef]
- Sartini, M.; Del Puente, F.; Oliva, M.; Carbone, A.; Bobbio, N.; Schinca, E.; Giribone, L.; Cristina, M.L. Preventive vitamin D supplementation and risk for COVID-19 infection: A systematic review and meta-analysis. Nutrients 2024, 16, 679. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitamin Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Polish Food Composition Tables [In Polish: Tabele Składu i Wartości Odżywczej Żywności]; PZWL: Warsaw, Poland, 2017. [Google Scholar]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- United States (US) National Institutes of Health. Office of Dietary Supplements Fact Sheet for Health Professionals—Vitamin D. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 22 February 2024).
- Royal Osteoporosis Society Vitamin D Supplements and Tests. Available online: https://strwebprdmedia.blob.core.windows.net/media/4jnlbttc/ros-vitamin-d-supplements-and-tests.pdf (accessed on 22 February 2024).
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Andersen, R.; Mølgaard, C.; Skovgaard, L.T.; Brot, C.; Cashman, K.D.; Chabros, E.; Charzewska, J.; Flynn, A.; Jakobsen, J.; Karkkainen, M.; et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur. J. Clin. Nutr. 2005, 59, 533–541. [Google Scholar] [CrossRef]
- Santé Publique France. Nutritional Status, Biology, Adults: ENNS Distribution Tables. Available online: https://www.santepubliquefrance.fr/determinants-de-sante/nutrition-et-activite-physique/articles/enns-etude-nationale-nutrition-sante/biologie-adultes-tableaux-de-distribution-enns (accessed on 22 February 2024).
- Sewerynek, E.; Cieślak, K.; Janik, M.; Gowin, E.; Stuss, M. Evaluation of vitamin D concentration in a population of young, healthy women—The effects of vitamin D supplementation. Endokrynol. Pol. 2015, 68, 533–540. [Google Scholar] [CrossRef][Green Version]
- Lucey, A.J.; Paschos, G.K.; Cashman, K.D.; Martínéz, J.A.; Thorsdottir, I.; Kiely, M. Influence of moderate energy restriction and seafood consumption on bone turnover in overweight young adults. Am. J. Clin. Nutr. 2008, 87, 1045–1052. [Google Scholar] [CrossRef][Green Version]
- Hansen, A.L.; Dahl, L.; Bakke, L.; Frøyland, L.; Thayer, J.F. Fish consumption and heart rate variability. J. Psychophysiol. 2010, 24, 41–47. [Google Scholar] [CrossRef]
- Manios, Y.; Moschonis, G.; Kafatos, A.; Tzanetakou, I.; Bountziouka, V.; Papadimitriou, A. Dietary intake of vitamin D, calcium, and phosphorus and the risk of osteoporosis in elderly men and women: The role of diet and supplementation. Nutrients 2017, 9, 405. [Google Scholar] [CrossRef]
- Bratlie, M.; Hagen, I.V.; Helland, A.; Midttun, Ø.; Ulvik, A.; Rosenlund, G.; Sveier, H.; Mellgren, G.; Ueland, P.M.; Gudbrandsen, O.A. Five salmon dinners per week were not sufficient to prevent the reduction in serum vitamin D in autumn at 60° north latitude: A randomised trial. Br. J. Nutr. 2020, 123, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Utri, Z.; Głąbska, D. Salmon intake intervention in the vulnerable group of young Polish women to maintain vitamin D status during the autumn season. Sustainability 2020, 12, 2829. [Google Scholar] [CrossRef]
- Lehmann, U.; Gjessing, H.R.; Hirche, F.; Mueller-Belecke, A.; Gudbrandsen, O.A.; Ueland, P.M.; Mellgren, G.; Lauritzen, L.; Lindqvist, H.; Hansen, A.L.; et al. Efficacy of fish intake on vitamin D status: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2015, 102, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Lofstedt, A.; de Roos, B.; Fernandes, P.G. Less than half of the European dietary recommendations for fish consumption are satisfied by national seafood supplies. Eur. J. Nutr. 2021, 60, 4219–4228. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Recommendations for the Polish Population and Their Application; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020; Available online: https://ncez.pl/abc-zywienia-/zasadyzdrowego-zywienia/normy-zywienia-2020 (accessed on 22 February 2024).
- Pasidi, E.; Vareltzis, P. Vitamin D3 bioaccessibility from supplements and foods—Gastric pH effect using a static in vitro gastrointestinal model. Molecules 2024, 29, 1153. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption: Rome, 25–29 January 2010; FAO/WHO: Rome, Italy, 2011. [Google Scholar]
- Jakobsen, J.; Smith, C.; Bysted, A.; Cashman, K.D. Vitamin D in wild and farmed Atlantic salmon (Salmo salar)—What do we know? Nutrients 2019, 11, 982. [Google Scholar] [CrossRef]
- EN 12821: 2009-08; Foodstuffs—Determination of Vitamin D by High Performance Liquid Chromatography—Measurement of Cholecalciferol (D3) or Ergocalciferol (D2). European Committee for Standardization: Brussels, Belgium, 2009.
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive stability study of vitamin D3 in aqueous solutions and liquid commercial products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]
- Taylor, P.N.; Davies, J.S. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef]
- International Society for Advancement of Kinanthropometry. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2001; ISBN 9780868037127. [Google Scholar]
- Nuttall, F.Q. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today 2015, 50, 117. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Sidor, P.; Włodarek, D. Vitamin D dietary intake questionnaire validation conducted among young Polish women. Nutrients 2016, 8, 36. [Google Scholar] [CrossRef]
- 2022—Climatological Summary. Center for Meteorological Modeling. Available online: https://modele.imgw.pl/cmm/?page_id=17143 (accessed on 5 September 2024). (In Polish).
- 2018—Climatological Summary. Center for Meteorological Modeling. Available online: https://modele.imgw.pl/cmm/?page_id=17652 (accessed on 5 September 2024). (In Polish).
- Statistics Poland—Demographic Yearbook of Poland 2023. Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-demograficzny-2023,3,17.html (accessed on 14 October 2024).
- Traczyk, I.; Kucharska, A.; Sińska, B.I.; Panczyk, M.; Wronka, L.; Raciborski, F.; Szostak-Węgierek, D.; Samoliński, B. Every second adult inhabitant of Poland (aged 18–64) is overweight—Results of representative cross-sectional studies conducted in 2017–2020. Ann. Agric. Environ. Med. 2023, 30, 322–330. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. jamovi (Version 2.3). 2024. Available online: https://www.jamovi.org (accessed on 2 April 2024).
- Raimundo, F.V.; Faulhaber, G.A.M.; Menegatti, P.K.; Marques, L.d.S.; Furlanetto, T.W. Effect of high- versus low-fat meal on serum 25-hydroxyvitamin D levels after a single oral dose of vitamin D: A single-blind, parallel, randomized trial. Int. J. Endocrinol. 2011, 2011, 809069. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, F.V.; Lang, M.A.B.; Scopel, L.; Marcondes, N.A.; Araújo, M.G.A.; Faulhaber, G.A.M.; Furlanetto, T.W. Effect of fat on serum 25-hydroxyvitamin D levels after a single oral dose of vitamin D in young healthy adults: A double-blind randomized placebo-controlled study. Eur. J. Nutr. 2015, 54, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Lichtenstein, A.H.; Dolnikowski, G.; Palermo, N.J.; Rasmussen, H. Dietary fat increases vitamin D-3 absorption. J. Acad. Nutr. Diet. 2015, 115, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S.; Palermo, N.J.; Ceglia, L.; Rasmussen, H. Meal conditions affect the absorption of supplemental vitamin D3 but not the plasma 25-hydroxyvitamin D response to supplementation. J. Bone Miner. Res. 2013, 28, 1778–1783. [Google Scholar] [CrossRef]
- Reboul, E.; Borel, P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 2011, 50, 388–402. [Google Scholar] [CrossRef]
- Tangpricha, V.; Koutkia, P.; Rieke, S.M.; Chen, T.C.; Perez, A.A.; Holick, M.F. Fortification of orange juice with vitamin D: A novel approach for enhancing vitamin D nutritional health. Am. J. Clin. Nutr. 2003, 77, 1478–1483. [Google Scholar] [CrossRef]
- Wagner, D.; Sidhom, G.; Whiting, S.J.; Rousseau, D.; Vieth, R. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J. Nutr. 2008, 138, 1365–1371. [Google Scholar] [CrossRef]
- Natri, A.M.; Salo, P.; Vikstedt, T.; Palssa, A.; Huttunen, M.; Kärkkäinen, M.U.M.; Salovaara, H.; Piironen, V.; Jakobsen, J.; Lamberg-Allardt, C.J. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J. Nutr. 2006, 136, 123–127. [Google Scholar] [CrossRef]
- Holmberg, I.; Aksnes, L.; Berlin, T.; Lindbäck, B.; Zemgals, J.; Lindeke, B. Absorption of a pharmacological dose of vitamin D3 from two different lipid vehicles in man: Comparison of peanut oil and a medium chain triglyceride. Biopharm. Drug Dispos. 1990, 11, 807–815. [Google Scholar] [CrossRef]
- Niramitmahapanya, S.; Harris, S.S.; Dawson-Hughes, B. Type of dietary fat is associated with the 25-hydroxyvitamin D3 increment in response to vitamin D supplementation. J. Clin. Endocrinol. Metab. 2011, 96, 3170–3174. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Outila, T.A.; Mattila, P.H.; Piironen, V.I.; Lamberg-Allardt, C.J. Bioavailability of vitamin D from wild edible mushrooms (Cantharellus tubaeformis) as measured with a human bioassay. Am. J. Clin. Nutr. 1999, 69, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Neill, H.R.; Gill, C.I.R.; McDonald, E.J.; McRoberts, W.C.; Loy, R.; Pourshahidi, L.K. Impact of cooking on vitamin D3 and 25(OH)D3 content of pork products. Food Chem. 2022, 397, 133839. [Google Scholar] [CrossRef]
- Jakobsen, J.; Knuthsen, P. Stability of vitamin D in foodstuffs during cooking. Food Chem. 2014, 148, 170–175. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, T.C.; Zhang, A.; Persons, K.S.; Kohn, N.; Berkowitz, R.; Martinello, S.; Holick, M.F. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J. Steroid. Biochem. Mol. Biol. 2007, 103, 642–644. [Google Scholar] [CrossRef]
- Żurek, G.; Przybyło, M.; Witkiewicz, W.; Langner, M. Novel approach for the approximation of vitamin D3 pharmacokinetics from in vivo absorption studies. Pharmaceutics 2023, 15, 783. [Google Scholar] [CrossRef]
- Maurya, V.K.; Aggarwal, M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef]
- Ramasamy, I. Vitamin D metabolism and guidelines for vitamin D supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef]
- Corring, T. The adaptation of digestive enzymes to the diet: Its physiological significance. Reprod. Nutr. Dev. 1980, 20, 1217–1235. [Google Scholar] [CrossRef]
- Lu, S.; Cao, Z.-B. Interplay between vitamin D and adipose tissue: Implications for adipogenesis and adipose tissue function. Nutrients 2023, 15, 4832. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Production, metabolism and mechanisms of action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278935/ (accessed on 7 October 2024).
- Anderson, P.H.; May, B.K.; Morris, H.A. Vitamin D metabolism: New concepts and clinical implications. Clin. Biochem. Rev. 2003, 24, 13. [Google Scholar]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D sources, metabolism, and deficiency: Available compounds and guidelines for its treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A.G. Vitamin D3 is more potent than vitamin D2 in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Sampels, S. Nutritional value of fish: Lipids, proteins, vitamins, and minerals. Rev. Fish. Sci. Aquac. 2018, 26, 243–253. [Google Scholar] [CrossRef]
- Thomsen, B.J.; Chow, E.Y.; Sapijaszko, M.J. The potential uses of omega-3 fatty acids in dermatology: A review. J. Cutan. Med. Surg. 2020, 24, 481–494. [Google Scholar] [CrossRef]
- Januszewski, J.; Forma, A.; Zembala, J.; Flieger, M.; Tyczyńska, M.; Dring, J.C.; Dudek, I.; Świątek, K.; Baj, J. Nutritional supplements for skin health—A review of what should be chosen and why. Medicina 2024, 60, 68. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid. Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef]
- Clifford, J.; Kozil, A. Fat-Soluble Vitamins: A, D, E, and K. Colorado State University Fact Sheet No 9.315. Available online: https://extension.colostate.edu/topic-areas/nutrition-food-safety-health/fat-soluble-vitamins-a-d-e-and-k-9-315/ (accessed on 24 September 2024).
- Jerome, S.P.; Sticka, K.D.; Schnurr, T.M.; Mangum, S.J.; Reynolds, A.J.; Dunlap, K.L. 25(OH)D levels in trained versus sedentary university students at 64° North. Int. J. Circumpolar Health 2017, 76, 1314414. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Agence Nationale de Sécurité Sanitaire de l’alimentation, de l’environnement et du Travail (ANSES). Vitamin D: Why Do I Need an Adequate Intake and How Can I Make Sure I Get It? Available online: https://www.anses.fr/en/content/vitamin-d-why-do-i-need-adequate-intake-and-how-can-i-make-sure-i-get-it (accessed on 26 February 2024).
- Voedingscentrum. Vitamine D. Available online: https://www.voedingscentrum.nl/encyclopedie/vitamine-d.aspx#blokhoeveel-vitamine-d-heb-je-per-dag-nodig? (accessed on 26 February 2024). (In Dutch).
- German Nutrition Society. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Finnish Food Authority—Ruokavirasro. Special Instructions and Restrictions. Vitamin D Supplementation. Available online: https://www.ruokavirasto.fi/en/foodstuffs/healthy-diet/nutrition-and-food-recommendations/special-instructions-and-restrictions/ (accessed on 26 February 2024).
- Ziegler, F.; Hilborn, R. Fished or farmed: Life cycle impacts of salmon consumer decisions and opportunities for reducing impacts. Sci. Total. Environ. 2023, 854, 158591. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Eilertsen, K.E.; Otnæs, C.H.A.; Mæhre, H.K.; Elvevoll, E.O. An update on the content of fatty acids, dioxins, PCBs and heavy metals in farmed, escaped and wild Atlantic salmon (Salmo salar L.) in Norway. Foods 2020, 9, 1901. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2016; UN: Rome, Italy, 2016; ISBN 9789210474559. [Google Scholar]
- Wang, T.; Masedunskas, A.; Willett, W.C.; Fontana, L. Vegetarian and vegan diets: Benefits and drawbacks. Eur. Heart J. 2023, 44, 3423–3439. [Google Scholar] [CrossRef]
| Smoked Salmon, 100 g | Salmon Sausage, 100 g | |
|---|---|---|
| Energy, kcal | 176.0 | 241.0 |
| Fat, g | 9.9 | 16.7 |
| Saturated fatty acids, g | 1.7 | 2.6 |
| Carbohydrates, g | 1.0 | 11.4 |
| Protein, g | 20.1 | 11.6 |
| Salt, g | 1.7 | 1.5 |
| Omega-3 fatty acids, g | 1.4 | - |
| EPA + DHA, mg | 610 | 360 |
| Vitamin D3, μg | 21.3 ± 5.55 | 4.41 ± 1.15 |
| Smoked Salmon Intervention Group n = 38 | Salmon Sausage Intervention Group n = 27 | Control Group n = 34 | p ** | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | ||
| Age, years | 21.6 ± 2.7 | 21 (20; 23) *a | 22.4 ± 2.8 | 21 (20; 23.5) *ab | 23.2 ± 2.9 | 23 (21; 25) b | 0.048 |
| Weight, kg | 58.3 ± 7.2 | 58.5 (51.8; 62.8) | 63.0 ± 9.6 | 61.5 (55.4; 69.4) * | 59.8 ± 8.9 | 60.3 (52.7; 65.9) | 0.172 |
| Height, cm | 168.1 ± 7.0 | 167.8 (163.7; 174.1) | 168.4 ± 6.5 | 168.2 (164.2; 171.8) | 168.3 ± 6.4 | 167.6 (164.5; 171.8) | 0.982 |
| BMI, kg/m2 | 20.6 ± 2.4 | 20.4 (19.2; 22.5) | 22.2 ± 3.0 | 21.2 (20.1; 23.7) * | 21.1 ± 3.0 | 20.9 (18.8; 22.8) | 0.118 |
| SLM, % | 70.8 ± 5.1 | 70.9 (66.8; 74.1) | 67.4 ± 4.9 | 67.3 (65.0; 69.9) | 68.9 ± 5.6 | 69.3 (64.9; 72.4) | 0.299 |
| TBW, % | 55.9 ± 4.0 | 56.0 (52.6; 58.4) | 53.1 ± 3.8 | 53.0 (51.1; 55.0) | 54.3 ± 4.4 | 54.6 (51.2; 57.1) | 0.291 |
| FM, % | 23.7 ± 5.5 | 23.7 (20.3; 28.0) a | 27.4 ± 5.2 | 27.5 (24.8; 30.0) b | 25.8 ± 6.0 | 25.2 (22.0; 30.0) ab | 0.014 |
| FFM, % | 76.3 ± 5.5 | 76.3 (72.0; 79.7) | 72.6 ± 5.2 | 72.5 (70.0; 75.2) | 74.2 ± 6.0 | 74.8 (70.0; 78.0) | 0.302 |
| SMM, % | 42.5 ± 3.1 | 42.5 (40.1; 44.4) | 40.4 ± 2.9 | 40.3 (38.9; 41.9) | 41.3 ± 3.4 | 41.6 (39.0; 43.4) | 0.298 |
| Weekly Intake of Fish and Fish Products, g/week | Smoked Salmon Intervention Group n = 38 | Salmon Sausage Intervention Group n = 27 | Control Group n = 34 | p ** | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | |||
| w0 (baseline) | 99.7 ± 58.7 | 87.5 (56.1; 137.0) * | 131.0 ± 75.5 | 128.0 (75.8; 187.0) | 108.0 ± 105.0 | 78.8 (54.0; 128.0) * | 0.119 | |
| w1 to w4 (4 first weeks of intervention) | including intervention | 240.0 ± 72.1 | 222.0 (187.0; 254.0) *a | 735.0 ± 85.7 | 712.0 (671.0; 776.0) *c | 82.9 ± 75.4 | 64.2 (24.8; 114.0) *b | <0.001 |
| excluding intervention | 76.8 ± 72.1 | 58.3 (23.3; 90.4) * | 79.5 ± 79.6 | 58.3 (17.5; 123.0) * | 82.9 ± 75.4 | 64.2 (24.8; 114.0) * | 0.902 | |
| w5 to w8 (4 last weeks of intervention) | including intervention | 229.0 ± 72.8 | 210.0 (171.0; 271.0) *a | 711.0 ± 58.2 c | 700.0 (665.0; 747.0) | 69.7 ± 57.1 | 58.3 (26.3; 102.0) *b | <0.001 |
| excluding intervention | 66.2 ± 72.7 | 46.7 (7.3; 108.0) * | 59.6 ± 55.3 | 46.7 (17.5; 93.3) * | 69.7 ± 57.1 | 58.3 (26.3; 102.0) * | 0.712 | |
| Daily Intake of Vitamin D, µg/day | Smoked Salmon Intervention Group n = 38 | Salmon Sausage Intervention Group n = 27 | Control Group n = 34 | p ** | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | |||
| w0 (baseline) | 3.4 ± 1.5 | 3.1 (2.4; 4.0) * | 3.7 ± 1.7 | 3.4 (2.3; 5.0) | 3.0 ± 1.2 | 2.7 (2.1; 3.4) * | 0.152 | |
| w1 to w4 (4 first weeks of intervention) | including intervention | 7.7 ± 1.2 | 7.3 (6.8; 8.4) *a | 7.1 ± 1.8 | 6.8 (6.0; 7.4) *b | 2.5 ± 1.1 | 2.3 (1.8; 3.1) c | <0.001 |
| excluding intervention | 2.7 ± 1.2 | 2.4 (1.9; 3.4) * | 2.9 ± 1.7 | 2.7 (1.9; 3.2) * | 2.5 ± 1.1 | 2.3 (1.8; 3.1) | 0.690 | |
| w5 to w8 (4 last weeks of intervention) | including intervention | 7.8 ± 1.9 | 7.3 (6.6; 8.2) *a | 6.7 ± 1.4 | 6.5 (5.9; 7.0) *b | 2.5 ± 1.3 | 2.3 (1.6; 2.8) *c | <0.001 |
| excluding intervention | 2.8 ± 1.9 | 2.3 (1.7; 3.2) * | 2.6 ± 1.4 | 2.4 (1.8; 2.9) * | 2.5 ± 1.3 | 2.3 (1.6; 2.8) * | 0.898 | |
| Variables | Time | Smoked Salmon Intervention Group n = 38 | Salmon Sausage Intervention Group n = 27 | Control Group n = 34 | p ** | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | Mean ± SD | Median (P25; P75) | |||
| 25(OH)D serum concentration, nmol/L | w0 | 64.2 ± 23.2 | 63.5 (47.8; 77.2) | 66.3 ± 24.9 | 65.8 (48.6; 80.4) | 65.1 ± 27.3 | 61.5 (47.9; 76.4) | 0.947 |
| w5 | 55.9 ± 18.6 | 56.9 (41.0; 68.9) | 61.9 ± 22.6 | 57.5 (46.8; 75.5) | 53.8 ± 23.4 | 56.4 (33.9; 69.8) | 0.323 | |
| w9 | 52.6 ± 18.8 | 53.3 (36.7; 63.0) | 62.4 ± 26.5 | 56.3 (45.8; 68.3) * | 48.3 ± 21.8 | 48.4 (32.6; 62.5) | 0.130 | |
| 25(OH)D serum concentration change, nmol/L | w0 to w5 | −8.2 ± 11.6 | −6.3 (−12.2; −2.1) *ab | −4.3 ± 8.2 | −2.5 (−11.4; 0.0) b | −11.4 ± 10.5 | −10.0 (−16.1; −4.5) a | 0.022 |
| w5 to w8 | −3.3 ± 6.8 | −3.1 (−6.1; −0.5) *ab | 0.4 ± 8.6 | −2.3 (−4.5; 3.8) *b | −5.5 ± 4.6 | −4.4 (−8.4; −2.3) a | 0.020 | |
| w0 to w9 | −11.6 ± 14.1 | −9.3 (−17.6; −3.9) *a | −3.9 ± 13.2 | −4.3 (−8.5; −0.8) *b | −16.9 ± 12.4 | −15.0 (−22.0; −8.4) *a | <0.001 | |
| Reference Value | Measurement Time | Vitamin D Status | Smoked Salmon Intervention Group n = 38 | Salmon sausage Intervention Group n = 27 | Control Group n = 34 | p * |
|---|---|---|---|---|---|---|
| 50 nmol/L 1 | w0 | Adequate | 27 (71%) | 18 (67%) | 24 (71%) | 0.922 |
| Inadequate | 11 (29%) | 9 (33%) | 10 (29%) | |||
| w5 | Adequate | 25 (66%) | 19 (70%) | 21 (62%) | 0.781 | |
| Inadequate | 13 (34%) | 8 (30%) | 13 (38%) | |||
| w9 | Adequate | 21 (55%) | 16 (59%) | 16 (47%) | 0.614 | |
| Inadequate | 17 (45%) | 11 (41%) | 18 (53%) | |||
| 75 nmol/L 2 | w0 | Adequate | 12 (32%) | 9 (33%) | 10 (29%) | 0.946 |
| Inadequate | 26 (68%) | 18 (67%) | 24 (71%) | |||
| w5 | Adequate | 6 (16%) | 7 (26%) | 5 (15%) | 0.470 | |
| Inadequate | 32 (84%) | 20 (74%) | 29 (85%) | |||
| w9 | Adequate | 6 (16%) | 6 (22%) | 3 (9%) | 0.346 | |
| Inadequate | 32 (84%) | 21 (78%) | 31 (91%) |
| Variables | Time | Vitamin D Status at Baseline | Smoked Salmon Intervention Group (Adequate 1 at Baseline: n = 27, Inadequate 2 at Baseline: n = 11) | Salmon Sausage Intervention Group (Adequate 1 at Baseline: n = 18, Inadequate 2 at Baseline: n = 9) | Control Group (Adequate 1 at Baseline: n = 24, Inadequate 2 at Baseline: n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (P25; P75) | p ** | Mean ± SD | Median (P25; P75) | p ** | Mean ± SD | Median (P25; P75) | p ** | |||
| 25(OH)D serum concentration, nmol/L | w0 | Adequate 1 | 74.6 ± 18.7 | 69.3 (62.5; 83.6) * | <0.001 | 79.0 ± 19.8 | 74.6 (66.0; 87.5) | <0.001 | 76.9 ± 22.8 | 71.5 (60.2; 84.7) * | <0.001 |
| Inadequate 2 | 38.5 ± 7.8 | 39.5 (34.0; 44.1) | 40.7 ± 8.8 | 41.8 (34.3; 47.5) | 36.8 ± 11.6 | 39.6 (32.4; 45.8) | |||||
| w5 | Adequate 1 | 63.8 ± 13.8 | 62.3 (54.9; 74.4) | <0.001 | 71.8 ± 20.6 | 67.3 (58.4; 79.8) * | <0.001 | 64.4 ± 18.5 | 61.3 (54.8; 72.9) | <0.001 | |
| Inadequate 2 | 36.7 ± 14.6 | 36.0 (25.8; 39.0) * | 42.2 ± 10.3 | 46.0 (38.8; 47.3) * | 28.1 ± 9.9 | 27.9 (21.6; 32.3) | |||||
| w9 | Adequate 1 | 59.8 ± 13.9 | 59.5 (50.0; 68.9) | <0.001 | 72.0 ± 26.9 | 63.8 (56.2; 84.6) * | <0.001 | 57.9 ± 18.0 | 56.1 (47.2; 67.8) | <0.001 | |
| Inadequate 2 | 34.9 ± 17.9 | 28.0 (26.8; 35.5) * | 43.1 ± 10.4 | 45.3 (44.5; 46.5) * | 25.1 ± 9.0 | 24.6 (19.1; 30.9) | |||||
| 25(OH)D serum concentration change, nmol/L | w0 to w5 | Adequate 1 | −10.8 ± 11.6 | −9.3 (−14.1; −3.6) * | 0.079 | −7.2 ± 7.7 | −3.8 (−13.1; −1.1) | 0.018 | −12.5 ± 12.0 | −12.0 (−17.9; −2.2) | 0.558 |
| Inadequate 2 | −1.8 ± 9.3 | −3.8 (−7.3; 0.0) | 1.5 ± 6.1 | 0.0 (−2.5; 3.8) | −8.7 ± 5.0 | −8.0 (−11.8; −4.6) | |||||
| w5 to w9 | Adequate 1 | −3.9 ± 5.0 | −3.3 (−6.8; −1.3) | 0.551 | 0.2 ± 10.0 | −3.5 (−4.9; 4.8) * | 0.217 | −6.6 ± 5.0 | −6.0 (−10.2; −2.8) | 0.576 | |
| Inadequate 2 | −1.8 ± 10.0 | −2.0 (−5.1; 0.1) | 0.8 ± 5.0 | −1.5 (−2.3; 1.5) * | −3.0 ± 1.8 | −3.5 (−4.4; −2.3) | |||||
| w0 to w9 | Adequate 1 | −14.8 ± 12.9 | −12.5 (−21.0; −5.3) * | 0.055 | −7.0 ± 13.9 | −5.4 (−16.3; −1.6) | 0.048 | −19.0 ± 13.9 | −18.5 (−24.0; −8.0) | 0.117 | |
| Inadequate 2 | −3.6 ± 14.3 | −5.8 (−11.0; −3.0) * | 2.3 ± 9.7 | −3.0 (−4.3; 7.8) * | −11.6 ± 4.8 | −12.8 (−14.6; −9.0) | |||||
| Time | 25(OH)D Serum Concentration Change | Smoked Salmon Intervention Group n = 38 | Salmon Sausage Intervention Group n = 27 | Control Group n = 34 | p * |
|---|---|---|---|---|---|
| w0 to w5 | Increase/maintained | 6 (16%) | 8 (30%) | 3 (9%) | 0.097 |
| Decrease | 32 (84%) | 19 (70%) | 31 (91%) | ||
| w5 to w9 | Increase/maintained | 9 (24%) | 9 (33%) | 2 (6%) | 0.024 |
| Decrease | 29 (76%) | 18 (67%) | 32 (94%) | ||
| w0 to w9 | Increase/maintained | 2 (5%) | 6 (22%) | 1 (3%) | 0.020 |
| Decrease | 36 (95%) | 21 (78%) | 33 (97%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utri-Khodadady, Z.; Głąbska, D.; Guzek, D. Effect of Consuming Salmon Products on Vitamin D Status of Young Caucasian Women in Autumn—A Randomized 8-Week Dietary VISA 2 (Vitamin D in Salmon Part 2) Intervention Study. Nutrients 2024, 16, 3565. https://doi.org/10.3390/nu16203565
Utri-Khodadady Z, Głąbska D, Guzek D. Effect of Consuming Salmon Products on Vitamin D Status of Young Caucasian Women in Autumn—A Randomized 8-Week Dietary VISA 2 (Vitamin D in Salmon Part 2) Intervention Study. Nutrients. 2024; 16(20):3565. https://doi.org/10.3390/nu16203565
Chicago/Turabian StyleUtri-Khodadady, Zofia, Dominika Głąbska, and Dominika Guzek. 2024. "Effect of Consuming Salmon Products on Vitamin D Status of Young Caucasian Women in Autumn—A Randomized 8-Week Dietary VISA 2 (Vitamin D in Salmon Part 2) Intervention Study" Nutrients 16, no. 20: 3565. https://doi.org/10.3390/nu16203565
APA StyleUtri-Khodadady, Z., Głąbska, D., & Guzek, D. (2024). Effect of Consuming Salmon Products on Vitamin D Status of Young Caucasian Women in Autumn—A Randomized 8-Week Dietary VISA 2 (Vitamin D in Salmon Part 2) Intervention Study. Nutrients, 16(20), 3565. https://doi.org/10.3390/nu16203565









