Abstract
Background/Objectives: Our objective was to review published studies of the intestinal barrier and permeability, the deleterious effects of dietary components (particularly fat), the impact of altered intestinal permeability in disease models and human diseases, the role of the microbiome and epigenomics in control of barrier function, and the opportunities to restore normal barrier function with dietary interventions and products of the microbiota. Methods: We conducted a literature review including the following keywords alone or in combination: intestinal barrier, permeability, microbiome, epigenomics, diet, irritable bowel syndrome, inflammatory bowel disease, probiotics. Results: Intestinal permeability is modified by a diet including fat, which increases permeability, and nutrients such as fiber, glutamine, zinc, vitamin D, polyphenols, emulsifiers, and anthocyanins, which decrease permeability. There is significant interaction of the microbiome and barrier function, including the inflammatory of luminal/bacterial antigens, and anti-inflammatory effects of commensals or probiotics and their products, including short-chain fatty acids. Epigenomic modification of barrier functions are best illustrated by effects on junction proteins or inflammation. Detailed documentation of the protective effects of diet, probiotics, prebiotics, and microbiota is provided. Conclusion: intestinal permeability is a critical factor in protection against gastrointestinal diseases and is impacted by nutrients that preserve or heal and repair the barrier and nurture anti-inflammatory effects.
1. Introduction
The objective of this review is to provide information regarding the intestinal barrier and permeability, the deleterious effects of dietary components (particularly fat), the impact of altered intestinal permeability in disease models or human diseases, the role of the microbiome and epigenomics in the control of barrier function, the opportunities to restore normal barrier function with dietary interventions, and products of microbiota.
Intestinal Barrier and Permeability
The gastrointestinal tract features one of the longest barriers between the environment and the systemic circulation, spanning up to 40 square meters. This barrier is composed of a mucus layer, commensal microbiota, the intestinal epithelium, and immune cells situated in the lamina propria [1,2,3]. The mucus layer over the intestinal epithelium safeguards it from contents within the intestinal lumen [4]. Commensal microbiota support barrier function, shape intestinal and systemic immune responses, and inhibit pathogenic bacteria by directly stimulating epithelial cells, producing nutrients and metabolites that are essential for enterocyte health, and influencing the priming of the immune system [5]. The epithelial semi-permeable barrier contributes to maintaining intestinal homeostasis by selectively allowing nutrients to cross from the intestinal lumen into the internal milieu, to reach the systemic circulation, while controlling the passage of bacterial pathogens, allergens, and toxins [2].
The epithelial cells are organized into a single layer of cells from four primary lineages: enterocytes (the most abundant), goblet cells, enteroendocrine cells, and Paneth cells [4,6,7]. Enterocytes are chiefly involved in absorption of nutrients, water and electrolytes from the lumen, goblet cells secret mucus that adds protection to the barrier layer, enteroendocrine cells secrete hormones that have diverse regulatory functions, and Paneth cells release antimicrobial peptides [2,6,8]. These cells function synergistically to maintain the gut barrier’s integrity [6].
Tight junctions (TJs) are a series of transmembrane proteins that connect intestinal epithelial cells and are essential for maintaining barrier integrity, particularly when the intestines are distended [4]. The recent literature has reported three main proteins that make up TJs and contribute to their function. Proteins found in the cytoplasm beneath the TJ membrane include cingulin (CGN), guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and zonula occludens proteins (ZOs) including ZO-1, ZO-2, and ZO-3 (which are also known as TJP1, TJP2, and TJP3, respectively) [9,10]. Other proteins that contribute to the permeability/barrier function of TJs comprise claudin polymers that are either barrier-forming or channel-forming. It is important to note that further interactions between claudin isoforms also exist but have yet to be established [9,11]. Additionally among the proteins that contribute to the permeability, barrier, and adhesion function of TJs, there is junctional adhesion molecule A (JAM-A, also known as F11R) [9].
These junctions are also crucial for regulating the transport of substances between the intestinal lumen and systemic circulation [12]; such transport is mediated in part by the permeability of TJs, leading to three types of functional gaps with different properties: pore pathway, leak pathway, and restricted pathway [13]. The pore pathway is of high capacity and charge-selective, permitting the passage of small ions and uncharged molecules (typically <8 Å); the leak pathway is low-capacity and nonselective (<100 Å); on the other hand, the unrestricted pathway is independent of the TJ and results from epithelial damage [9,12,14]. Damage to these pathways alters the barrier integrity and may contribute to the development of disease [15,16].
Intestinal permeability refers to the functional properties of the intestinal barrier and is characterized primarily by paracellular and transcellular transport mechanisms [7]. The paracellular route, regulated by tight junctions, allows the passage of ions, water, and large hydrophilic compounds [17], while the transcellular route facilitates the passage of proteins, sugars, amino acids, and bacteria [18], typically by carrier-mediated transport. Disruption in either route can affect barrier homeostasis, alter permeability and potentially lead to disease [3,18,19].
Various factors, including dietary components, genetic predisposition, medications (e.g., NSAIDs and antibiotics), alcohol consumption, strenuous physical activity, psychological or environmental stress, pregnancy, pathogens, systemic diseases, inflammatory conditions and cytokines, metabolic disorders including obesity, and surfactants (e.g., bile acids and emulsifiers) can alter barrier homeostasis [3,17,18,19,20] and increase permeability, leading to “leaky gut” [18,21]. This “leakiness” allows passage of microorganisms, allergens, and toxins inciting inflammation locally in the gut, and passage into the systemic circulation [3,21]. “Leaky gut” has been implicated in a wide range of systemic diseases (including type 2 diabetes mellitus, obesity), neuropsychiatric diseases (e.g., Alzheimer disease, Parkinson disease, autism spectrum disorders, major depressive disorder), and autoimmune disease (e.g., psoriasis, rheumatologic diseases, and uveitis), in addition to intestinal diseases (e.g., inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), celiac disease) [1,4,18,20,22].
2. Epigenetic Mechanisms Related to Intestinal Permeability
Recent data suggest epigenetic mechanisms are involved in regulating intestinal permeability. Epigenetic mechanisms include how the environment alters the expression of DNA without changing DNA nucleotides. Examples of epigenetic mechanisms include DNA methylation, histone modification, non-coding RNA activity, and chromatin remodeling, which all affect how genes are expressed. Environmental exposures, stress, toxins, medications, development, aging, and diet can induce epigenetic changes. Environmental states with increased levels of stress are thought to effect change via epigenetic mechanisms, related to the corticotropin releasing factor/hypothalamic-pituitary-adrenal axis [23]. The published data on epigenetic changes on intestinal permeability are primarily from stressed animal models which have increased intestinal paracellular permeability and visceral hypersensitivity [23]. In a chronic preclinical stress model, which is relevant to pathophysiological states like irritable bowel syndrome, the mice had increased levels of cytokine IL-6, which is pro-inflammatory, and correlated to a decrease in tight junction proteins in the colon, documented by an inverse correlation of IL-6 level and the expression of occludins [24]. IL-6 acted via epigenetic mechanisms (as evidenced by increased methylation of the repressive histone H3K9) with an observed increase in paracellular permeability [24]. In biopsies taken from patients with diarrhea-predominant irritable bowel syndrome, there were increased levels of micro-RNA 29A and B and reduced levels of claudin-1 (CLDN1) and nuclear factor-kB-repressing factor (NKRF), which was replicated in a knockout animal model that documented the fact that increased micro-RNA 29A and B decreased the levels of CLDN1 and NKRF mRNA [25].
Network enrichment analysis of RNA-sequencing data in a chronic stress model of rats identified that super enhancers play an important role in intestinal barrier dysfunction [26]. Super enhancers are sites in the genome with increased areas for transcription factors to bind (enhancers) and enhance genetic expression of an associated gene up to one million base pairs away. Super enhancers have recently been considered an epigenetic mechanism as they regulate gene expression without modifying DNA. In particular, in the rat chronic stress model, it was shown that super enhancers played a role in the downregulation of adherens and intestinal epithelial tight junction genes (e-cadherin (CDH1), tight junction protein 3 or ZO-3 (TJP3), p-cadherin (CDH3), m-cadherin (CDH15), claudin-2 (CLDN2), claudin-3 (CLDN3), claudin-7 (CLDN7), and tight junction associated protein 1 or 4 (TJAP1)), which are linked to increased paracellular permeability [26]. The microRNAs involved in intestinal permeability include microRNA-16 (downregulated in IBS), microRNA 125b (downregulated in jejunal IBS-D tissue), microRNA 144 (upregulated in a colon IBS_D rat model), and microRNA-29a (upregulated in colon and duodenum of IBS and rat models) [23,27,28,29,30,31].
Irritable bowel syndrome is not the only phenotype where intestinal permeability and epigenetic mechanisms have been studied. Paracellular membrane permeability was influenced by DNA methyltransferase 3A activity (which is involved in DNA methylation) in the colon epithelial cells from patients with IBD in studies conducted in vitro [32]. Colon tissue samples and organoids from patients with ulcerative colitis had increased levels of IL1B mRNA and microRNA 200C-3p that decreased the expression of occludin and increased intestinal permeability [33]. In patients with systemic lupus erythematosus, there is also evidence of association of possibly increased intestinal permeability, with DNA methylation levels being highest in 926 CpG sites [34].
Additionally, the microbiome is also thought to contribute to local changes in intestinal permeability and maintenance of membrane barrier function [35,36,37]. Increased intestinal permeability can result from altered expression of tight junction related proteins with changes in intestinal microbiota profiles [38]. The host phenotype is influenced by the epigenome–microbiome axis. This includes how the environment and host genotype affect both the host epigenotype and the microbiome composition and activity. It is also known that the microbiome and epigenotype contribute to the host phenotype [39]. Microbiota can induce modification of gene expression through epigenetic mechanisms [39]. For example, disruption to the barrier function was repaired though the activation of epithelial histone deacetylase 3 (HDAC3) via microbiota-derived IP3 (inositol triphosphate) in a murine model and intestinal organoid model [37]. Histone deacetylases remove acetyl groups from histones (and non-histones), decreasing transcription. Short chain fatty acids (SCFAs) like butyrate, acetate, and propionate inhibit host histone deacetylases, leading to silencing of transcription, chromatin condensation, histone acylation, and DNA methylation [39].
Chronic stress and the microbiome are not the only ways via which epigenetic modulation affects intestinal permeability. Ingested substances (discussed more extensively in subsequent sections and Table 1 also influence epigenetic mechanisms. For example, anthocyanins, which are part of the flavonoid group of polyphenols, naturally occur in many colorful fruits and vegetables. Anthocyanins appear to play a role in epigenetic modulation of the expression of key membrane integrity proteins, such as ZO-1 and occludins, in animal models [40]. Zinc also influences the gene expression of tight junction proteins, claudin 1 and 2, through histone deacetylase activity and chromatin remodeling [41,42].

Table 1.
Effects of high-fat diet on intestinal barrier function in humans in vivo or in vitro (adapted and updated from Camilleri and Vella [2]).
3. Effect of Food Substances and Disease States on Intestinal Permeability
3.1. Food Substances That Decrease Intestinal Barrier Integrity or Increase Permeability
In view of the most recent extensive literature regarding the deleterious effect of fats on intestinal barrier function, and the associations of Western diet on diverse diseases, Table 1 summarizes the effects of high-fat diet on intestinal barrier function [21,43,44,45,46]. The table shows that, in vivo, fat intake is associated with serological markers of increased permeability such as plasma lipopolysaccharide and serum endotoxin; in addition, there was a trend to increased permeability measured using the ratio of the urinary excretion of oral sugar probe molecules (lactulose and mannitol). There is also evidence, based on in vitro studies of modulators from human Peyer’s patches, that the emulsifier polysorbate-80 increased translocation of E. coli.
3.2. Altered Permeability and Disease States
Altered permeability has been investigated in vivo in preclinical models of disease, as well as in human disease states, and these are summarized in Table 2 and Table 3 and elaborated further in Section 4 [21,47,48,49,50,51,52,53,54,55].

Table 2.
Summary of preclinical models of altered permeability investigated in vivo (adapted and updated from Khoshbin and Camilleri [42]).
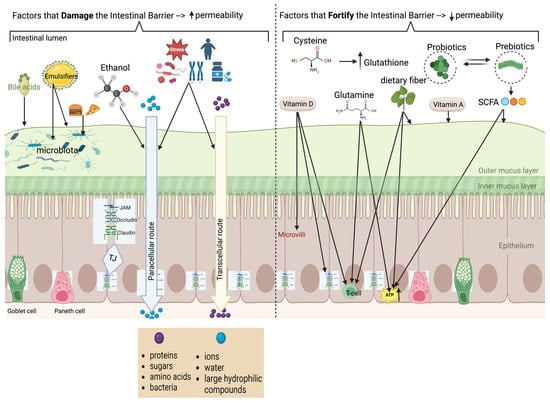
Figure 1.
Composition of barrier, physiology of permeability, and factors which help or harm permeability (created in BioRender. matar, a. (2024) BioRender.com/m56y070 (accessed on 10 October 2024)). The left side of the figure summarizes the factors that damage the intestinal barrier resulting in increased permeability; the right side summarizes factors that enhance the intestinal barrier resulting in reduced intestinal permeability. Several amino acids, exemplified by glutamine in the figure, are associated with fortification of the intestinal barrier (as detailed in the text).

Table 3.
Summary of preclinical models investigating human disease states of altered permeability (adapted and updated from Khoshbin and Camilleri [42]).
4. Effects of Dietary Components That Enhance or Damage the Intestinal Barrier
4.1. Nutrients
Table 4 provide a summary of the nutrients that either increase or decrease intestinal permeability as well as the mechanisms proposed for the changes in intestinal permeability

Table 4.
Summary of nutrients increasing or decreasing intestinal permeability and proposed mechanisms.
4.1.1. Substances That Help Increase Intestinal Barrier Integrity
Numerous strategies have been suggested to address gut barrier dysfunction, such as direct immune therapies, blocking signaling factors, introducing specific microbes, fecal microbiota transplantation, using microbial metabolites, probiotics, targeting disease-induced regulatory factors, hormone treatments, treating underlying conditions, dietary supplementation, and maintaining a healthy diet and overall wellness [71]. This section focuses on dietary factors and microbial metabolites and nutrients, based on a review of the recent literature and updated versions of reports written by Khoshbin and Camilleri [42] and Camilleri and Vella [21].
4.1.2. Dietary Fiber
Dietary fiber is a carbohydrate polymer made up of 10 or more monomers that are neither absorbed nor digested in the small intestine [56,57]. The four subgroups of dietary fiber that research primarily focuses on are resistant oligosaccharides, non-starch polysaccharides, resistant starches, and associated substances [57]. Dietary sources of resistant oligosaccharides include various legumes, vegetables, fruits, and specific plant products like chicory root and soybeans. Non-starch polysaccharides, such as cellulose, hemicellulose, and pectins are found in cereals, grains, fruits, and vegetables, while resistant starches come from foods like grains, green bananas, and processed products. Additionally, associated non-carbohydrate substances like lignin, waxes, and chitins are present in cereal grain outer layers, insect secretions, and the exoskeletons of crustaceans. These types of fiber are considered as prebiotics since stimulate bacteria beneficial for the gut health and aid in the increase of short chains of fatty acids (SCFAs) which also enhances the integrity of the intestinal barrier [47].
SCFAs, produced from dietary fiber fermentation by gut microbiota, play a crucial role in colon health and microbiota–gut–brain communication, providing 5–10% of human basal energy needs [58,59]. Research has focused around three SCFAs: acetate, butyrate, and propionate [60]. Acetate, the most abundant SCFA, supports ATP production, while SCFAs overall lower colon pH to inhibit pathogenic bacteria and modulate inflammation through various cellular mechanisms [60,61]. Butyrate, in particular, has significant health benefits, including cancer prevention, while propionate may reduce blood cholesterol, and both contribute to communication along the microbiota–gut–brain axis due to their neuro-active properties [62].
Resistant oligosaccharides include fructo-oligosaccharides (short chain inulin), which are β-(2 → 1) linear fructans derived from enzymatic hydrolysis of long-chain inulin or synthesized from sucrose [58]. Galacto-oligosaccharides consist of two to eight saccharide units with glucose and galactose and can be found in human milk or synthesized from lactose [58]. Other resistant oligosaccharides include non-digestible types like konjac oligosaccharide. Incorporating a wide range of fruits, vegetables, various legumes, and soybeans (resistant oligosaccharide products) can go along way toward enhancing gut health, especially considering recent Westernized diets that have become widely popular. Mistry et al. showed in an in vivo study on male mice fed a Western-type diet that resistant oligosaccharide supplementation significantly reduced bodyweight gain, fat accumulation, insulin resistance, and plasma cholesterol levels, while also altering gut microbiota composition in a way that may benefit metabolic health. These findings suggest that resistant oligosaccharides could have therapeutic potential for improving metabolic markers and reducing the risk of metabolic syndrome [47].
Non-starch polysaccharides include cellulose, the main structural component of plant cell walls, and hemicelluloses, which are diverse cell-wall polysaccharides found in fruits, vegetables, and cereals [58]. Pectins, found in plant cell walls, and various gums and mucilages from plants, microbes, and seaweed, are also key non-starch polysaccharides. Additionally, β-glucans and β-fructans, such as inulin from chicory roots, are non-digestible fibers that are resistant to enzymatic digestion but fermentable in the colon [58]. In an in vivo study with healthy male volunteers, inulin was shown to lower the lactulose–mannitol (L/M) ratio and serum zonulin while increasing mucosal GLP-2 after 8 weeks [21]. However, the study’s methods were suboptimal, including the use of inappropriate sugar probes, limited urine collection time, and serum levels that may not have accurately indicated intestinal permeability.
Resistant starches (RS) come in various forms, including physically inaccessible starch (RS1), granular native starch (RS2), retrograded starch (RS3), chemically modified starch (RS4), and resistant maltodextrins like Nutriose (RS5) [58]. Gondalia et al. [48] showed that in an in vivo study of 80 healthy adults who were either given high-amylose wheat (HAW) product or low-amylose wheat (LAW) product, HAW and LAW had similar effects on fecal output and total SCFA excretion, but the HAW-R group showed 38% higher fecal butyrate excretion and more SCFA-producing bacteria at 4 weeks. While LAW-R increased fecal p-cresol levels, which disrupts the epithelial barrier function, and also increased the abundance of a p-cresol-producing bacterium (Clostridium difficile, Clostridium scatologenes, Clostridium bolteae), these were reduced by HAW-R, with no impact on fecal consistency or digestive comfort from the amylose level [48].
4.1.3. Polyphenols, Anthocyanins, and Ellagitannins
Recent studies have focused on polyphenols, particularly flavonoids (such as anthocyanins, flavonols, and flavanols), condensed and hydrolysable tannins (like ellagitannins), phenolic acids, stilbenes, and lignans, due to their potential benefits for the intestinal barrier [63,64,65]. These polyphenols are predominantly found in various types of berries, including blueberries, strawberries, and blackberries [65]. Berries are a rich source of flavonoids, particularly anthocyanins, which give them their vibrant colors and have strong antioxidant properties [65]. These pigments, along with flavonols and flavan-3-ols, are not only capable of crossing the blood–brain barrier but also offer potential health benefits, including cardioprotective, neuroprotective, anti-inflammatory, and anticancer effects [65].
Ellagitannins (ETs) are hydrolysable tannins abundantly found in raspberries, walnuts, strawberries, and pomegranate [64]. They are hydrolyzed in the intestinal lumen and release ellagic acid (EA), which is then further metabolized with ellagitannins into urolithins [64,66]. Due to the interindividual variability of metabolization of ETs, three Uro metabotypes (UM) that are microbiome-specific have been identified and are most relevant to this paper, metabotype A, metabotype B, and metabotype 0, yielding UM-A, UM-B, and UM-0, respectively [65,66].
Urolithin A (Uro-A) and to a lesser extent Urothilin B (Uro-B) exhibit anti-inflammatory, neuroprotective, cardioprotective, and anti-obesity activites, as well as improving gut microbiota and tight junction protein expression, cognitive function, and muscle function, while also demonstrating potential in reducing oxidative stress, protecting against organ damage, and modulating the immune response in various animal models [67]. A recent in vivo study found that administering Uro-A and Uro-B intraperitoneally for 4 weeks in rats on a high-fat diet altered gut microbiota composition, leading to a decrease in microbes associated with body weight, lipid metabolism issues, and inflammation [53].
4.1.4. Glutamine
In an in vivo study investigating the effects of dietary glycyl-glutamine (GlyGln) supplementation, weaned piglets were given either a basal diet or the same diet supplemented with 0.25% GlyGln for 3 weeks [54]. Following intraperitoneal injections of lipopolysaccharide (LPS) to induce inflammation, the GlyGln-supplemented piglets showed improved ileum morphology, reduced inflammation, and enhanced oxidative status, with increased levels of interleukin 10 and tight junction proteins, as well as higher superoxide dismutase activity [54]. Additionally, GlyGln supplementation restored gut microbiota diversity and function, enriching beneficial bacteria and short-chain fatty acid producers, and improving intestinal integrity and microbial balance disrupted by the LPS challenge [54].
In another study that investigated the effects of oral supplementation with Ala-Gln and glutamine (Gln) on dextran sulfate sodium (DSS)-induced colitis in mice [55], both supplements significantly improved colitis symptoms, such as bodyweight loss and colon damage, reduced inflammation markers and tissue apoptosis, and enhanced gut microbiota diversity and function, with Ala-Gln demonstrating superior efficacy over Gln in preserving intestinal integrity and microbiota composition [55].
4.1.5. Vitamin D
Research shows that vitamin D is essential for maintaining gut barrier integrity, with VDR-deficient mice study displaying increased susceptibility to bacteria and LPS in vivo due to weakened tight junctions [49]. Vitamin D supplementation strengthens the epithelial barrier by enhancing the expression of tight junction proteins like ZO-1, occludin, and claudin-1, as demonstrated in studies where 1,25(OH)2D3 treatment partially rescued these proteins’ reduced expression in DSS-treated Caco-2 cells [49,68]. Additionally, vitamin D upregulates antimicrobial peptides, which are crucial for maintaining a balanced microbiome and preventing harmful bacterial colonization [68]. Thomas et al. conducted an in vivo study where 567 men provided stool samples, to study their microbiome and correlate it with vitamin D metabolite levels. This study showed that men with the highest levels of active vitamin D (1,25(OH)2D) or activation ratios were more likely to have gut bacteria associated with butyrate production, which helps increase the integrity of the intestinal barrier, whereas serum 25(OH)D levels showed no strong association with microbiota diversity or specific bacteria [50]. This suggests that the regulation of vitamin D metabolism, rather than overall body stores of vitamin D, may have more significant health implications.
It is worth noting that Yamamoto et al. showed that improper regulation of vitamin D, whether through deficiency or excessive supplementation, can result in “leaky” junctions, which may partially contribute to the development of colitis [49]. They highlighted the importance of balancing vitamin D levels to ensure a therapeutic effect and not a damaging one [49]. However, more evidence is needed to fully understand the extent of vitamin D’s role in this process.
4.1.6. Zinc
Recent studies have shown that zinc is a double-edged sword. In other words, both the deficiency and the overdosing of zinc can cause dysbiosis in the gut microbiome [69]. The deficiency of zinc has been correlated with multiple gastrointestinal disorders such as gastric cancer, Crohn’s disease, impaired intestinal permeability, and irritable bowel syndrome [51,70]. Omry et al. show in an in vivo study with chicks divided into two treatment groups based on sex and body weight, “high Zn” and “low Zn,” that Zn deficiency led to significant changes in microbial taxonomy, reduced species richness and diversity, and substantial reductions in KEGG pathways for nutrient uptake and beneficial short-chain fatty acids, which can impair optimal host Zn availability [51].
Consequently, supplementation with the appropriate dose of Zn has been shown to increase gut bacteria biodiversity, improve intestinal barrier integrity, and thereby limit the number of bacteria that can pass through the barrier into the systemic circulation [52]. The overdosing of Zn has been hypothesized to be related to the presence of “free flowing” Zn, thus altering immunity and causing dysbiosis [52]. An in vivo study in mice of chronic toxic exposure to ZnSO4 for 7 weeks showed reduced body and organ weight and increased AST activity [52]. Thus, excessive Zn doses can induce oxidative stress, compromise barrier integrity, and increase intestinal permeability, leading to a higher risk of the progression of other diseases [52,70]. Therefore, the right dosage of Zn should be provided and monitored to ensure its beneficial outcomes.
4.1.7. Emulsifiers
Emulsifiers, commonly known as surfactants, are widely used as food additives in the food industry and are highly prevalent in ultra-processed foods [42]. Previous research conducted primarily on mice in vitro indicated that emulsifiers increased intestinal permeability and were proinflammatory [72,73,74,75]. The study details were as follows:
Levine et al. [72] and Bancil et al. [74] conducted reviews of the existing literature, including both in vivo and in vitro studies, which showed that emulsifiers such as carboxymethylcellulose (CMS) and polysorbate 80 (P80) disrupt the intestinal mucosal layer leading to increased intestinal permeability or “leaky gut”.
Naimi et al. studied the effects of CMC and P80 on the gut microbiota using in vitro fermentation of healthy human fecal samples. This study showed that exposure to either CMC or P80 caused a reduction in microbial diversity, an increase in pro-inflammatory bacteria (Proteobacteria), a decrease in butyrate production, decreased barrier integrity, and increased permeability in an in vitro epithelial model.
Ogulur et al. [75] conducted both in vitro and in vivo studies with polysorbate 20 (P20) and P80. In vitro studies were carried out on human intestinal epithelial cell lines (Caco-2 and HT29 cells), indicating that P80 and P20 compromised barrier function and disrupted TJ proteins. In vivo studies were conducted on mice and also showed an increase in intestinal permeability.
However, a recent in vivo and in vitro study by Fitzpatrick et al. involving 22 healthy adults who were fed either a high- or low-emulsifier diet (CMC, P80) produced unexpected results [76]. The study found that, based on diverse measurements (specifically, 2 h urinary lactulose–rhamnose ratio, serum concentrations of lipopolysaccharide-binding protein, soluble CD14, markers of epithelial injury and inflammation), diets high in emulsifiers actually improved intestinal barrier function in unstressed conditions, but increased sensitivity of intestinal permeability in response to stress [76]. Neither effect was associated with signs of inflammation [76]. More studies should be conducted to further solidify this finding and to appraise the interaction of experimental stress and exposure to emulsifiers.
4.2. Microbial Nutrients and Metabolites
4.2.1. Prebiotics
Descriptions and Mechanisms of Action: Prebiotics are defined as “substrate[s] that [are] selectively utilized by host microorganisms conferring a health benefit” [77]. While many different classes of compounds function as prebiotics, the most common types are poorly or non-digestible carbohydrates that are resistant to endogenous intestinal digestive enzymes. Non-digestible oligosaccharides represent a broader category that includes any oligosaccharides that are not digested nor absorbed in the small intestine; this group encompasses resistant or non-resistant oligosaccharides from the perspective of microbial fermentation. These oligosaccharides, such as fructans and galactans, reach the colon and are selectively metabolized by beneficial bacteria such as bifidobacteria. For instance, fructo-oligosaccharides (FOSs) and galacto-oligosaccharides (GOSs) are not digestible by human enzymes and can serve as substrates for fermentation by beneficial gut bacteria. Thus, they are degraded by β-fructanosidase and β-galactosidase enzymes, respectively. This selective utilization results in the production of short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate, which are crucial for maintaining intestinal health and can influence metabolic activities, immune functions, and overall homeostasis. Other examples of prebiotics include human milk oligosaccharides (HMOs), which are vital for the development of the newborn intestinal microbiota and immune system. HMOs are selectively utilized by specific Bifidobacterium species, promoting the growth of beneficial bacteria and protecting against pathogens. Plant polyphenols are another class of compounds that meet prebiotic criteria, undergoing extensive biotransformation in the colon to produce health-promoting metabolites [77].
Prebiotics exert significant protective effects on the intestinal barrier through multiple mechanisms. One primary effect is the modulation of microbial communities within the gut. Fermentation of prebiotic fibers leads to the production of SCFAs, which play a crucial role in maintaining the integrity of the intestinal barrier. Butyrate, in particular, is a key energy source for colonocytes and helps strengthen the epithelial barrier by promoting mucus production and secretion. This mucus layer acts as a protective shield, preventing microbial invasion and reducing susceptibility to infections and inflammatory conditions. Additionally, acetate and propionate support goblet cell function and mucin production, further enhancing the barrier function [78]. Prebiotics can also enhance the intestinal microenvironment in mechanisms independent of SCFAs. For instance, fiber can bind and transport essential minerals like calcium, zinc, and copper to the distal gut, where they are released for absorption. This process not only supports the microbiota but also improves mineral bioavailability for the host. Moreover, fiber may facilitate interactions between bacteria and biomolecules such as bile acids, enhancing microbial metabolism [78]. In addition to a large body of pre-clinical evidence detailing the mechanisms of prebiotics, there is also considerable pre-clinical evidence that these effects are anti-inflammatory in the intestines. For example, numerous studies have found that prebiotics can reduce inflammation in animal models of colitis [79]. A comprehensive summary of clinical trials that have assessed the effects of prebiotics on intestinal permeability is presented in Table 5 [3,80,81,82,83,84,85,86,87,88,89,90,91,92].

Table 5.
Study characteristics and findings of clinical trials of prebiotics where effects on intestinal permeability were among the outcomes assessed.
In summary, the evidence for prebiotics to reduce intestinal permeability is mixed. Some studies, such as those involving formula-fed infants and healthy young men, demonstrated significant reductions in intestinal permeability with prebiotic supplementation. However, other studies, particularly those involving vulnerable populations like preterm infants, burn patients, and individuals with type 2 diabetes or obesity, showed no significant improvements in intestinal permeability. The methodologies for assessing intestinal permeability varied across studies, with most involving the lactulose–mannitol ratio or other sugar-based tests. Other heterogeneity among the studies resulted from study design, population variability, and differences in prebiotic formulations (as well as dosing and duration). While prebiotics show promise in reducing intestinal permeability, further research is needed to identify which populations benefit most and the optimal prebiotic formulation for the given indication.
4.2.2. Probiotics
Descriptions and Mechanisms of Action: Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [93]. They encompass a wide range of single and multi-strain formulations of microorganisms (usually bacteria and/or fungi). While many of the specific health benefits derived from probiotics are strain-specific, there are general mechanisms thought to be shared among the most common probiotic organisms. These include the production of active metabolites (such as short-chain fatty acids, SCFAs), secretion of compounds (such as secretory IgA), interaction with the immune system, contribution to digestion and bile acid metabolism, manufacturing of systemically active molecules (such as serotonin), communication with the endogenous gut microbiota, and maintenance of the gut epithelial barrier [94]. Probiotics influence intestinal barrier function by modulating tight junction (TJ) proteins in intestinal epithelial cells (IECs), which are crucial for maintaining barrier integrity and preventing pathogen entry. Certain probiotics such as Lactobacillus reuteri and Lactobacillus rhamnosus enhance the expression and localization of TJ proteins such as ZO1, occludin, and claudin, thereby improving intestinal permeability and barrier function. They also regulate immune responses by promoting the maturation of dendritic cells, enhancing IgA secretion, and modulating inflammatory pathways. Additionally, probiotics can alter the gut microbiome composition, increase beneficial bacteria, and produce metabolites like SCFAs that further support barrier function and immune regulation [95]. The evidence for effects on intestinal permeability comes mainly from pre-clinical models. Lacticaseibacillus rhamnosus GG (LGG) normalizes intestinal permeability in rats with cow’s milk-induced increase in permeability [96], and it also normalizes the increased permeability caused by acute alcohol exposure [97]. This effect may be mediated in part by LGG’s propensity to adhere to the intestinal mucus layer with the adhesive protein LGG-0186 and its pili [98]. A systematic review and meta-analysis of nine studies found that probiotics and synbiotics significantly reduces serum zonulin levels, indicating improved intestinal permeability [99]. A summary of clinical trials of probiotics that have evaluated their effects on intestinal permeability is presented in Table 6 [100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132].

Table 6.
Study characteristics and findings of clinical trials of probiotics where effect on intestinal permeability was among the outcomes assessed. Specific information about the microbiota in diverse, commercial formulations is included in the Supplementary Material.
In summary, the evidence for probiotics in reducing intestinal permeability is mixed. Heterogeneity deriving from differences in study protocols, target population or disease state, probiotic formulation (as well as dosing and duration), and assessment of intestinal permeability makes it challenging to draw definitive conclusions regarding probiotics and permeability. Further large-scale, well-designed trials are needed to clarify the role of different probiotic formulations, optimal doses, and intervention durations in improving intestinal permeability across specific populations.
4.2.3. Synbiotics
Descriptions and Mechanisms of Action: Synbiotics are defined as “mixture[s] comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” [133]. Synbiotics can be categorized into two types: complementary and synergistic. Complementary synbiotics involve a mixture where the probiotic and prebiotic components work independently to provide health benefits, whereas synergistic synbiotics involve a specific substrate that enhances the effects of the co-administered live microorganism. To be considered a synbiotic, the formulation must show evidence of health benefits when compared against a placebo and demonstrate either selective utilization by the endogenous microbiota or the administered microorganism [133].
Synbiotics support the gut epithelial barrier and intestinal permeability through multiple mechanisms, derived from their combined pre- and probiotic constituents. They enhance microbial balance by providing beneficial microorganisms (probiotics) and substrates (prebiotics), which promote the growth of beneficial bacteria and protection against pathogens. This balance leads to the production SCFAs like butyrate, which serve as an energy source for epithelial cells, enhance mucin production, and maintain tight junctions, thereby reducing intestinal permeability. Synbiotics may also regulate tight junction proteins, support mucosal immunity, reduce inflammation, and competitively exclude pathogens. These combined effects help protect and strengthen the gut barrier, ensuring its integrity and proper function [134]. A summary of clinical trials of synbiotics where effects on intestinal permeability were assessed is displayed in Table 7 [90,135,136,137,138,139,140,141,142,143,144,145,146,147,148].

Table 7.
Study characteristics and findings of clinical trials of synbiotics where effects on intestinal permeability were among the outcomes assessed. Specific information about the microbiota in diverse, commercial formulations is included in the Supplementary Material.
The available evidence on synbiotics to reduce intestinal permeability shows varied results depending on the population and condition studied. Several studies have reported improvements in intestinal permeability with synbiotic interventions, while others have found no significant effects. Once again, interpretation is limited by considerable heterogeneity among study methodologies, target population or disease state, intervention (including dosing and duration), and assessment of intestinal permeability. Further well-designed, large-scale trials with standardized methods are needed to better understand the efficacy of synbiotics in enhancing intestinal permeability and to establish clearer guidelines for the use of specific formulations in specific conditions.
5. Recommendations and Conclusions
Intestinal permeability is an important component of the barrier between intraluminal antigens and the establishment of diseases of the gastrointestinal tract as well as other organs. It is significantly modified by diet, including fat and emulsifiers that increase permeability, and nutrients such as fiber, glutamine, zinc, vitamin D, polyphenols, and anthocyanins that decrease permeability. There is significant interaction of the microbiome and barrier function, including the inflammatory effects of luminal/bacterial antigens and the anti-inflammatory effects of commensals or probiotics and their products including short chain fatty acids. Epigenomic modifications of barrier functions are best illustrated by effects on junction proteins or inflammation. Further understanding of the barrier function and its potential amelioration by dietary, microbial, or, in the future, pharmacological agents will have the potential to reverse the pathobiology underpinning gastrointestinal diseases, as well as the multitude of other systemic diseases that are attributed to the leaky gut phenomenon. It is essential to use accurate measurements of intestinal permeability, particularly in vivo, and to ascertain the longitudinal changes in the barrier that occur with perturbations, disease, and amelioration through treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16203494/s1, File S1.
Author Contributions
A.M.: literature review, writing and revising manuscript; J.A.D.: literature review, writing and revising manuscript; K.J.J.: literature review, writing and revising manuscript; M.C.: conceptualization, senior author, revising manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
R01-DK135440 grant from National Institutes of Health to Michael Camilleri.
Data Availability Statement
This narrative review is based on the reported literature search, with no original data presented.
Acknowledgments
The authors thank Cindy Stanislav for excellent secretarial assistance.
Conflicts of Interest
The authors have no relevant conflicts of interest.
References
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef] [PubMed]
- Farré, R.; Fiorani, M.; Rahiman, S.A.; Matteoli, G. Intestinal permeability, inflammation and the role of nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- de Souza Marinho do Nascimento, D.; Costa Campos Mota, A.C.; da Cruz Carvalho, M.C.; de Oliveira Andrade, E.D.; de Oliveira, E.P.S.F.; Galvão, L.L.P.; Maciel, B.L.L. Can diet alter the intestinal barrier permeability in healthy people? A systematic review. Nutrients 2024, 16, 1871. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Castonguay-Paradis, S.; Veilleux, A. Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 2020, 21, 6402. [Google Scholar] [CrossRef]
- Ahern, P.P.; Maloy, K.J. Understanding immune–microbiota interactions in the intestine. Immunology 2020, 159, 4–14. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Dunleavy, K.A.; Raffals, L.E.; Camilleri, M. Intestinal barrier dysfunction in inflammatory bowel disease: Underpinning pathogenesis and therapeutics. Dig. Dis. Sci. 2023, 68, 4306–4320. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shang, W.; Bao, L.; Peng, Z.; Wu, C. Epithelial-immune cell crosstalk for intestinal barrier homeostasis. Eur. J. Immunol. 2024, 54, e2350631. [Google Scholar] [CrossRef]
- Citi, S.; Fromm, M.; Furuse, M.; González-Mariscal, L.; Nusrat, A.; Tsukita, S.; Turner, J.R. A short guide to the tight junction. J. Cell Sci. 2024, 137, jcs261776. [Google Scholar] [CrossRef]
- Imafuku, K.; Iwata, H.; Natsuga, K.; Okumura, M.; Kobayashi, Y.; Kitahata, H.; Kubo, A.; Nagayama, M.; Ujiie, H. Zonula occludens-1 distribution and barrier functions are affected by epithelial proliferation and turnover rates. Cell Prolif. 2023, 56, e13441. [Google Scholar] [CrossRef]
- AlMarzooqi, S.K.; Almarzooqi, F.; Sadida, H.Q.; Jerobin, J.; Ahmed, I.; Abou-Samra, A.-B.; Fakhro, K.A.; Dhawan, P.; Bhat, A.A.; Al-Shabeeb Akil, A.S. Deciphering the complex interplay of obesity, epithelial barrier dysfunction, and tight junction remodeling: Unraveling potential therapeutic avenues. Obes. Rev. 2024, 25, e13766. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Kuo, W.-T.; Turner, J.R. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef]
- Oami, T.; Yumoto, T.; Shimazui, T.; Sarmiento, S.; Klingensmith, N.J.; Chen, C.-W.; Otani, S.; Liang, Z.; Burd, E.M.; Mahdi, Z.K.; et al. Chronic ethanol use worsens gut permeability and alters tight junction expression in a murine sepsis model. Shock 2023, 60, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Lacy, B.E.; Wise, J.L.; Cangemi, D.J. Leaky gut syndrome: Myths and management. Gastroenterol. Hepatol. 2024, 20, 265. [Google Scholar]
- Perez-Diaz-del-Campo, N.; Castelnuovo, G.; Ribaldone, D.G.; Caviglia, G.P. Fecal and circulating biomarkers for the non-invasive assessment of intestinal permeability. Diagnostics 2023, 13, 1976. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Meyer, F.; Wendling, D.; Demougeot, C.; Prati, C.; Verhoeven, F. Cytokines and intestinal epithelial permeability: A systematic review. Autoimmun. Rev. 2023, 22, 103331. [Google Scholar] [CrossRef]
- Camilleri, M.; Vella, A. What to do about the leaky gut. Gut 2022, 71, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, A.V. The intestinal barrier and current techniques for the assessment of gut permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Mahurkar-Joshi, S.; Chang, L. Epigenetic mechanisms in irritable bowel syndrome. Front. Psychiatry 2020, 11, 805. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.W.; Zong, Y.; Zheng, G.; Zhu, S.; Hong, S. Histone H3K9 methylation regulates chronic stress and IL-6-induced colon epithelial permeability and visceral pain. Neurogastroenterol. Motil. 2020, 32, e13941. [Google Scholar] [CrossRef]
- Zhou, Q.; Costinean, S.; Croce, C.M.; Brasier, A.R.; Merwat, S.; Larson, S.A.; Basra, S.; Verne, G.N. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 2015, 148, 158–169.e8. [Google Scholar] [CrossRef]
- Wiley, J.W.; Higgins, G.A.; Hong, S. Chronic psychological stress alters gene expression in rat colon epithelial cells promoting chromatin remodeling, barrier dysfunction and inflammation. PeerJ 2022, 10, e13287. [Google Scholar] [CrossRef]
- Zhou, Q.; Souba, W.W.; Croce, C.M.; Verne, G.N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010, 59, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Chao, G.; Wang, Y.; Zhang, S.; Yang, W.; Ni, Z.; Zheng, X. MicroRNA-29a increased the intestinal membrane permeability of colonic epithelial cells in irritable bowel syndrome rats. Oncotarget 2017, 8, 85828–85837. [Google Scholar] [CrossRef]
- Wohlfarth, C.; Schmitteckert, S.; Härtle, J.D.; Houghton, L.A.; Dweep, H.; Fortea, M.; Assadi, G.; Braun, A.; Mederer, T.; Pöhner, S.; et al. miR-16 and miR-103 impact 5-HT(4) receptor signalling and correlate with symptom profile in irritable bowel syndrome. Sci. Rep. 2017, 7, 14680. [Google Scholar] [CrossRef]
- Martínez, C.; Rodiño-Janeiro, B.K.; Lobo, B.; Stanifer, M.L.; Klaus, B.; Granzow, M.; González-Castro, A.M.; Salvo-Romero, E.; Alonso-Cotoner, C.; Pigrau, M.; et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017, 66, 1537–1538. [Google Scholar] [CrossRef]
- Hou, Q.; Huang, Y.; Zhu, S.; Li, P.; Chen, X. MiR-144 increases intestinal permeability in IBS-D rats by targeting OCLN and ZO1. Cell. Physiol. Biochem. 2017, 44, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Bordoni, D.; Kuiper, J.W.P.; Weber-Stiehl, S.; Stengel, S.T.; Arnold, P.; Ellinghaus, D.; Ito, G.; Tran, F.; Messner, B.; et al. DNA methyltransferase 3A controls intestinal epithelial barrier function and regeneration in the colon. Nat. Commun. 2022, 13, 6266. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriva, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B increases intestinal tight junction permeability by up-regulation of MIR200C-3p which degrades occludin, m.R.N.A. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef]
- Bowes, M.M.; Casares-Marfil, D.; Sawalha, H. Intestinal permeability correlates with disease activity and DNA methylation changes in lupus patients. Clin. Immunol. 2024, 262, 110173. [Google Scholar] [CrossRef]
- Higgins, G.A.; Hong, S.; Wiley, J.W. The role of epigenomic regulatory pathways in the gut-brain axis and visceral hyperalgesia. Cell. Mol. Neurobiol. 2022, 42, 361–376. [Google Scholar] [CrossRef]
- Yuan, X.; Tan, Y.; Bajinka, O.; Jammeh, M.L.; Dukureh, A.; Obiegbusi, C.N.; Abdelhalim, K.A. The connection between epigenetics and gut microbiota-current perspective. Cell Biochem. Funct. 2024, 42, e3941. [Google Scholar] [CrossRef]
- Wu, S.-E.; Hashimoto-Hill, S.; Woo, V.; Eshleman, E.M.; Whitt, J.; Engleman, L.; Karns, R.; Denson, L.A.; Haslam, D.B.; Alenghat, T. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 2020, 586, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Pathak, R.; Garg, S.; Skinner, C.M.; Melnyk, S.; Pavliv, O.; Hendrickson, H.; Landes, R.D.; Lumen, A.; Tackett, A.J.; et al. Short-term dietary methionine supplementation affects one-carbon metabolism and DNA methylation in the mouse gut and leads to altered microbiome profiles, barrier function, gene expression and histomorphology. Genes Nutr. 2017, 12, 22. [Google Scholar] [CrossRef]
- Pepke, M.L.; Hansen, S.B.; Limborg, M.T. Unraveling host regulation of gut microbiota through the epigenome-microbiome axis. Trends Microbiol. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Speciale, A.; Molonia, M.S.; Muscarà, C.; Cristani, M.; Salamone, F.L.; Saija, A.; Cimino, F. An overview on the cellular mechanisms of anthocyanins in maintaining intestinal integrity and function. Fitoterapia 2024, 175, 105953. [Google Scholar] [CrossRef]
- Jimenez-Rondan, F.R.; Ruggiero, C.H.; Lobean McKinley, K.; Koh, J.; Roberts, J.F.; Triplett, E.W.; Cousins, R.J. Enterocyte-specific deletion of metal transporter Zip14 (Slc39a14) alters intestinal homeostasis through epigenetic mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G159–G176. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, K.; Camilleri, M. Effects of dietary components on intestinal permeability in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G589–G608. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Burcelin, R.; Ruidavets, J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energy intake is associated with endotoxemia in apparently healthy men. Am. J. Clin. Nutr. 2008, 87, 1219–1223. [Google Scholar] [CrossRef]
- Leutner, M.; Göbl, C.; Wielandner, A.; Howorka, E.; Prünner, M.; Bozkurt, L.; Schlager, O.; Charwat-Resl, S.; Kautzky-Willer, A. Clinical and metabolic characteristics of treated hyperlipidemic patients additionally affected by subclinical hyperglycemia. Lipids Health Dis. 2016, 15, 10. [Google Scholar] [CrossRef]
- Bowser, S.M.; McMillan, R.P.; Boutagy, N.E.; Tarpey, M.D.; Smithson, A.T.; Osterberg, K.L.; Neilson, A.P.; Davy, B.M.; Davy, K.P.; Hulver, M.W. Serum endotoxin, gut permeability and skeletal muscle metabolic adaptations following a short term high fat diet in humans. Metabolism 2020, 103, 154041. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, A.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohns disease Escherichia coli across M-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef]
- Mistry, R.H.; Liu, F.; Borewicz, K.; Lohuis, M.A.M.; Smidt, H.; Verkade, H.J.; Tietge, U.J.F. Long-term β-galacto-oligosaccharides supplementation decreases the development of obesity and insulin resistance in mice fed a western-type diet. Mol. Nutr. Food Res. 2020, 64, e1900922. [Google Scholar] [CrossRef]
- Gondalia, S.V.; Wymond, B.; Benassi-Evans, B.; Berberzy, P.; Bird, A.R.; Belobrajdic, D.P. Substitution of refined conventional wheat flour with wheat high in resistant starch modulates the intestinal microbiota and fecal metabolites in healthy adults: A randomized, controlled trial. J. Nutr. 2022, 152, 1426–1437. [Google Scholar] [CrossRef]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front. Immunol. 2020, 10, 499337. [Google Scholar] [CrossRef]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 5997. [Google Scholar] [CrossRef]
- Koren, O.; Tako, E. Chronic dietary zinc deficiency alters gut microbiota composition and function. Proceedings 2020, 61, 16. [Google Scholar] [CrossRef]
- Skalny, A.V.; Aschner, M.; Lei, X.G.; Gritsenko, V.A.; Santamaria, A.; Alekseenko, S.I.; Prakash, N.T.; Chang, J.-S.; Sizova, E.A.; Chao, J.C.J.; et al. Gut microbiota as a mediator of essential and toxic effects of zinc in the intestines and other tissues. Int. J. Mol. Sci. 2021, 22, 13074. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.O.; Alzubaidi, M.Y.; Nadeem, M.S.; Khan, J.A.; Rather, I.A.; Khan, M.I. Effects of urolithins on obesity-associated gut dysbiosis in rats fed on a high-fat diet. Int. J. Food Sci. Nutr. 2021, 72, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, Y.; Yin, B.; Zhang, L.; Qin, W.; Niu, Y.; Tang, Y.; Zhou, S.; Yan, X.; Ma, L. Dietary glycyl-glutamine supplementation ameliorates intestinal integrity, inflammatory response, and oxidative status in association with the gut microbiota in LPS-challenged piglets. Food Funct. 2021, 12, 3539–3551. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, M.; Li, M.; Hou, J.; Zhang, X.; Gao, Y.; Chachar, B.; Li, X. Dietary bioactive peptide alanyl-glutamine attenuates dextran sodium sulfate-induced colitis by modulating gut microbiota. Oxid. Med. Cell Longev. 2021, 2021, 5543003. [Google Scholar] [CrossRef]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary fiber and its potential role in obesity: A focus on modulating the gut microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A critical review on interplay between dietary fibers and gut microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Nogal, A.; Louca, P.; Zhang, X.; Wells, P.M.; Steves, C.J.; Spector, T.D.; Falchi, M.; Valdes, A.M.; Menni, C. Circulating levels of the short-chain fatty acid acetate mediate the effect of the gut microbiome on visceral fat. Front. Microbiol. 2021, 12, 711359. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; El Hachlafi, N.; El Jemly, M.; Hakkour, M.; Balahbib, A.; El Menyiy, N.; Bakrim, S.; Mrabti, H.N.; Khouchlaa, A.; et al. Chemical compounds of berry-derived polyphenols and their effects on gut microbiota, inflammation, and cancer. Molecules 2022, 27, 3286. [Google Scholar] [CrossRef]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2023, 89, 101109. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A comprehensive update on their metabolism, bioactivity, and associated gut microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The association between vitamin D and gut microbiota: A systematic review of human studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, P.; Yu, X.; Ding, H.; Wang, Z.; Feng, J. Effect of long-term and short-term imbalanced Zn manipulation on gut microbiota and screening for microbial markers sensitive to zinc status. Microbiol. Spectr. 2021, 9, e00483-21. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace metals and animal health: Interplay of the gut microbiota with iron, manganese, zinc, and copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of intestinal barrier function by microbial metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.R.; Picoraro, J.A.; Dorfzaun, S.; LeLeiko, N.S. Emulsifiers and intestinal health: An introduction. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Naimi, S.; Viennois, E.; Gewirtz, A.T.; Chassaing, B. Direct impact of commonly used dietary emulsifiers on human gut Microbiota. Microbiome 2021, 9, 66. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: Mechanistic insights in inflammatory bowel disease. J. Crohns. Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- Ogulur, I.; Yazici, D.; Pat, Y.; Bingöl, E.N.; Babayev, H.; Ardicli, S.; Heider, A.; Rückert, B.; Sampath, V.; Dhir, R.; et al. Mechanisms of gut epithelial barrier impairment caused by food emulsifiers polysorbate 20 and polysorbate 80. Allergy 2023, 78, 2441–2455. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.A.; Gibson, P.R.; Taylor, K.M.; Halmos, E.P. The effect of dietary emulsifiers and thickeners on intestinal barrier function and its response to acute stress in healthy adult humans: A randomised controlled feeding study. Aliment. Pharmacol. Ther. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Looijer-Van Langen, M.A.C.; Dieleman, L.A. Prebiotics in chronic intestinal inflammation. Inflamm. Bowel Dis. 2008, 15, 454–462. [Google Scholar] [CrossRef]
- Francavilla, R.; Castellaneta, S.; Masciale, A.; Straziuso, S.; Polimeno, L.; Gagliardi, F.; Bohem, G.; Miniello, V. Intestinal permeability and faecal flora of infants fed with a prebiotic supplemented formula: A double blind placebo controlled study. J. Pediatr. Gastroenterol. Nutr. 2006, 42, E96–E97. [Google Scholar] [CrossRef]
- Westerbeek, E.A.; van den Berg, J.P.; Lafeber, H.N.; Fetter, W.P.; Boehm, G.; Twisk, J.W.; van Elburg, R.M. Neutral and acidic oligosaccharides in preterm infants: A randomized, double-blind, placebo-controlled trial1234. Am. J. Clin. Nutr. 2010, 91, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schick, A.; Meddings, J.; Reimer, R.A.; Huang, C. Effect of prebiotic on microbiota, intestinal permeability, and glycemic control in children with type 1 diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef]
- Olguin, F.; Araya, M.; Hirsch, S.; Brunser, O.; Ayala, V.; Rivera, R.; Gotteland, M. Prebiotic ingestion does not improve gastrointestinal barrier function in burn patients. Burns 2005, 31, 482–488. [Google Scholar] [CrossRef]
- Russo, F.; Linsalata, M.; Clemente, C.; Chiloiro, M.; Orlando, A.; Marconi, E.; Chimienti, G.; Riezzo, G. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr. Res. 2012, 32, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Salden, B.N.; Troost, F.J.; Wilms, E.; Truchado, P.; Vilchez-Vargas, R.; Pieper, D.H.; Jáuregui, R.; Marzorati, M.; van de Wiele, T.; Possemiers, S.; et al. Reinforcement of intestinal epithelial barrier by arabinoxylans in overweight and obese subjects: A randomized controlled trial: Arabinoxylans in gut barrier. Clin. Nutr. 2018, 37, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hermes, G.D.A.; Emauel, E.C.; Holst, J.J.; Zoetendal, E.G.; Smidt, H.; Troost, F.; Schaap, F.G.; Damink, S.O.; Jocken, J.W.E.; et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: A randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef]
- Ganda Mall, J.P.; Löfvendahl, L.; Lindqvist, C.M.; Brummer, R.J.; Keita, Å.V.; Schoultz, I. Differential effects of dietary fibres on colonic barrier function in elderly individuals with gastrointestinal symptoms. Sci. Rep. 2018, 8, 13404. [Google Scholar] [CrossRef]
- Machado, A.M.; da Silva, N.B.M.; de Freitas, R.M.P.; de Freitas, M.B.D.; Chaves, J.B.P.; Oliveira, L.L.; Martino, H.S.D.; de Cássia Goncalves Alfenas, R. Effects of yacon flour associated with an energy restricted diet on intestinal permeability, fecal short chain fatty acids, oxidative stress and inflammation markers levels in adults with obesity or overweight: A randomized, double blind, placebo controlled clinical trial. Arch. Endocrinol. Metab. 2021, 64, 597–607. [Google Scholar]
- Pedersen, C.; Gallagher, E.; Horton, F.; Ellis, R.J.; Ijaz, U.Z.; Wu, H.; Jaiyeola, E.; Diribe, O.; Duparc, T.; Cani, P.D.; et al. Host–microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br. J. Nutr. 2016, 116, 1869–1877. [Google Scholar] [CrossRef]
- Jain, P.K.; McNaught, C.E.; Anderson, A.D.G.; MacFie, J.; Mitchell, C.J. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: A randomised controlled trial. Clin. Nutr. 2004, 23, 467–475. [Google Scholar]
- Cao, S.; Shaw, E.L.; Quarles, W.R.; Sasaki, G.Y.; Dey, P.; Hodges, J.K.; Pokala, A.; Zeng, M.; Bruno, R.S. Daily inclusion of resistant starch-containing potatoes in a dietary guidelines for Americans dietary pattern does not adversely affect cardiometabolic risk or intestinal permeability in adults with metabolic syndrome: A randomized controlled trial. Nutrients 2022, 14, 1545. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mehrabani, E.; Harouni, R.; Behrooz, M.; Ranjbar, F.; Asghari-Jafarabadi, M.; Ebrahimi-Mameghani, M. Effects of inulin supplementation on inflammatory biomarkers and clinical symptoms of women with obesity and depression on a calorie-restricted diet: A randomised controlled clinical trial. Br. J. Nutr. 2023, 129, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Isolauri, E.; Majamaa, H.; Arvola, T.; Rantala, I.; Virtanen, E.; Arvilommi, H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology 1993, 105, 1643–1650. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Sidhu, A.; Ma, Z.; McClain, C.; Feng, W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G32–G41. [Google Scholar] [CrossRef]
- Capurso, L. Thirty years of Lactobacillus rhamnosus GG: A review. J. Clin. Gastroenterol. 2019, 53 (Suppl. 1), S1–S41. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Ahmadi, A.; Sadeghian, M.; Alipour, M.; Ahmadi Taheri, S.; Rahmani, S.; Abbasnezhad, A. The effects of probiotic/synbiotic on serum level of zonulin as a biomarker of intestinal permeability: A systematic review and meta-analysis. Iran J. Public Health 2020, 49, 1222–1231. [Google Scholar] [CrossRef]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef]
- Sindhu, K.N.C.; Sowmyanarayanan, T.V.; Paul, A.; Babji, S.; Ajjampur, S.S.R.; Priyadarshini, S.; Sarkar, R.; Balasubramanian, K.A.; Wanke, C.A.; Ward, H.D.; et al. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: A randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 2014, 58, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Andrew, H.; Kirschner, B.S.; Guandalini, S. Is Lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J. Pediatr. Gastroenterol. Nutr. 2000, 31, 453–457. [Google Scholar]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Paerregaard, A.; Fleischer Michaelsen, K. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef]
- Sentongo, T.A.; Cohran, V.; Korff, S.; Sullivan, C.; Iyer, K.; Zheng, X. Intestinal permeability and effects of Lactobacillus rhamnosus therapy in children with short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 41–47. [Google Scholar] [CrossRef]
- Gotteland, M.; Cruchet, S.; Verbeke, S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment. Pharmacol. Ther. 2001, 15, 11–17. [Google Scholar] [CrossRef]
- Montalto, M.; Gallo, A.; Curigliano, V.; D’Onofrio, F.; Santoro, L.; Covino, M.; Dalvai, S.; Gasbarrini, A.; Gasbrarrini, G. Clinical trial: The effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy—A randomized, double-blind, cross-over, placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Akama, F.; Nishino, R.; Makino, S.; Kobayashi, K.; Kamikaseda, K.; Nagano, J.; Koga, Y. The effect of probiotics on gastric mucosal permeability in humans administered with aspirin. Scand. J. Gastroenterol. 2011, 46, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Judkins, T.C.; Solch-Ottaiano, R.J.; Ceretto-Clark, B.; Nieves, C., Jr.; Colee, J.; Wang, Y.; Tompkins, T.A.; Caballero-Calero, S.E.; Langkamp-Henken, B. The effect of an acute aspirin challenge on intestinal permeability in healthy adults with and without prophylactic probiotic consumption: A double-blind, placebo-controlled, randomized trial. BMC Gastroenterol. 2024, 24, 4. [Google Scholar] [CrossRef]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Da Gama Torres, H.O.; Gurerra Pinto, A.; Carneiro Aguirre, A.C.; Martins, F.P.; Andrade Goulart, E.M.; Da Cunha, A.S. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; de Roos, N.M.; Hovenier, R.; Meijerink, J.; Besseling-van der Vaart, I.; van Hemert, S.; Witteman, B.J.M. Intestinal permeability measured by urinary sucrose excretion correlates with serum zonulin and faecal calprotectin concentrations in UC patients in remission. J. Nutr. Metab. 2019, 2019, 2472754. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.-Q.; Zuo, X.-L.; Zhen, Y.-B.; Yang, J.; Liu, C.-H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Algert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef] [PubMed]
- Boonma, P.; Shapiro, J.M.; Hollister, E.B.; Badu, S.; Wu, Q.; Weidler, E.M.; Abraham, B.P.; Devaraj, S.; Luna, R.A.; Versalovic, J.; et al. Probiotic VSL#3 treatment reduces colonic permeability and abdominal pain symptoms in patients with irritable bowel syndrome. Front. Pain Res. 2021, 2, 691689. [Google Scholar]
- Ait Abdellah, S.; Gal, C.; Laterza, L.; Velenza, V.; Settanni, C.R.; Napoli, M.; Schiavoni, E.; Mora, V.; Petito, V.; Gasbarrini, A. Effect of a multistrain probiotic on leaky gut in patients with diarrhea-predominant irritable bowel syndrome: A pilot study. Dig. Dis. 2023, 41, 489–499. [Google Scholar] [CrossRef]
- Marchix, J.; Quénéhervé, L.; Bordron, P.; Aubert, P.; Durand, T.; Oullier, T.; Blondeau, C.; Ait Abdellah, S.; Bruley des Varannes, S.; Chaffron, S.; et al. Could the microbiota be a predictive factor for the clinical response to probiotic supplementation in IBS-D? A cohort study. Microorganisms 2023, 11, 277. [Google Scholar] [CrossRef]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for preventing and treating small intestinal bacterial overgrowth: A meta-analysis and systematic review of current evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Leber, B.; Tripolt, N.J.; Bladdl, D.; Eder, M.; Wascher TCPieber, T.R.; Stauber, R.; Sourij, H.; Oettl, K.; Stadlbauer, V. The influence of probiotic supplementation on gut permeability in patients with metabolic syndrome: An open label, randomized pilot study. Eur. J. Clin. Nutr. 2012, 66, 1110–1115. [Google Scholar] [CrossRef]
- DiMattia, Z.; Damani, J.J.; Van Syoc, E.; Rogers, C.J. Effect of probiotic supplementation on intestinal permeability in overweight and obesity: A systematic review of randomized controlled trials and animal studies. Adv. Nutr. 2024, 15, 100162. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Moncrief, K.; Madsen, K.; Arrieta, M.C.; Owen, R.J.; Bain, V.G.; Wong, W.W.; Ma, M.M. Effects of probiotic therapy on portal pressure in patients with cirrhosis: A pilot study. Liver Int. 2009, 29, 1110–1115. [Google Scholar] [CrossRef]
- Jayakumar, S.; Carbonneau, M.; Hotte, N.; Befus, A.D.; St Laurent, C.; Owen, R.; McCarthy, M.; Madsen, K.; Bailey, R.J.; Ma, M.; et al. VSL#3® probiotic therapy does not reduce portal pressures in patients with decompensated cirrhosis. Liver Int. 2013, 33, 1470–1477. [Google Scholar]
- Kwak, D.S.; Jun, D.W.; Seo, J.G.; Chung WSPark, S.-E.; Lee, K.N.; Khalid-Saeed, W.; Lee, H.L.; Lee, O.Y.; Yoon, B.C.; Choi, H.S. Short-term probiotic therapy alleviates small intestinal bacterial overgrowth, but does not improve intestinal permeability in chronic liver disease. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.; et al. Randomised clinical trial: The effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Durdevic, M.; Leber, B.; di Vora, K.; Rainer, F.; Krones, E.; Douschan, P.; Spindelboeck, W.; Durchschein, F.; Zollner, G.; et al. Changes in the intestinal microbiome during a multispecies probiotic intervention in compensated cirrhosis. Nutrients 2020, 12, 1874. [Google Scholar] [CrossRef] [PubMed]
- Ayob, N.; Nawawi, K.N.M.; Nor, M.H.M.; Ali, R.A.F.; Ahmad, H.F.; Oon, S.F.; Mokhtar, N.M. The effects of probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines 2023, 11, 640. [Google Scholar] [CrossRef]
- Qin, H.-L.; Zheng, J.-J.; Tong, D.-N.; Chen, W.-X.; Fan, X.-B.; Hang, X.-M.; Jiang, Y.-Q. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur. J. Clin. Nutr. 2008, 62, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.; van Santvoort, H.C.; Renooij, W.; de Smet, M.B.; Boermeester, M.A.; Fischer, K.; Timmerman Hmali, U.A.; Cirkel, G.A.; Bollen, T.L.; van Ramshorst, B.; et al. Intestinal barrier dysfunction in a randomized trial of a specific probiotic composition in acute pancreatitis. Ann. Surg. 2009, 250, 712–719. [Google Scholar] [CrossRef]
- McNaught, C.E.; Woodcock, N.P.; Anderson, A.D.G.; MacFie, J. A prospective randomised trial of probiotics in critically ill patients. Clin. Nutr. 2005, 24, 211–219. [Google Scholar] [CrossRef]
- Alberda, C.; Gramlich, L.; Meddings, J.; Field, C.; McCargar, L.; Kutsogiannis, D.; Fedorak, R.; Madsen, K. Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2007, 85, 816–823. [Google Scholar] [CrossRef]
- Presti, R.M.; Yeh, E.; Williams, B.; Landay, A.; Jacobson, J.M.; Wilson, C.; Fichtenbaum, C.J.; Utay, N.S.; Dube, M.P.; Klingman, K.L.; et al. A randomized, placebo-controlled trial assessing the effect of VISBIOME ES probiotic in people with HIV on antiretroviral therapy. Open Forum Infect. Dis. 2021, 8, ofab550. [Google Scholar] [CrossRef]
- Mokkala, K.; Pussinen, P.; Houttu, N.; Koivuniemi, E.; Vahlberg, T.; Laitinen, K. The impact of probiotics and n-3 long-chain polyunsaturated fatty acids on intestinal permeability in pregnancy: A randomised clinical trial. Benef. Microbes 2018, 9, 199–208. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Sivamaruthi, B.S.; Lailerd, N.; Sirilun, S.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Saelee, M.; Chaiyasut, K.; Kesika, P.; et al. Probiotics supplementation improves intestinal permeability, obesity index and metabolic biomarkers in elderly Thai subjects: A randomized controlled trial. Foods 2022, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- de Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The effects of a multispecies probiotic on migraine and markers of intestinal permeability–results of a randomized placebo-controlled study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott KPHolscher, H.D.; Azad, M.B.; Delzenne, N.M.; Sanders, M.E. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Camilleri, M. Human intestinal barrier: Effects of stressors, diet, prebiotics, and probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Macfie, J.; Gatt, M.; Larsen, C.N.; Jensen, S.S.; Leser, T.D. Randomized clinical trial of effect of synbiotics, neomycin and mechanical bowel preparation on intestinal barrier function in patients undergoing colectomy. Br. J. Surg. 2007, 94, 546–554. [Google Scholar] [CrossRef]
- Spindler-Vesel, A.; Bengmark, S.; Vovk, I.; Cerovic, O.; Kompan, L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: A randomized study in trauma patients. J. Parenter. Enter. Nutr. 2007, 31, 119–126. [Google Scholar] [CrossRef]
- Sharma, B.; Srivastava, A.; Singh, N.; Sachdev, V.; Kapur, S.; Saraya, A. Role of probiotics on gut permeability and endotoxemia in patients with acute pancreatitis: A double-blind randomized controlled trial. J. Clin. Gastroenterol. 2011, 45, 442–448. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.W.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Nishigaki, E.; Abe, T.; Fukaya, M.; Asahara, T.; Nomoto, K.; Nagino, M. Randomized clinical trial of the effect of perioperative synbiotics versus no synbiotics on bacterial translocation after oesophagectomy. Br. J. Surg. 2014, 101, 189–199. [Google Scholar] [CrossRef]
- Wilms, E.; Gerritsen, J.; Smidt, H.; Besseling-van der Vaart, I.; Rijkers, G.T.; Garcia Fuentes, A.R.; Masclee, A.A.M.; Troost, F.J. Effects of supplementation of the synbiotic Ecologic® 825/FOS P6 on intestinal barrier function in healthy humans: A randomized controlled trial. PLoS ONE 2016, 11, e0167775. [Google Scholar] [CrossRef]
- Stenman, L.K.; Lehtinen, M.J.; Meland, N.; Christensen, J.E.; Yeung, N.; Saarinen, M.T.; Courtney, M.; Burcelin, R.; Lähdeaho, M.-L.; Linros, J.; et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine 2016, 13, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, S.M.; Couto, C.A.; Costa-Silva, L.; Armiliato, G.N.A.; Pereira, C.A.S.; Martins, F.S.; de Lourdes AFerrari, M.; Vilela, E.G.; Tores, H.O.G.; Cunha, A.S.; et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients 2016, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.M.; Spindelboeck, W.; Halwachs, B.; Strohmaier, H.; Kump, P.; Gorkiewicz, G.; Högenauer, C. Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur. J. Nutr. 2019, 58, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Horvath, A.; Leber, B.; Feldbacher, N.; Steinwender, M.; Komarova, I.; Rainer, F.; Blesl, A.; Stadlbauer, V. The effects of a multispecies synbiotic on microbiome-related side effects of long-term proton pump inhibitor use: A pilot study. Sci. Rep. 2020, 10, 2723. [Google Scholar] [CrossRef]
- Horvath, A.; Leber, B.; Feldbacher, N.; Tripolt, N.; Rainer, F.; Blesl, A.; Trieb, M.; Marsche, G.; Sourij, H.; Stadlbauer, V. Effects of a multispecies synbiotic on glucose metabolism, lipid marker, gut microbiome composition, gut permeability, and quality of life in diabesity: A randomized, double-blind, placebo-controlled pilot study. Eur. J. Nutr. 2020, 59, 2969–2983. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; di Bari, I.; Acquaviva, P.M.; Maranzano, V.; Corciulo, S.; Di Ciaula, A.; Di Palo, D.M.; La Forgia, F.M.; Fontana, S.; et al. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins 2021, 13, 334. [Google Scholar] [CrossRef]
- Ghavami, A.; Khorvash, F.; Heidari, Z.; Khalesi, S.; Askari, G. Effect of synbiotic supplementation on migraine characteristics and inflammatory biomarkers in women with migraine: Results of a randomized controlled trial. Pharmacol. Res. 2021, 169, 105668. [Google Scholar] [CrossRef]
- Liu, M.; Tandorost, A.; Moludi, J.; Dey, P. Prebiotics plus probiotics may favorably impact on gut permeability, endocannabinoid receptors, and inflammatory biomarkers in patients with coronary artery diseases: A clinical trial. Food Sci. Nutr. 2024, 12, 1207–1217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).