Abstract
Background: Vitamin D, through the activation of its receptor (VDR), plays an immunomodulatory role in the gastrointestinal tract. Single-nucleotide polymorphisms (SNPs) in the VDR gene have been associated with Crohn’s disease (CD) risk, and patients carrying the TaqI polymorphism in this gene run a higher risk of developing a penetrating behavior. Aims: We analyzed the association of BsmI, ApaI, TaqI, and FokI SNPs in the VDR gene with the clinical characteristics of CD. Methods: Four polymorphisms identified in the VDR gene (BsmI, FokI, ApaI, and TaqI) were genotyped in blood samples from CD patients (n = 115) by using PCR-RFLP. The disease’s location and behavior and the presence of perianal fistulas were collected from each patient. Intestinal fibroblasts from ileal resections of CD patients (n = 10) were genotyped, and the expression of fibrotic and inflammatory markers was analyzed by RT-PCR. Results: The data reveal no association between any of the polymorphisms and CD risk. A strong linkage disequilibrium was detected between TaqI and both ApaI and BsmI, which in turn were strongly associated. Homozygosis or heterozygosis for the a allele of the ApaI SNP or b allele of the BsmI SNP was significantly associated with a lower risk of a penetrating behavior, while the aa genotype was associated with a lower risk of perianal fistulas. Fibroblasts carrying the aa genotype expressed lower levels of fibrotic and inflammatory markers. Conclusion: The aa genotype of the ApaI SNP in the VDR gene is associated with a lower risk of perianal fistulas in CD and a reduced expression of fibrotic and inflammatory markers in intestinal fibroblasts.
1. Introduction
Crohn’s disease is a chronic inflammatory disorder of the gastrointestinal (GI) tract associated with transmural inflammation affecting the entire thickness of the bowel wall, and it is characterized by periods of relapse and clinical remission. Although some doubts arise [1], the Montreal Classification is the most widely accepted and integrates disease location, disease behavior, and the presence of perianal disease. The colon and ileum constitute the main organs affected, even though CD can affect any part of the GI tract. Disease behavior is classified into three phenotypes: inflammatory (B1), stenotic (B2), and penetrating (B3). Additionally, perianal disease must be noted separately, as it is not necessarily associated with fistulizing disease [2,3,4]. While disease location tends to remain relatively static, both disease behavior and perianal disease can vary over time. In fact, patients with an inflammatory phenotype at diagnosis are very likely to develop either fistulizing or stricturing complications within a few years.
Both the etiopathogenesis of CD and the reasons why some patients evolve toward more complicated behaviors are unknown. Genetic studies have identified susceptibility genes for CD [5], among which, the human VDR gene, located on chromosome 12q13.11 is situated within one of the candidate regions for inflammatory bowel disease (IBD) susceptibility [6,7]. This nuclear receptor mediates most of the biological functions of VD and it has been associated with an immunomodulatory effect in the gastrointestinal tract [6]. Four common single-nucleotide polymorphisms (SNPs) recognized by restriction enzymes have been identified in the VDR gene, including the FokI (rs2228570), BsmI (rs1544410), ApaI (rs7975232), and TaqI (rs731236), and their association with CD risk is still a matter of debate [8,9,10,11,12,13,14]. Ethnicity and the sample size of some of the studies are among the main reasons that explain the discrepancies reported. Of interest, in several cohorts of patients in which no association was found with CD risk, some of these polymorphisms were linked to more aggressive disease and a higher incidence of surgery [7,15,16]. In particular, the t allele in the TaqI polymorphism has been associated with reduced VDR protein levels in both epithelial cells and fibroblasts, which were increased by treatment with vitamin D [17].
Here, we aim to investigate the allelic and genotypic distributions of four common SNPs in the VDR gene, FokI, BsmI, ApaI, and TaqI, in a cohort of Caucasian CD patients and their association with clinical characteristics of the disease. Our data reveal a strong linkage disequilibrium between TaqI and ApaI, which in turn is associated with BsmI, and the results show that homozygosis for the a allele of the ApaI SNP is associated with a lower risk of both a penetrating behavior and perianal fistulas in CD patients and also with lower levels of fibrotic and inflammatory markers in primary intestinal fibroblasts.
2. Materials and Methods
2.1. Patients
In all cases, patients included in this study were recruited from the Gastroenterology Service of the Hospital of Manises (Valencia, Spain) following the Helsinki declaration recommendations. All patients were of European Caucasian ethnicity and had been followed up and treated in the hospital. This study was approved by the CEIM-Hospital Universitario y Politécnico la Fe (Valencia, Spain), with the protocol approval number 2021-284-1 (12 May 2021). Written informed consent was obtained from all patients.
Blood samples were obtained from a cohort of 115 CD patients (Table 1). We collected demographic and clinical data from them, including age, gender, age at diagnosis, family history, smoking status, disease location, disease behavior, presence of perianal fistulas, development of extraintestinal manifestations, and treatment history. In this study, we also included blood samples from non-IBD patients as control samples (55% female, 45% male). The mean ± SEM age of the controls was 39.8 ± 9.1 years, which was not significantly different than that of the CD patients (38.6 ± 1.2).

Table 1.
Clinical characteristics of Crohn’s disease patients from whom blood was obtained.
2.2. Primary Fibroblast Isolation
Primary intestinal fibroblasts were isolated from the surgical resections obtained from a second cohort of patients (Table 2), as previously reported [17,18]. In brief, the tissue was cut into small pieces and incubated with agitation in HBSS-EDTA for 30 min at 37 °C. After this step, a digestion of the pieces was performed with collagenase I (1 mg/mL), hyaluronidase (2 mg/mL), and DNAse (1 µL/mL) in PBS for 30 min at 37 °C. Finally, the explants were seeded in a Petri dish with the culture medium. The medium (DMEM high glucose, Sigma-Aldrich, Steinheim, Germany) was supplemented with FCS 20%, penicilin/streptomycin (100 µg/mL), gentamycin (100 µg/mL), amphotericin B (2 µg/mL), and ciprofloxacin (16 µg/mL). Fibroblasts from passages 6 to 8 were used.

Table 2.
Clinical characteristics of CD patients from whom fibroblasts were obtained.
2.3. DNA Extraction and Genotyping
Blood samples were genotyped for the four SNPs in the VDR gene, ApaI (rs7975232), FokI (rs2228570), BsmI (rs1544410), and TaqI (rs731236), while intestinal fibroblasts were only genotyped for the ApaI SNP. From both samples, genomic DNA was extracted from EDTA or citrated blood samples with the QIAamp® DNA Mini kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. After extraction, DNA concentrations were measured by spectrophotometry with a NanoDrop®, ND1000 (Thermo Fisher Scientific, Waltham, MA, USA). Genotyping was carried out by polymerase chain reaction–restriction fragment length polymorphism (RFLP-PCR) [15,17]. DNA fragments containing the SNPs were amplified by PCR using 0.25 units of rTaq™ DNA Polymerase (Takara Bio Inc., Shiga, Japan) and the sense/antisense primers (Table 3). PCR products were restricted with the specific restriction enzyme (New England Biolabs, Ipswich, MA, USA) (Table 3) and resolved in 2% agarose gels to visualize the restriction fragments. The presence of restriction sites was coded with lower-case letters (ApaI: a; BsmI: b; FokI: f; and TaqI: t), and the absence of restriction sites with upper-case letters (ApaI: A; BsmI: B; FokI: F; and TaqI: T). We observed that the genotyped SNPs were in Hardy–Weinberg equilibrium in the CD patients and in the controls.

Table 3.
Primers and restriction enzymes used.
2.4. Real Time-PCR
Total RNA from the fibroblasts was isolated with an Illustra RNAspin Mini RNA isolation Kit (GE Healthcare Life Science, Amersham, UK), and 1 µg was used to obtain cDNA with the PrimeScript RT reagent Kit (Takara Bio Inc.). Real-time PCR was performed with the SYBR® Premix Ex Taq (Takara Bio Inc.) in a LightCycler thermocycler (Roche Diagnostics, Penzberg, Germany). Specific oligonucleotides were designed according to the reported sequences and are shown in Table 4. β-actin was used as a housekeeping gene, and the results are expressed as the ΔCT between the CT gene and CT β-actin (CT: Threshold Cycle).

Table 4.
Sequences of primers used in real time-PCR.
2.5. Statistical Analysis
For clinical data analysis, contingency tables considering the 3 genotypes of the specific SNP were analyzed by the χ2 test (3 × 2 tables). In some cases, we also compared them using Fisher’s exact test (2 × 2 tables), with one genotype vs. the other two of the specific SNP, as well as between haplotypes. Data obtained from cultured fibroblasts are presented as mean ± SEM, and normality tests were used to check the normal distribution of data. For the statistical analyses of gene expression between AA and aa genotypes in isolated fibroblasts, we used the t-test or Mann–Whitney test for independent samples, as appropriate. A p value < 0.05 was statistically significant.
3. Results
3.1. No Association Is Detected between CD Risk and the VDR FokI, BsmI, ApaI, and TaqI Gene Polymorphisms
To study a potential association between several SNPs in the VDR gene and CD risk, we genotyped a cohort of 115 CD patients and 20 healthy donors. The results showed that the genotypic frequencies of VDR FokI, ApaI, BsmI, and TaqI were not significantly different between the CD patients and healthy controls (Table 5).

Table 5.
Genotype frequencies of the vitamin D receptor variants in the control and CD patients.
3.2. A Linkage Disequilibrium between BsmI, ApaI, and TaqI Is Detected
Next, we proceeded to compare the presence of the different polymorphisms with each other to see if there were any possible relationships of inheritance in “clusters” or haplotypes (Table 6). The results reveal that the FokI SNP was not in linkage disequilibrium with any of the other three. In contrast, we detected a clear and significant association between the BB and AA genotypes and between these genotypes and the tt genotype (Table 6).

Table 6.
Distribution of CD patients according to the different genotypes for each SNP in the VDR gene.
3.3. CD Patients Carrying the aa Genotype Have a Lower Risk of Developing a Perianal Fistula
Next, we analyzed the association of genetic VDR polymorphisms with clinical characteristics of the disease. Our results demonstrate that patients carrying the aa genotype have a lower risk of presenting with perianal fistulas when compared with any of the other two genotypes (Table 7). Regarding the risk of suffering a penetrating behavior, we detected significant differences (p = 0.0347) when comparing the AA genotype vs. the combination of the other two genotypes (Figure 1A). No significant differences were detected for any of the other variables analyzed (Table 7).

Table 7.
Classification of Crohn’s disease patients by genotypes in the ApaI SNP on the VDR gene.
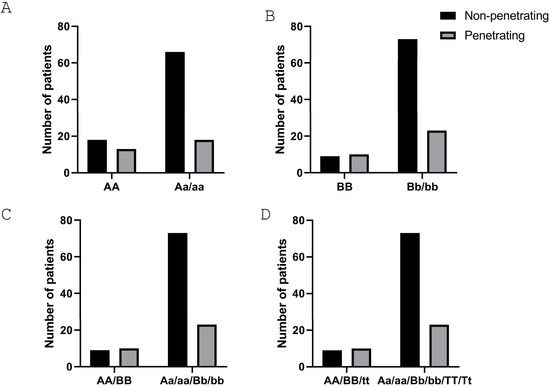
Figure 1.
CD patients carrying the a, b, or T alleles in the ApaI, BsmI, and TaqI SNPs of the VDR gene, respectively, have a lower risk of a penetrating behavior. (A,B) Graphs show the association between the penetrating behavior in CD and the number of patients carrying the AA genotype (A) (p = 0.0347) and the BB genotype (B) (p = 0.0235). (C) Graph shows the association between the penetrating behavior in CD and the number of patients carrying the AA/BB haplotype (p = 0.0235). (D) Graph shows the association between the penetrating behavior in CD and the number of patients carrying the triple haplotype AA/BB/tt (p = 0.0235). The p-value corresponds to statistical analysis by the χ2 test of a contingency table for the two different conditions in each graph.
3.4. CD Patients Carrying the bb Genotype Have a Lower Risk of Requiring Surgery
Regarding the VDR BsmI SNP, data show that patients carrying the bb genotype had a lower risk of surgery when compared with any of the other two genotypes (Table 8). In a similar manner to that obtained with the ApaI SNP, the risk of suffering a penetrating behavior was statistically significant (p = 0.0235) when comparing the BB genotype with the combination of the other two genotypes (Figure 1B). No significant differences were detected for any of the other variables analyzed (Table 8).

Table 8.
Classification of Crohn’s disease patients by genotypes in the BsmI SNP on the VDR gene.
Finally, when we analyzed patients carrying the haplotype AA/BB vs. all the others (Aa/Bb/aa/bb), we detected a lower and significant risk (p = 0.0235) of suffering a penetrating behavior in the latter ones (Figure 1C). This was also observed when we compared patients carrying the triple haplotype AA/BB/tt vs. all the other ones (p = 0.0235) (Figure 1D).
3.5. No Differences Were Detected in Any of the Clinical Parameters among the Three Genotypes in the FokI SNP of the VDR Gene
As shown in Table 9, the FokI genotypes in the VDR gene were not significantly associated with any clinical characteristic of the disease.

Table 9.
Classification of Crohn’s disease patients by genotypes in the FokI SNP on the VDR gene.
3.6. Fibroblasts Carrying the aa Genotype Expressed Lower Levels of Fibrotic Markers
Fibroblasts were isolated from resections of CD patients and classified according to the genotype for the ApaI SNP. The analyses of the mRNA expression of different cellular markers in these cells showed remarkable differences. As shown in Figure 2, the mRNA expression of COL1A1, αSMA (involved in the contractile apparatus), and IL6 was significantly higher in fibroblasts carrying the AA genotype than in those carrying the aa genotype. In contrast, the former exhibited significantly lower mRNA levels of both VIMENTIN and FSP1 than the latter ones. A non-significant reduction in the mRNA expression of VDR was detected and associated with the AA genotype (Figure 2).
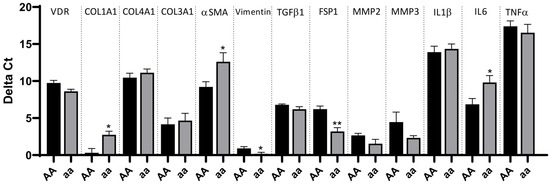
Figure 2.
Increased expression of pro-inflammatory and profibrotic genes in intestinal fibroblasts from CD patients carrying the AA genotype. Intestinal fibroblasts were obtained from ileal surgical resections from CD patients homozygous for alleles A and a in the ApaI SNP of the VDR gene. Graphs show the delta Ct (Ct gene–Ct actin) of several cytokines, fibrotic genes, and VDR in fibroblasts homozygous for the a allele vs. the A allele. Bars represent mean ± S.E.M. (n = 5 for AA and n = 5 for aa). * p < 0.05 or ** p < 0.01 vs. the AA genotype in each specific gene by Student’s t-test or the Mann–Whitney test, as appropriate.
4. Discussion
Our results reveal an association between patients carrying the a allele of ApaI SNP in the VDR gene and a lower risk of suffering both a penetrating behavior and perianal fistula in CD. Isolated intestinal fibroblasts from CD patients carrying the aa genotype exhibited a lower gene expression of fibrotic markers, such as COL1A1, α-SMA, and IL6, and a higher expression of FSP1.
Our results in a cohort of Caucasian patients reveal that the genotypic frequencies of the four VDR SNPs analyzed, FokI, BsmI, ApaI, and TaqI, did not significantly differ between controls and CD patients, which suggests that the VDR polymorphisms are not associated with a higher CD risk. Controversy has been reported regarding this association, and the sample size and ethnicity have been argued as the reasons for these discrepancies [8,9,11,12]. We agree that the sample sizes of the present and previous studies constitute a limitation to answer this query, but it is important to note that the VDR rare allele frequencies obtained in our cohort of patients (FokI = 57.5%, BsmI = 44.5%, ApaI = 58%, and TaqI = 39.5%) were comparable to other studies, some of which included a larger sample size of the Caucasian population [19]. Our data also reveal that FokI is not associated with any other SNP, while ApaI is strongly associated with BsmI, and both exhibited a linkage disequilibrium with the TaqI SNP, as previously reported [20]. In line with this, we have detected an association between the risk of penetrating behavior and the genotypes AA and BB, which reinforces the reported association of this behavior with the tt genotype in the same cohort of patients [15]. The association of these genotypes with penetrating behavior in CD is further demonstrated in patients carrying the haplotype AA/BB/tt in the VDR gene, a haplotype that has also been associated with other diseases, such as depressive disorders [20]. Of interest, our data extend these observations and show for the first time that the aa genotype reduces the risk of perianal fistulas. This observation seems to be independent of the association found between the AA genotype with the penetrating behavior since, in line with previous studies [2], the percentage of patients with perianal fistulas in our cohort is similar among CD behaviors (36% for penetrating, 40% for stenotic, and 31% for inflammatory). Furthermore, our data reveal that patients carrying the BB genotype did not exhibit that risk, but they were associated with a higher surgery risk. Further studies with a larger population are required to better understand the association of the ApaI, BsmI, and TaqI SNPs with two characteristics of the disease, perianal fistulas and surgery, which are intimately related.
The etiopathogenic mechanisms of perianal fistulas are poorly understood, but matrix remodelling, the epithelial–mesenchymal transition, and a high inflammatory burden seem to be involved [21]. Fibroblasts not only play an essential role in matrix deposition but also as a source of cytokines involved in the perpetuation of inflammation [22,23]. Our data reveal in fibroblasts isolated from intestinal resections of CD patients a higher mRNA expression of a profibrotic gene, αSMA in cells carrying the AA genotype than in those carrying the aa genotype. αSMA is a molecule associated with the differentiation of fibroblasts to myofibroblasts [24], a process that is crucial for ECM deposition, which agrees with the higher mRNA expression of COL1A1 also detected in these cells. Considering the anti-inflammatory and antifibrotic effects attributed to VDR [6] and the linkage disequilibrium between ApaI and TaqI SNPs, this observation may be related to the reduced VDR protein levels previously reported in patients carrying the tt genotype in the TaqI SNP [17]. Of interest, our data also show increased expression of IL6 in fibroblasts carrying the AA genotype, suggesting higher levels of inflammatory cytokines associated with this variant, which not only matches with the higher TNFα levels detected in the serum of healthy children carrying this genotype [25] but also with the increased expression of IL1β previously detected in PBMC carrying the tt genotype [15]. Finally, fibroblasts homozygous for the A allele of ApaI exhibited lower levels of the apoptosis inducing factor, FSP1, than those carrying the aa genotype. The fact that fibroblasts analyzed in the present study were obtained from the intestines of CD patients with complications, suggests that a higher viability of these cells may be involved in CD complications [26].
5. Conclusions
In conclusion, our data reveal an association between CD patients carrying the a allele of ApaI SNP in the VDR gene and both a lower risk of perianal fistulas and a penetrating behavior, as well as with a reduced expression of fibrotic and inflammatory markers in intestinal fibroblasts. These findings suggest that the characterization of VDR polymorphisms may help clinicians better predict the risk of CD complications and optimize treatment, including VD supplementation, which has been shown to restore the reduced VDR protein levels detected in fibroblasts from CD patients [27], including those carrying the TaqI polymorphism [17].
Author Contributions
M.D.B. designed this study; J.H. took responsibility for patient care and follow-up; and L.G.-F. and J.L. performed the experiments and performed the statistics. All authors analyzed the data; M.D.B. and L.G.-F. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Regional Development Fund of the European Union [ERDF] (PID2019-108996RB-I00, MCIN/AEI/10.13039/501100011033), by MCIN/AEI/10.13039/501100011033, by “ERDF A way of making Europe” (PID2022-141011OB-I00), by CIBERehd (EHD19PI05), and by Generalitat Valenciana [CIPROM2021/044].
Institutional Review Board Statement
This study was approved by the CEIM-Hospital Universitario y Politécnico la Fe (Valencia, Spain), with the protocol approval number 2021-284-1 (date 12 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data used in this manuscript are not publicly available because of participants’ privacy concerns but are available upon reasonable request.
Acknowledgments
We thank Brian Normanly for his English language.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verstockt, B.; Bressler, B.; Martinez-Lozano, H.; McGovern, D.; Silverberg, M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology 2022, 162, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Householder, S.; Picoraro, J.A. Diagnosis and Classification of Fistula from Inflammatory Bowel Disease and Inflammatory Bowel Disease-Related Surgery. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Geldof, J.; Iqbal, N.; LeBlanc, J.F.; Anandabaskaran, S.; Sawyer, R.; Buskens, C.; Bemelman, W.; Gecse, K.; Lundby, L.; Lightner, A.L.; et al. Classifying perianal fistulising Crohn’s disease: An expert consensus to guide decision-making in daily practice and clinical trials. Lancet Gastroenterol. Hepatol. 2022, 7, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Panaccione, R.; Nugent, Z.; Marshall, D.A.; Kaplan, G.G.; Vanner, S.; Dieleman, L.A.; Graff, L.A.; Otley, A.; Jones, J.; et al. Crohn’s Disease Phenotypes and Associations with Comorbidities, Surgery Risk, Medications and Nonmedication Approaches: The MAGIC in IMAGINE Study. Inflamm. Bowel Dis. 2024, izae055. [Google Scholar] [CrossRef] [PubMed]
- Annese, V. Genetics and epigenetics of IBD. Pharmacol. Res. 2020, 159, 104892. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR with New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef]
- Martin, K.; Radlmayr, M.; Borchers, R.; Heinzlmann, M.; Folwaczny, C. Candidate genes colocalized to linkage regions in inflammatory bowel disease. Digestion 2002, 66, 121–126. [Google Scholar] [CrossRef]
- Simmons, J.D.; Mullighan, C.; Welsh, K.I.; Jewell, D.P. Vitamin D receptor gene polymorphism: Association with Crohn’s disease susceptibility. Gut 2000, 47, 211–214. [Google Scholar] [CrossRef]
- Xue, L.N.; Xu, K.Q.; Zhang, W.; Wang, Q.; Wu, J.; Wang, X.Y. Associations between vitamin D receptor polymorphisms and susceptibility to ulcerative colitis and Crohn’s disease: A meta-analysis. Inflamm. Bowel Dis. 2013, 19, 54–60. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.T.; Hu, J.J.; Fan, R.; Zhou, J.; Zhong, J. Polymorphisms of the vitamin D receptor gene and the risk of inflammatory bowel disease: A meta-analysis. Genet. Mol. Res. 2014, 13, 2598–2610. [Google Scholar] [CrossRef]
- Xia, S.L.; Lin, X.X.; Guo, M.D.; Zhang, D.G.; Zheng, S.Z.; Jiang, L.J.; Jin, J.; Lin, X.Q.; Ding, R.; Jiang, Y. Association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with Crohn’s disease in Chinese patients. J. Gastroenterol. Hepatol. 2016, 31, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Farnood, A.; Habibi, M.; Derakhshan, F.; Balaii, H.; Motahari, Z.; Agah, M.R.; Firouzi, F.; Rad, M.G.; Aghazadeh, R.; et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2008, 23, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Shu, X.L.; Zhao, H.; Yu, J.D.; Ma, M.; Chen, J. Association between vitamin D receptor gene polymorphisms and pediatric Crohn’s disease in China: A study based on gene sequencing. Zhongguo Dang Dai Er Ke Za Zhi 2013, 15, 1006–1008. [Google Scholar]
- Limketkai, B.N.; Singla, M.B.; Rodriguez, B.; Veerappan, G.R.; Betteridge, J.D.; Ramos, M.A.; Hutfless, S.M.; Brant, S.R. Levels of Vitamin D Are Low After Crohn’s Disease Is Established but Not Before. Clin. Gastroenterol. Hepatol. 2020, 18, 1769–1776 e1761. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Ferrandiz, L.; Salvador, P.; Ortiz-Masia, D.; Macias-Ceja, D.C.; Orden, S.; Esplugues, J.V.; Calatayud, S.; Hinojosa, J.; Barrachina, M.D.; Hernandez, C. A Single Nucleotide Polymorphism in the Vitamin D Receptor Gene Is Associated with Decreased Levels of the Protein and a Penetrating Pattern in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Cusato, J.; Cafasso, C.; Antonucci, M.; Palermiti, A.; Manca, A.; Caviglia, G.P.; Vernero, M.; Armandi, A.; Saracco, G.M.; D’Avolio, A.; et al. Correlation between Polymorphisms of Vitamin D Metabolism Genes and Perianal Disease in Crohn’s Disease. Biomedicines 2024, 12, 320. [Google Scholar] [CrossRef]
- Gisbert-Ferrandiz, L.; Cosin-Roger, J.; Hernandez, C.; Macias-Ceja, D.C.; Ortiz-Masia, D.; Salvador, P.; Wildenberg, M.E.; Esplugues, J.V.; Alos, R.; Navarro, F.; et al. The vitamin D receptor Taq I polymorphism is associated with reduced VDR and increased PDIA3 protein levels in human intestinal fibroblasts. J. Steroid Biochem. Mol. Biol. 2020, 202, 105720. [Google Scholar] [CrossRef]
- Macias-Ceja, D.C.; Ortiz-Masia, D.; Salvador, P.; Gisbert-Ferrandiz, L.; Hernandez, C.; Hausmann, M.; Rogler, G.; Esplugues, J.V.; Hinojosa, J.; Alos, R.; et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019, 12, 178–187. [Google Scholar] [CrossRef]
- Hughes, D.J.; McManus, R.; Neary, P.; O’Morain, C.; O’Sullivan, M. Common variation in the vitamin D receptor gene and risk of inflammatory bowel disease in an Irish case-control study. Eur. J. Gastroenterol. Hepatol. 2011, 23, 807–812. [Google Scholar] [CrossRef]
- Lye, M.S.; Tor, Y.S.; Tey, Y.Y.; Shahabudin, A.; Loh, S.P.; Ibrahim, N.; Stanslas, J.; Rosli, R.; Ling, K.H. BsmI-ApaI-TaqI TAC (BAt) Haplotype of Vitamin D Receptor Gene Is Associated with Increased Risk of Major Depressive Disorder. J. Mol. Neurosci. 2021, 71, 981–990. [Google Scholar] [CrossRef]
- Cao, S.; Colonna, M.; Deepak, P. Pathogenesis of Perianal Fistulising Crohn’s Disease: Current Knowledge, Gaps in Understanding, and Future Research Directions. J. Crohn’s Colitis 2023, 17, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Santacroce, G.; Broglio, G.; Rossi, C.M.; Di Sabatino, A. Recent advances in intestinal fibrosis. Mol. Asp. Med. 2024, 96, 101251. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Cong, Y. Stromal Cell Regulation of Intestinal Inflammatory Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Wen, D.; Wang, J. Noncoding RNAs: Master Regulator of Fibroblast to Myofibroblast Transition in Fibrosis. Int. J. Mol. Sci. 2023, 24, 1801. [Google Scholar] [CrossRef]
- Ferrer-Suay, S.; Alonso-Iglesias, E.; Tortajada-Girbes, M.; Carrasco-Luna, J.; Codoner-Franch, P. Vitamin D receptor gene ApaI and FokI polymorphisms and its association with inflammation and oxidative stress in vitamin D sufficient Caucasian Spanish children. Transl. Pediatr. 2021, 10, 103–111. [Google Scholar] [CrossRef]
- Seco-Cervera, M.; Ortiz-Masia, D.; Macias-Ceja, D.C.; Coll, S.; Gisbert-Ferrandiz, L.; Cosin-Roger, J.; Bauset, C.; Ortega, M.; Heras-Moran, B.; Navarro-Vicente, F.; et al. Resistance to apoptosis in complicated Crohn’s disease: Relevance in ileal fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166966. [Google Scholar] [CrossRef]
- Gisbert-Ferrándiz, L.; Cosín-Roger, J.; Hernández, C.; Macias-Ceja, D.C.; Ortiz-Masiá, D.; Salvador, P.; Esplugues, J.V.; Hinojosa, J.; Navarro, F.; Calatayud, S.; et al. Diminished Vitamin D Receptor Protein Levels in Crohn’s Disease Fibroblasts: Effects of Vitamin D. Nutrients 2020, 12, 973. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).