Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Cultures
2.3. Macrophages Differentiation and Co-Culture Setup
2.4. Cytotoxicity Assay
2.5. RNA Extraction and Real-Time PCR
2.6. Quantitation of Cytokines by Cytometric Bead Array
2.7. Quantitation of Cytokines by ELISA
2.8. Assessment of Intracellular ROS by Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Differentiation, Polarization of Macrophages, and a Co-Culture of Endometriotic 12Z Cells and M1-Polarized THP-1 Cells
3.2. Effect of Resveratrol and Its Derivatives on 12Z Cell and THP-1-Derived Macrophage Proliferation
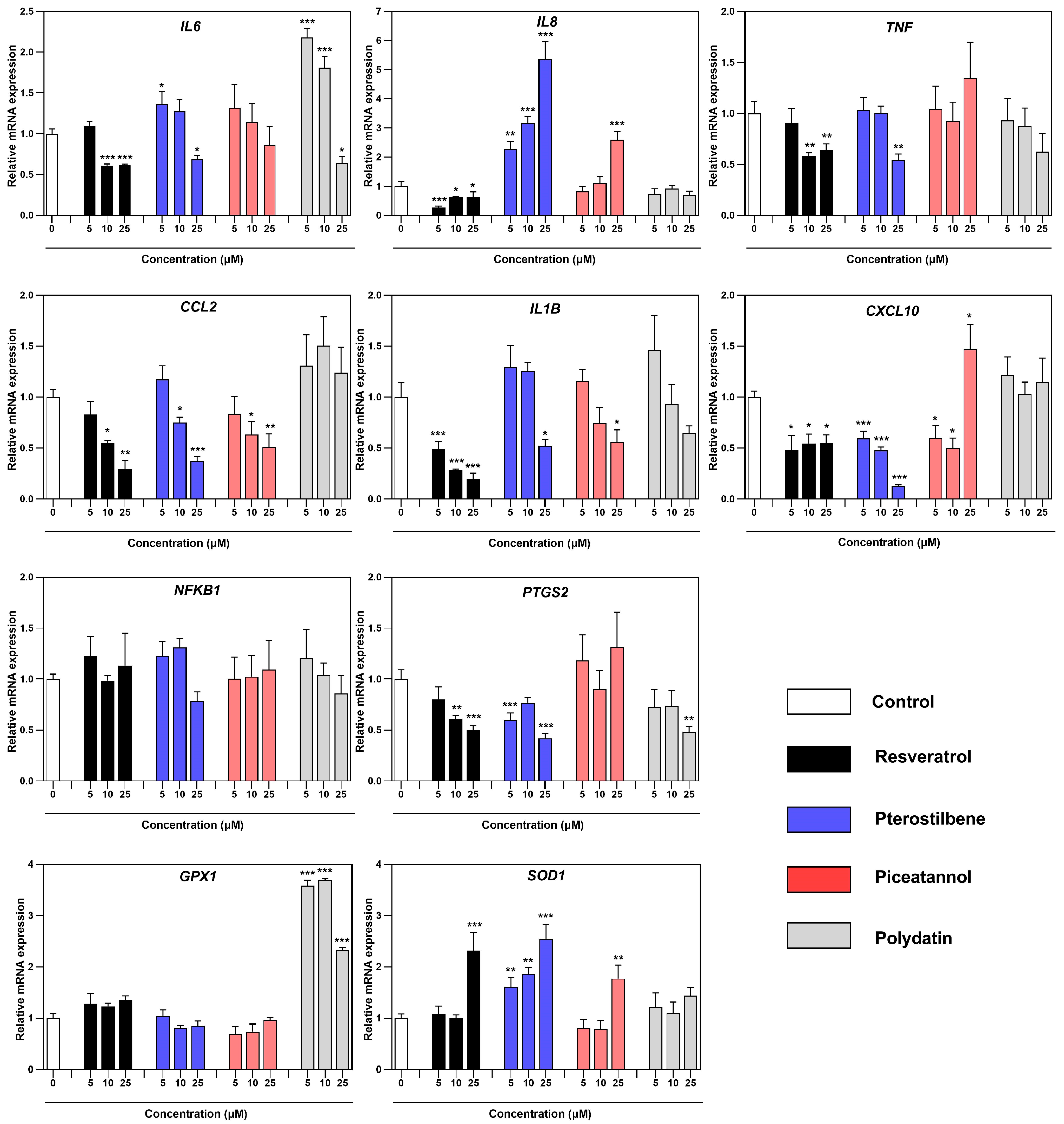
3.3. Effect of Resveratrol and Its Derivatives on the Expression of Genes Related to the Inflammatory and Oxidative Profile of Macrophages Co-Cultured with Endometriotic Cells
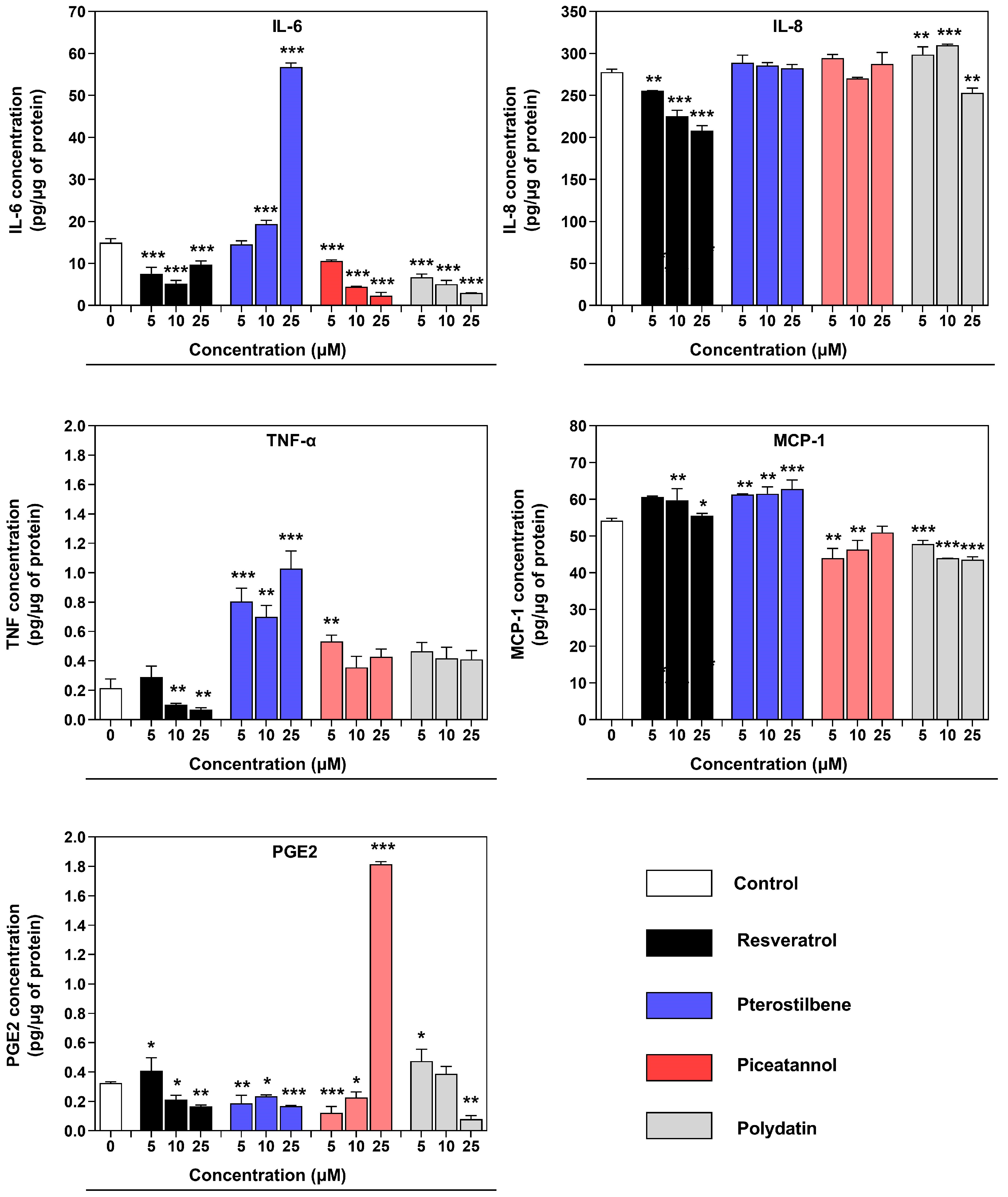
3.4. Effect of Resveratrol and Its Derivatives on the Cytokine/Chemokine Secretion in an Inflamed Co-Culture Model of Macrophages and Endometriotic Cells
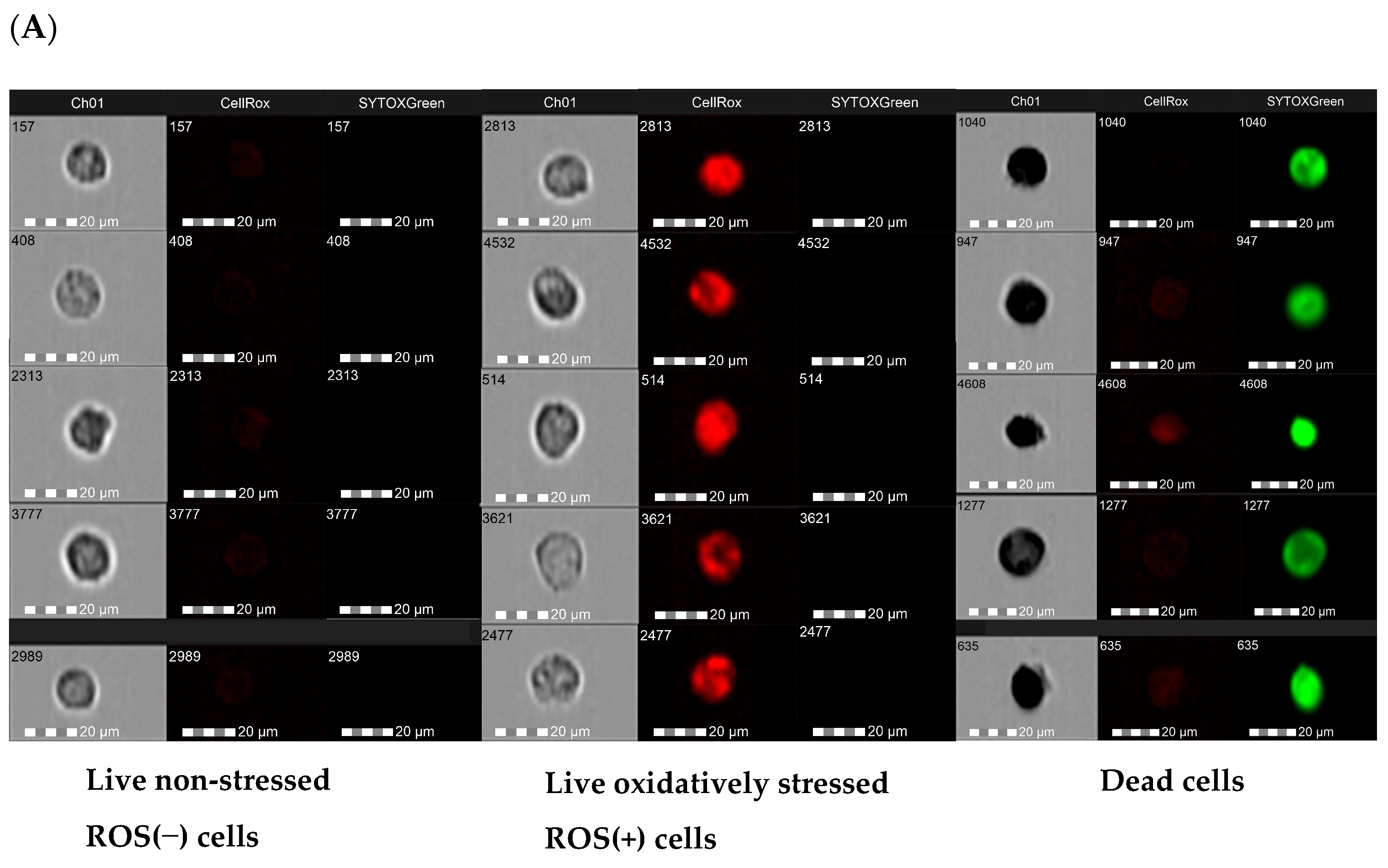
3.5. Effect of Resveratrol and Its Derivatives on the Intracellular ROS Generation in an Inflamed Co-Culture Model of Macrophages and Endometriotic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Critchley, H.O.D.; Babayev, E.; Bulun, S.E.; Clark, S.; Garcia-Grau, I.; Gregersen, P.K.; Kilcoyne, A.; Kim, J.-Y.J.; Lavender, M.; Marsh, E.E.; et al. Menstruation: Science and Society. Am. J. Obstet. Gynecol. 2020, 223, 624–664. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.; Rees, A.; Cronin, J.G.; Nair, M.; Jones, N.; Thornton, C.A. Macrophage Plasticity in Reproduction and Environmental Influences on Their Function. Front. Immunol. 2021, 11, 607328. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Temp, J.; Esnal-Zufiurre, A.; Mechsner, S.; Horne, A.W.; Saunders, P.T.K. Estradiol Is a Critical Mediator of Macrophage-Nerve Cross Talk in Peritoneal Endometriosis. Am. J. Pathol. 2015, 185, 2286–2297. [Google Scholar] [CrossRef]
- Yang, H.-L.; Zhou, W.-J.; Chang, K.-K.; Mei, J.; Huang, L.-Q.; Wang, M.-Y.; Meng, Y.; Ha, S.-Y.; Li, D.-J.; Li, M.-Q. The Crosstalk between Endometrial Stromal Cells and Macrophages Impairs Cytotoxicity of NK Cells in Endometriosis by Secreting IL-10 and TGF-β. Reproduction 2017, 154, 815–825. [Google Scholar] [CrossRef]
- Gołąbek, A.; Kowalska, K.; Olejnik, A. Polyphenols as a Diet Therapy Concept for Endometriosis-Current Opinion and Future Perspectives. Nutrients 2021, 13, 1347. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, M.; Li, X.; Wang, D.; Yu, L.; Ma, F.; Jiang, J.; Zhang, L.; Li, P. Contributions of Common Foods to Resveratrol Intake in the Chinese Diet. Foods 2024, 13, 1267. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, Prooxidant and Cytotoxic Activity of Hydroxylated Resveratrol Analogues: Structure–Activity Relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Krambeck, K.; Oliveira, A.; Santos, D.; Pintado, M.M.; Baptista Silva, J.; Sousa Lobo, J.M.; Amaral, M.H. Identification and Quantification of Stilbenes (Piceatannol and Resveratrol) in Passiflora Edulis By-Products. Pharmaceuticals 2020, 13, 73. [Google Scholar] [CrossRef]
- Tanzil, A.D.; Al, E. Effects of Oral Intake of Piceatannol on Fat Burning―A Randomized, Double—Blind, Placebo—Controlled Crossover Comparison Study―. Jpn. Pharmacol. Ther. 2020, 48, 1235–1240. [Google Scholar]
- Yan, W.; Ren, D.; Feng, X.; Huang, J.; Wang, D.; Li, T.; Zhang, D. Neuroprotective and Anti-Inflammatory Effect of Pterostilbene Against Cerebral Ischemia/Reperfusion Injury via Suppression of COX-2. Front. Pharmacol. 2021, 12, 770329. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef] [PubMed]
- Ravagnan, G.; De Filippis, A.; Cartenì, M.; De Maria, S.; Cozza, V.; Petrazzuolo, M.; Tufano, M.A.; Donnarumma, G. Polydatin, A Natural Precursor of Resveratrol, Induces β-Defensin Production and Reduces Inflammatory Response. Inflammation 2013, 36, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Potdar, S.; Parmar, M.S.; Ray, S.D.; Cavanaugh, J.E. Protective Effects of the Resveratrol Analog Piceid in Dopaminergic SH-SY5Y Cells. Arch. Toxicol. 2018, 92, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, Q.; Duan, H.; Ye, X.; Liu, J.; Peng, W.; Wu, C. Polydatin: A Critical Promising Natural Agent for Liver Protection via Antioxidative Stress. Oxid. Med. Cell Longev. 2022, 2022, 9218738. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.-M. Oxidative Stress in the Pelvic Cavity and Its Role in the Pathogenesis of Endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Young, H.; Hurlstone, A.; Wellbrock, C. Differentiation of THP1 Cells into Macrophages for Transwell Co-Culture Assay with Melanoma Cells. Bio Protoc. 2015, 5, e1638. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 Macrophages Derived from THP-1 Cells Differentially Modulate the Response of Cancer Cells to Etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef]
- Olejnik, A.; Rychlik, J.; Kidoń, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyński, R.; Moyer, M.P. Antioxidant Effects of Gastrointestinal Digested Purple Carrot Extract on the Human Cells of Colonic Mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Dembczyński, R.; Gołąbek, A.; Olkowicz, M.; Olejnik, A. ROS Modulating Effects of Lingonberry (Vaccinium Vitis-Idaea L.) Polyphenols on Obese Adipocyte Hypertrophy and Vascular Endothelial Dysfunction. Nutrients 2021, 13, 885. [Google Scholar] [CrossRef] [PubMed]
- Golabek, A.; Kaczmarek, M.; Dondajewska, E.; Sakrajda, K.; Mackiewicz, A.; Dams-kozlowska, H. Application of a Three-dimensional (3D) Breast Cancer Model to Study Macrophage Polarization. Exp. Ther. Med. 2021, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Oală, I.E.; Mitranovici, M.-I.; Chiorean, D.M.; Irimia, T.; Crișan, A.I.; Melinte, I.M.; Cotruș, T.; Tudorache, V.; Moraru, L.; Moraru, R.; et al. Endometriosis and the Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Pathophysiology: A Narrative Review of the Literature. Diagnostics 2024, 14, 312. [Google Scholar] [CrossRef]
- Ding, S.; Guo, X.; Zhu, L.; Wang, J.; Li, T.; Yu, Q.; Zhang, X. Macrophage-Derived Netrin-1 Contributes to Endometriosis-Associated Pain. Ann. Transl. Med. 2021, 9, 29. [Google Scholar] [CrossRef]
- Woo, J.-H.; Yang, Y.-I.; Ahn, J.-H.; Choi, Y.S.; Choi, J.-H. Interleukin 6 Secretion from Alternatively Activated Macrophages Promotes the Migration of Endometriotic Epithelial Cells. Biol. Reprod. 2017, 97, 660–670. [Google Scholar] [CrossRef][Green Version]
- Sekulovski, N.; Whorton, A.E.; Shi, M.; MacLean, J.A.; Hayashi, K. Endometriotic Inflammatory Microenvironment Induced by Macrophages Can Be Targeted by Niclosamide. Biol. Reprod. 2019, 100, 398–408. [Google Scholar] [CrossRef]
- Kämpfer, A.A.M.; Urbán, P.; Gioria, S.; Kanase, N.; Stone, V.; Kinsner-Ovaskainen, A. Development of an in Vitro Co-Culture Model to Mimic the Human Intestine in Healthy and Diseased State. Toxicol. Vitr. 2017, 45, 31–43. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Farhan, F.; Al Kury, L.T. Resveratrol and Tumor Microenvironment: Mechanistic Basis and Therapeutic Targets. Molecules 2020, 25, 4282. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pavez, T.N.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Marín-Sánchez, P.; Machado-Linde, F.; García-Peñarrubia, P. The Role of Peritoneal Macrophages in Endometriosis. Int. J. Mol. Sci. 2021, 22, 10792. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, Y.; Zhong, Z.; Wei, C.; Liu, Y.; Zhu, X. Peritoneal Immune Microenvironment of Endometriosis: Role and Therapeutic Perspectives. Front. Immunol. 2023, 14, 1134663. [Google Scholar] [CrossRef]
- Connolly, T.P. Cyclooxygenase-2 Inhibitors in Gynecologic Practice. Clin. Med. Res. 2003, 1, 105–110. [Google Scholar] [CrossRef]
- Tassinari, V.; Smeriglio, A.; Stillittano, V.; Trombetta, D.; Zilli, R.; Tassinari, R.; Maranghi, F.; Frank, G.; Marcoccia, D.; Di Renzo, L. Endometriosis Treatment: Role of Natural Polyphenols as Anti-Inflammatory Agents. Nutrients 2023, 15, 2967. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, A.; Futyma, K.; Nowakowski, Ł.; Gogacz, M.; Rechberger, T. Progress on Macrophage’s Proinflammatory Products as Markers of Acute Endometriosis. J. Acute Dis. 2015, 4, 169–172. [Google Scholar] [CrossRef][Green Version]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-Kappa B, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef]
- Benitez, D.A.; Hermoso, M.A.; Pozo-Guisado, E.; Fernández-Salguero, P.M.; Castellón, E.A. Regulation of Cell Survival by Resveratrol Involves Inhibition of NF Kappa B-Regulated Gene Expression in Prostate Cancer Cells. Prostate 2009, 69, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-Y.; Silver, M.; Parnes, A.; Nikiforow, S.; Berliner, N.; Vanasse, G.J. Resveratrol Ameliorates TNFα-Mediated Suppression of Erythropoiesis in Human CD34+ Cells via Modulation of NF-κB Signalling. Br. J. Haematol. 2011, 155, 93–101. [Google Scholar] [CrossRef]
- Xu, L.; Botchway, B.O.A.; Zhang, S.; Zhou, J.; Liu, X. Inhibition of NF-κB Signaling Pathway by Resveratrol Improves Spinal Cord Injury. Front. Neurosci. 2018, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, N.; Zhu, Y.; Yang, Y.; Chen, X.; Fan, S.; Chen, Q.; Zhou, H.; Zheng, J. Inhibition of Extracellular Calcium Influx Results in Enhanced IL-12 Production in LPS-Treated Murine Macrophages by Downregulation of the CaMKKβ-AMPK-SIRT1 Signaling Pathway. Mediators Inflamm. 2016, 2016, 6152713. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and Its Metabolite, Isorhapontigenin, Induce SIRT1 Expression in THP-1 Human Monocytic Cell Line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef]
- Yamamoto, T.; Li, Y.; Hanafusa, Y.; Yeh, Y.; Maruki-Uchida, H.; Kawakami, S.; Sai, M.; Goto, T.; Ito, T.; Kawada, T. Piceatannol Exhibits Anti-inflammatory Effects on Macrophages Interacting with Adipocytes. Food Sci. Nutr. 2016, 5, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional Profiling of the LPS Induced NF-κB Response in Macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol Distinctively Modulates the Inflammatory Profiles of Immune and Endothelial Cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef]
- Feng, L.; Yasmeen, R.; Schoene, N.W.; Lei, K.Y.; Wang, T.T.Y. Resveratrol Differentially Modulates Immune Responses in Human THP-1 Monocytes and Macrophages. Nutr. Res. 2019, 72, 57–69. [Google Scholar] [CrossRef]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A. Resveratrol Treatment Reduces Expression of MCP-1, IL-6, IL-8 and RANTES in Endometriotic Stromal Cells. J. Cell. Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef]
- Taguchi, A.; Wada-Hiraike, O.; Kawana, K.; Koga, K.; Yamashita, A.; Shirane, A.; Urata, Y.; Kozuma, S.; Osuga, Y.; Fujii, T. Resveratrol Suppresses Inflammatory Responses in Endometrial Stromal Cells Derived from Endometriosis: A Possible Role of the Sirtuin 1 Pathway. J. Obstet. Gynaecol. Res. 2014, 40, 770–778. [Google Scholar] [CrossRef]
- Didziokaite, G.; Biliute, G.; Gudaite, J.; Kvedariene, V. Oxidative Stress as a Potential Underlying Cause of Minimal and Mild Endometriosis-Related Infertility. Int. J. Mol. Sci. 2023, 24, 3809. [Google Scholar] [CrossRef] [PubMed]
- Mulgund, A.; Doshi, S.; Agarwal, A. Chapter 25—The Role of Oxidative Stress in Endometriosis. In Handbook of Fertility; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 273–281. ISBN 978-0-12-800872-0. [Google Scholar]
- Ekarattanawong, S.; Tanprasertkul, C.; Somprasit, C.; Chamod, P.; Tiengtip, R.; Bhamarapravatana, K.; Suwannarurk, K. Possibility of Using Superoxide Dismutase and Glutathione Peroxidase as Endometriosis Biomarkers. Int. J. Womens Health 2017, 9, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gołąbek-Grenda, A.; Olejnik, A. In Vitro Modeling of Endometriosis and Endometriotic Microenvironment—Challenges and Recent Advances. Cell. Signal. 2022, 97, 110375. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Salmeri, F.M.; Ban Frangež, H.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 Macrophages in Ovarian Endometriomas from Women Affected by Endometriosis at Different Stages of the Disease. Gynecol. Endocrinol. 2020, 36, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S. Understanding the Molecular Mechanisms of Macrophage Polarization and Metabolic Reprogramming in Endometriosis: A Narrative Review. Reprod. Med. Biol. 2022, 21, e12488. [Google Scholar] [CrossRef]
- Wang, T.T.Y.; Hudson, T.S.; Wang, T.-C.; Remsberg, C.M.; Davies, N.M.; Takahashi, Y.; Kim, Y.S.; Seifried, H.; Vinyard, B.T.; Perkins, S.N.; et al. Differential Effects of Resveratrol on Androgen-Responsive LNCaP Human Prostate Cancer Cells in Vitro and in Vivo. Carcinogenesis 2008, 29, 2001–2010. [Google Scholar] [CrossRef]
- Sienko, A.; Cichosz, A.; Urban, A.; Smolarczyk, R.; Czajkowski, K.; Sienko, J. The Effect of Two Anti-Inflammatory Dietary Components, Omega-3 and Resveratrol, on Endometriosis. Ginekol. Pol. 2024, 95, 573–583. [Google Scholar] [CrossRef]
- Indraccolo, U.; Indraccolo, S.R.; Mignini, F. Micronized Palmitoylethanolamide/Trans-Polydatin Treatment of Endometriosis-Related Pain: A Meta-Analysis. Ann. Ist. Super. Sanita 2017, 53, 125–134. [Google Scholar] [CrossRef]
- Mendes da Silva, D.; Gross, L.A.; de Neto, E.P.G.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]






| Gene | Accession | No. Sequence (5′-3′) | Amplicon (bp) |
|---|---|---|---|
| CCL2 | NM_002982.4 | F: AGAATCACCAGCAGCAAGTGTCC R: TCCTGAACCCACTTCTGCTTGG | 98 |
| CXCL10 | NM_001565.4 | F: GAACTGTACGCTGTACCTGCA R: TTGATGGCCTTCGATTCTGGA | 172 |
| GAPDH | NM_002046.7 | F: GTCTCCTCTGACTTCAACAGCG R:ACCACCCTGTTGCTGTAGCCAA | 131 |
| GPX1 | NM_000581.4 | F: GTGCTCGGCTTCCCGTGCAAC R: CTCGAAGAGCATGAAGTTGGGC | 123 |
| IL1B | NM_000576.3 | F: CCACAGACCTTCCAGGAGAATG R: GTGCAGTTCAGTGATCGTACAGG | 131 |
| IL6 | NM_000600.5 | F: AGACAGCCACTCACCTCTTCAG R: TTCTGCCAGTGCCTCTTTGCTG | 132 |
| IL8 | NM_001354840.3 | F: GAGAGTGATTGAGAGTGGACCAC R: CACAACCCTCTGCACCCAGTTT | 112 |
| NFKB1 | NM_003998.4 | F: GCAGCACTACTTCTTGACCACC R: TCTGCTCCTGAGCATTGACGTC | 130 |
| PTGS2 | NM_000963.4 | F: CGGTGAAACTCTGGCTAGACAG R: GCAAACCGTAGATGCTCAGGGA | 156 |
| SOD1 | NM_000454.5 | F: CTCACTCTCAGGAGACCATTGC R: CCACAAGCCAAACGACTTCCAG | 129 |
| TNF | NM_000594.4 | F: CTCTTCTGCCTGCTGCACTTTG R: ATGGGCTACAGGCTTGTCACTC | 135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołąbek-Grenda, A.; Juzwa, W.; Kaczmarek, M.; Olejnik, A. Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages. Nutrients 2024, 16, 3483. https://doi.org/10.3390/nu16203483
Gołąbek-Grenda A, Juzwa W, Kaczmarek M, Olejnik A. Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages. Nutrients. 2024; 16(20):3483. https://doi.org/10.3390/nu16203483
Chicago/Turabian StyleGołąbek-Grenda, Agata, Wojciech Juzwa, Mariusz Kaczmarek, and Anna Olejnik. 2024. "Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages" Nutrients 16, no. 20: 3483. https://doi.org/10.3390/nu16203483
APA StyleGołąbek-Grenda, A., Juzwa, W., Kaczmarek, M., & Olejnik, A. (2024). Resveratrol and Its Natural Analogs Mitigate Immune Dysregulation and Oxidative Imbalance in the Endometriosis Niche Simulated in a Co-Culture System of Endometriotic Cells and Macrophages. Nutrients, 16(20), 3483. https://doi.org/10.3390/nu16203483








