The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Results
3.1. Red Wine General Characteristics
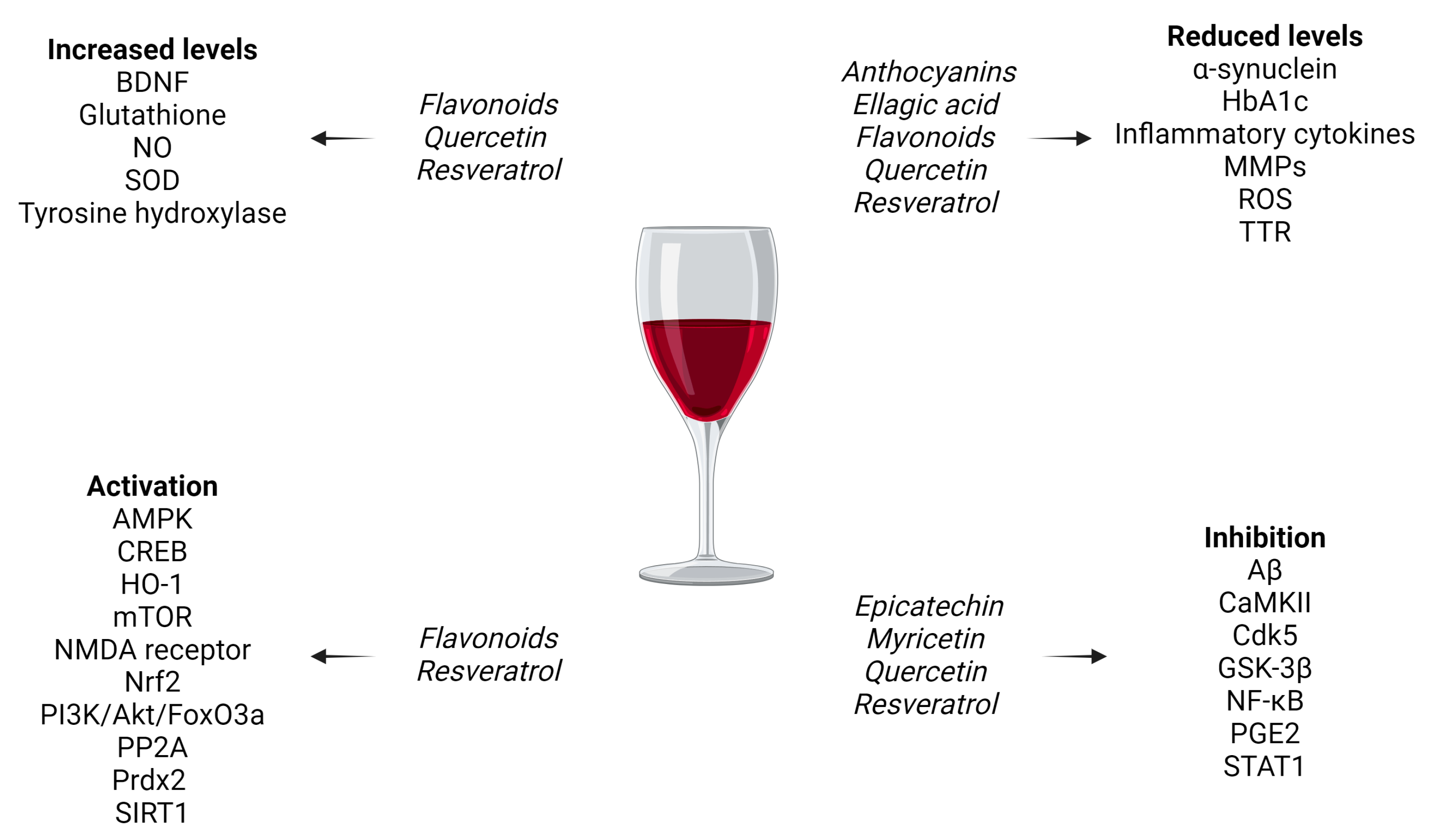
3.2. General Neuroprotective Effects of Red-Wine-Derived Compounds
3.3. The Impact of Red Wine and Its Components on Mild Cognitive Impairment
3.4. The Impact of Red Wine and Its Components on Alzheimer’s Disease
3.5. The Impact of Red Wine and Its Components on Vascular Dementia
3.6. The Impact of Red Wine and Its Components on Parkinson’s Disease Dementia
3.7. The Impact of Red Wine and Its Components on Metabolic Syndrome and Diabetic-Associated Cognitive Impairment
3.8. Preclinical Data on the Effect of Red Wine Compounds on Other Neurocognitive Disorders
3.9. Dose–Response Correlation between Alcoholic Beverages and Dementia
4. Discussion
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Bouaziz, B.; Müller, P.; MGlenn, J.; Bott, N.T.; Müller, N.; Chtourou, H.; Driss, T.; et al. Effects of Polyphenol-Rich Interventions on Cognition and Brain Health in Healthy Young and Middle-Aged Adults: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1598. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.; Tsiountsioura, M.; Meixner-Goetz, L.; Cvirn, G.; Lamprecht, M. Polyphenols’ Impact on Selected Biomarkers of Brain Aging in Healthy Middle-Aged and Elderly Subjects: A Review of Clinical Trials. Nutrients 2023, 15, 3770. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. Ageing and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Bobadilla, M.; Hernández, C.; Ayala, M.; Alonso, I.; Iglesias, A.; García-Sanmartín, J.; Mirpuri, E.; Barriobero, J.I.; Martínez, A. A Grape Juice Supplemented with Natural Grape Extracts Is Well Accepted by Consumers and Reduces Brain Oxidative Stress. Antioxidants 2021, 10, 677. [Google Scholar] [CrossRef]
- Mazzanti, G.; Di Giacomo, S. Curcumin and Resveratrol in the Management of Cognitive Disorders: What is the Clinical Evidence? Molecules 2016, 21, 1243. [Google Scholar] [CrossRef]
- Goni, L.; Fernández-Matarrubia, M.; Romanos-Nanclares, A.; Razquin, C.; Ruiz-Canela, M.; Martínez-González, M.Á.; Toledo, E. Polyphenol intake and cognitive decline in the Seguimiento Universidad de Navarra (SUN) Project. Br. J. Nutr. 2021, 126, 43–52. [Google Scholar] [CrossRef]
- Féart, C.; Samieri, C.; Barberger-Gateau, P. Mediterranean diet and cognitive function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean Diet and Mild Cognitive Impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre-Arbogast, S.; Gaudout, D.; Bensalem, J.; Letenneur, L.; Dartigues, J.-F.; Hejblum, B.P.; Féart, C.; Delcourt, C.; Samieri, C. Pattern of polyphenol intake and the long-term risk of dementia in older persons. Neurology 2018, 90, e1979–e1988. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Alshatwi, A.A.; Paladino, N.; Guerrera, I.; Grosso, G.; Castellano, S.; Godos, J. Association between alcoholic (poly)phenol-rich beverage consumption and cognitive status in older adults living in a Mediterranean area. Int. J. Food Sci. Nutr. 2023, 74, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; Goulart, R.D.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Boushey, C.; Ard, J.; Bazzano, L.; Heymsfield, S.; Mayer-Davis, E.; Sabaté, J.; Snetselaar, L.; Van Horn, L.; Schneeman, B.; English, L.; et al. Dietary Patterns and Neurocognitive Health: A Systematic Review; U.S. Department of Agriculture, Food and Nutrition Service, Center for Nutrition Policy and Promotion, Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2020. [Google Scholar]
- Lamport, D.J.; Lawton, C.L.; Mansfield, M.W.; Dye, L. Impairments in glucose tolerance can have a negative impact on cognitive function: A systematic research review. Neurosci. Biobehav. Rev. 2009, 33, 394–413. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Castellano-Escuder, P.; Carmona, F.; Lefèvre-Arbogast, S.; Low, D.Y.; Du Preez, A.; Ruigrok, S.R.; Manach, C.; Urpi-Sarda, M.; Korosi, A.; et al. Food and Microbiota Metabolites Associate with Cognitive Decline in Older Subjects: A 12-Year Prospective Study. Mol. Nutr. Food Res. 2021, 65, 2100606. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300. [Google Scholar] [CrossRef]
- Ammar, A.; Trabelsi, K.; Müller, P.; Bouaziz, B.; Boukhris, O.; Glenn, J.M.; Bott, N.; Driss, T.; Chtourou, H.; Müller, N.; et al. The Effect of (Poly)phenol-Rich Interventions on Cognitive Functions and Neuroprotective Measures in Healthy Aging Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 835. [Google Scholar] [CrossRef]
- Rabassa, M.; Cherubini, A.; Zamora-Ros, R.; Urpi-Sarda, M.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C. Low Levels of a Urinary Biomarker of Dietary Polyphenol Are Associated with Substantial Cognitive Decline over a 3-Year Period in Older Adults: The Invecchiare in Chianti Study. J. Am. Geriatr. Soc. 2015, 63, 938–946. [Google Scholar] [CrossRef]
- Lyu, W.; Rodriguez, D.; Ferruzzi, M.G.; Pasinetti, G.M.; Murrough, J.W.; Simon, J.E.; Wu, Q. Chemical, Manufacturing, and Standardization Controls of Grape Polyphenol Dietary Supplements in Support of a Clinical Study: Mass Uniformity, Polyphenol Dosage, and Profiles. Front. Nutr. 2021, 8, 780226. [Google Scholar] [CrossRef] [PubMed]
- Chou, E.J.; Keevil, J.G.; Aeschlimann, S.; Wiebe, D.A.; Folts, J.D.; Stein, J.H. Effect of ingestion of purple grape juice on endothelial function in patients with coronary heart disease. Am. J. Cardiol. 2001, 88, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Williams, C.M. Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. Brain Plast. 2021, 6, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Does phytoestrogen supplementation improve cognition in humans? A systematic review. Ann. N. Y. Acad. Sci. 2017, 1403, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef]
- Marx, W.; Kelly, J.T.; Marshall, S.; Cutajar, J.; Annois, B.; Pipingas, A.; Tierney, A.; Itsiopoulos, C. Effect of resveratrol supplementation on cognitive performance and mood in adults: A systematic literature review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 432–443. [Google Scholar] [CrossRef]
- Fischer, K.; Melo Van Lent, D.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; Van Den Bussche, H.; Wiese, B.; et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Agosti, P.; Lozupone, M.; Custodero, C.; Schilardi, A.; Valiani, V.; Sardone, R.; Dibello, V.; Di Lena, L.; Lamanna, A.; et al. Nutritional Intervention as a Preventive Approach for Cognitive-Related Outcomes in Cognitively Healthy Older Adults: A Systematic Review. J. Alzheimer's Dis. 2018, 64, S229–S254. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef]
- Noguer, M.A.; Cerezo, A.B.; Donoso Navarro, E.; Garcia-Parrilla, M.C. Intake of alcohol-free red wine modulates antioxidant enzyme activities in a human intervention study. Pharmacol. Res. 2012, 65, 609–614. [Google Scholar] [CrossRef]
- Singh, A.; Naidu, P.S.; Kulkarni, S.K. Reversal of Aging and Chronic Ethanol-induced Cognitive Dysfunction by Quercetin a Bioflavonoid. Free Radic. Res. 2003, 37, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord Grape Juice Supplementation and Neurocognitive Function in Human Aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.-K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Montandon, M.-L.; Rodriguez, C.; Herrmann, F.; Giannakopoulos, P. Impact of Coffee, Wine, and Chocolate Consumption on Cognitive Outcome and MRI Parameters in Old Age. Nutrients 2018, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Nooyens, A.C.J.; Bueno-de-Mesquita, H.B.; Van Gelder, B.M.; Van Boxtel, M.P.J.; Verschuren, W.M.M. Consumption of alcoholic beverages and cognitive decline at middle age: The Doetinchem Cohort Study. Br. J. Nutr. 2014, 111, 715–723. [Google Scholar] [CrossRef]
- Li, T.; Willette, A.A.; Wang, Q.; Pollpeter, A.; Larsen, B.A.; Mohammadiarvejeh, P.; Fili, M. Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank. Nutrients 2023, 15, 3390. [Google Scholar] [CrossRef]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults With or Without Mild Cognitive Impairment. JAMA Netw. Open 2019, 2, e1910319. [Google Scholar] [CrossRef]
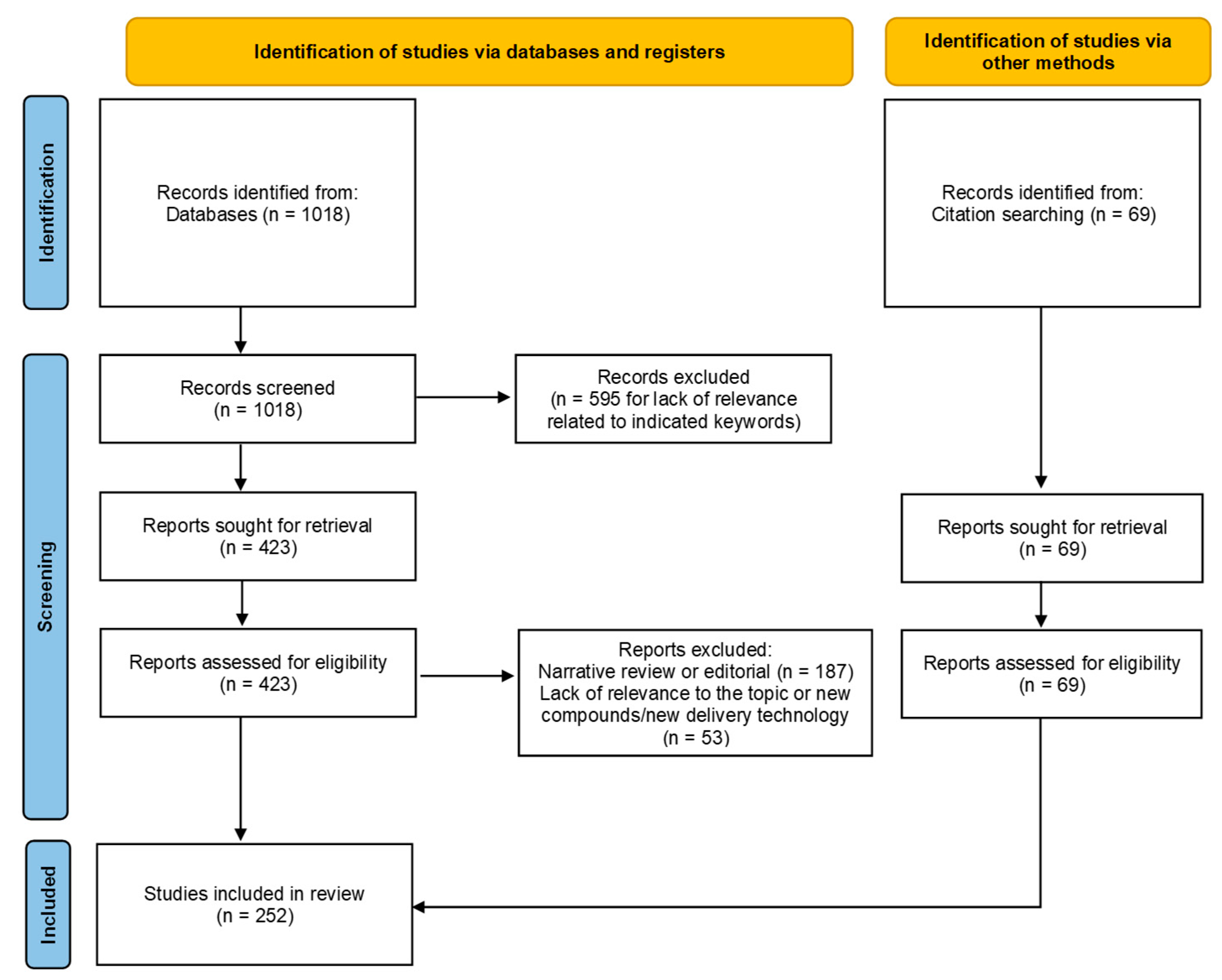
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Camajani, E.; Caprio, M.; Armani, A. Health Effects of Red Wine Consumption: A Narrative Review of an Issue That Still Deserves Debate. Nutrients 2023, 15, 1921. [Google Scholar] [CrossRef]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol Extracts from Red Wine and Grapevine: Potential Effects on Cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; Imbriani, G.; Acito, M.; Moretti, M.; Fanizzi, F.P.; De Donno, A.; Valacchi, G. Moderate red wine intake and cardiovascular health protection: A literature review. Food Funct. 2023, 14, 6346–6362. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Delgado, A.M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Ouslati, A.; Chammem, N. Phenols, Flavors, and the Mediterranean Diet. J. AOAC Int. 2020, 103, 915–924. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated Knowledge About the Presence of Phenolic Compounds in Wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef]
- Cozzolino, D. Phenolics and spectroscopy: Challenges and successful stories in the grape and wine industry. J. Sci. Food Agric. 2023. early view. [Google Scholar] [CrossRef]
- Tofalo, R.; Suzzi, G.; Perpetuini, G. Discovering the Influence of Microorganisms on Wine Color. Front. Microbiol. 2021, 12, 790935. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R. Mass spectrometry in grape and wine chemistry. Part I: Polyphenols. Mass Spectrom. Rev. 2003, 22, 218–250. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Sinha, K.; Ghosh, J.; Sil, P.C. Morin and Its Role in Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 453–471. [Google Scholar]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as Neuroprotective Agent: From Bench to Bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Zhu, J. A quarter century of wine pigment discovery. J. Sci. Food Agric. 2020, 100, 5093–5101. [Google Scholar] [CrossRef]
- Li, L.; Sun, B. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2019, 59, 563–579. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins Their Variation in Red Wines, I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-D.; Zhang, D.; Xiao, C.-L.; Zhou, Y.; Li, X.; Wang, L.; He, Z.; Reilly, J.; Xiao, Z.-Y.; Shu, X. P-Coumaric Acid Reverses Depression-Like Behavior and Memory Deficit Via Inhibiting AGE-RAGE-Mediated Neuroinflammation. Cells 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Mo, L.; Zou, Y.; Zhao, G. Tyrosinase inhibitory mechanism and the anti-browning properties of piceid and its ester. Food Chem. 2022, 390, 133207. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a Therapeutic Agent for Neurodegenerative Diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef]
- Khakimov, B.; Engelsen, S.B. Resveratrol in the foodomics era: 1:25,000. Ann. N. Y. Acad. Sci. 2017, 1403, 48–58. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Hasan, M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Busconi, M.; Flamini, R.; De Rosso, M. Wine Resveratrol: From the Ground Up. Nutrients 2016, 8, 222. [Google Scholar] [CrossRef]
- Yang, A.J.T.; Bagit, A.; MacPherson, R.E.K. Resveratrol, Metabolic Dysregulation, and Alzheimer’s Disease: Considerations for Neurogenerative Disease. Int. J. Mol. Sci. 2021, 22, 4628. [Google Scholar] [CrossRef] [PubMed]
- Aires, V.; Delmas, D. Common Pathways in Health Benefit Properties of RSV in Cardiovascular Diseases, Cancers and Degenerative Pathologies. Curr. Pharm. Biotechnol. 2015, 16, 219–244. [Google Scholar] [CrossRef] [PubMed]
- Mazué, F.; Delmas, D.; Murillo, G.; Saleiro, D.; Limagne, E.; Latruffe, N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014, 5, 663. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a Chemopreventive Agent: A Promising Molecule for Fighting Cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef]
- Donovan, J.L.; Lee, A.; Manach, C.; Rios, L.; Morand, C.; Scalbert, A.; Rémésy, C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br. J. Nutr. 2002, 87, 299–306. [Google Scholar] [CrossRef]
- Bianchi, M.G.; Chiu, M.; Taurino, G.; Brighenti, F.; Del Rio, D.; Mena, P.; Bussolati, O. Catechin and Procyanidin B2 Modulate the Expression of Tight Junction Proteins but Do Not Protect from Inflammation-Induced Changes in Permeability in Human Intestinal Cell Monolayers. Nutrients 2019, 11, 2271. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Bastianetto, S.; Zheng, W.; Quirion, R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000, 131, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.J.; Kang, N.J.; Lee, K.W.; Lee, H.J. Protective Effects of Red Wine Flavonols on 4-Hydroxynonenal-Induced Apoptosis in PC12 Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, N.; Hulick, W.; Winter, A.; Ross, E.; Linseman, D. Neuroprotective effects of anthocyanins on apoptosis induced by mitochondrial oxidative stress. Nutr. Neurosci. 2011, 14, 249–259. [Google Scholar] [CrossRef]
- Ben Khadher, T.; Aydi, S.; Mars, M.; Bouajila, J. Study on the Chemical Composition and the Biological Activities of Vitis vinifera Stem Extracts. Molecules 2022, 27, 3109. [Google Scholar] [CrossRef]
- Yammine, A.; Zarrouk, A.; Nury, T.; Vejux, A.; Latruffe, N.; Vervandier-Fasseur, D.; Samadi, M.; Mackrill, J.J.; Greige-Gerges, H.; Auezova, L.; et al. Prevention by Dietary Polyphenols (Resveratrol, Quercetin, Apigenin) Against 7-Ketocholesterol-Induced Oxiapoptophagy in Neuronal N2a Cells: Potential Interest for the Treatment of Neurodegenerative and Age-Related Diseases. Cells 2020, 9, 2346. [Google Scholar] [CrossRef]
- Bobadilla, M.; García-Sanmartín, J.; Martínez, A. Natural Food Supplements Reduce Oxidative Stress in Primary Neurons and in the Mouse Brain, Suggesting Applications in the Prevention of Neurodegenerative Diseases. Antioxidants 2021, 10, 46. [Google Scholar] [CrossRef]
- Abdenour, B.; Charles, R. Innovative anthocyanins formulation protects neuronal-like cells against oxidative stress-induced damage: Pharmacotherapeutic application for Alzheimer’s disease. Free Radic. Biol. Med. 2014, 75, S45. [Google Scholar] [CrossRef]
- Belkacemi, A.; Ramassamy, C. Anthocyanins Protect SK-N-SH Cells Against Acrolein-Induced Toxicity by Preserving the Cellular Redox State. J. Alzheimer's Dis. 2016, 50, 981–998. [Google Scholar] [CrossRef]
- CTenore, G.; Morisco, F.; Lembo, V.; Ritieni, A. Effect of Red Wine Polyphenols on the Expression of Transthyretin in Murine Choroid Plexus. Curr. Pharm. Biotechnol. 2016, 17, 1008–1015. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Bobermin, L.D.; Tramontina, A.C.; Wartchow, K.M.; Tagliari, B.; Souza, D.O.; Wyse, A.T.S.; Gonçalves, C.-A. Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: Neuroprotective effect of resveratrol. Toxicol. Vitr. 2014, 28, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, R.; Wang, Y.; Hu, Y.; Li, X.; Cheng, X.; Yin, Y.; Li, W.; Huang, H. Early-Life Exposure to Lead Induces Cognitive Impairment in Elder Mice Targeting SIRT1 Phosphorylation and Oxidative Alterations. Front. Physiol. 2017, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Kim, J.N.; Kim, M.K.; Lee, J.; Ignarro, L.J.; Kim, H.; Shin, C.Y.; Han, S. Melatonin synergistically increases resveratrol-induced heme oxygenase-1 expression through the inhibition of ubiquitin-dependent proteasome pathway: A possible role in neuroprotection. J. Pineal Res. 2011, 50, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Khodaie, N.; Tajuddin, N.; Mitchell, R.M.; Neafsey, E.J.; Collins, M.A. Combinatorial Preconditioning of Rat Brain Cultures with Subprotective Ethanol and Resveratrol Concentrations Promotes Synergistic Neuroprotection. Neurotox. Res. 2018, 34, 749–756. [Google Scholar] [CrossRef]
- Lavoie, S.; Chen, Y.; Dalton, T.P.; Gysin, R.; Cuénod, M.; Steullet, P.; Do, K.Q. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: Importance of the glutamate cysteine ligase modifier subunit. J. Neurochem. 2009, 108, 1410–1422. [Google Scholar] [CrossRef]
- Ho, C.-L.; Kao, N.-J.; Lin, C.-I.; Cross, T.-W.L.; Lin, S.-H. Quercetin Increases Mitochondrial Biogenesis and Reduces Free Radicals in Neuronal SH-SY5Y Cells. Nutrients 2022, 14, 3310. [Google Scholar] [CrossRef]
- Sutcliffe, T.; Winter, A.; Punessen, N.; Linseman, D. Procyanidin B2 Protects Neurons from Oxidative, Nitrosative, and Excitotoxic Stress. Antioxidants 2017, 6, 77. [Google Scholar] [CrossRef]
- Scapagnini, G.; Butterfield, D.A.; Colombrita, C.; Sultana, R.; Pascale, A.; Calabrese, V. Ethyl Ferulate, a Lipophilic Polyphenol, Induces HO-1 and Protects Rat Neurons Against Oxidative Stress. Antioxid. Redox Signal. 2004, 6, 811–818. [Google Scholar]
- Zhang, F.; Wang, H.; Wu, Q.; Lu, Y.; Nie, J.; Xie, X.; Shi, J. Resveratrol Protects Cortical Neurons against Microglia-mediated Neuroinflammation. Phytother. Res. 2013, 27, 344–349. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; De Oliveira, A.C.P.; Gräf, S.; Bhatia, H.S.; Hüll, M.; Muñoz, E.; Fiebich, B.L. Resveratrol potently reduces prostaglandin E2production and free radical formation in lipopolysaccharide-activated primary rat microglia. J. Neuroinflammation 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Kim, G.-Y.; Park, K.-Y.; Choi, Y.H. Resveratrol Inhibits Nitric Oxide and Prostaglandin E2 Production by Lipopolysaccharide-Activated C6 Microglia. J. Med. Food 2007, 10, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Amontree, M.; Nelson, M.; Stefansson, L.; Pak, D.; Maguire-Zeiss, K.; Turner, R.S.; Conant, K. Resveratrol differentially affects MMP-9 release from neurons and glia; implications for therapeutic efficacy. J. Neurochem. 2024, 168, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Porro, C.; Moda, P.; De Nuccio, F.; Nicolardi, G.; Giannotti, L.; Panaro, M.A.; Lofrumento, D.D. Formyl Peptide Receptor (FPR)1 Modulation by Resveratrol in an LPS-Induced Neuroinflammatory Animal Model. Nutrients 2021, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Boriero, D.; Carcereri De Prati, A.; Antonini, L.; Ragno, R.; Sohji, K.; Mariotto, S.; Butturini, E. The anti-STAT1 polyphenol myricetin inhibits M1 microglia activation and counteracts neuronal death. FEBS J. 2021, 288, 2347–2359. [Google Scholar] [CrossRef]
- Namsi, A.; Nury, T.; Hamdouni, H.; Yammine, A.; Vejux, A.; Vervandier-Fasseur, D.; Latruffe, N.; Masmoudi-Kouki, O.; Lizard, G. Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases 2018, 6, 67. [Google Scholar] [CrossRef]
- Menard, C.; Bastianetto, S.; Quirion, R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front. Cell. Neurosci. 2013, 7, 281. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, T.; Ma, Y.; Huang, S.; Lei, L.; Wen, A.; Ding, Y. Resveratrol: Multi-Targets Mechanism on Neurodegenerative Diseases Based on Network Pharmacology. Front. Pharmacol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Yang, L.; Ji, T.; Zhu, C.; Liu, B.; Zhang, H.; Xu, C.; Zhang, N.; Huang, S.; et al. Neuroprotection of resveratrol against cadmium-poisoning acts through dual inhibition of mTORC1/2 signaling. Neuropharmacology 2022, 219, 109236. [Google Scholar] [CrossRef]
- Guida, N.; Laudati, G.; Anzilotti, S.; Secondo, A.; Montuori, P.; Di Renzo, G.; Canzoniero, L.M.T.; Formisano, L. Resveratrol via sirtuin-1 downregulates RE1-silencing transcription factor (REST) expression preventing PCB-95-induced neuronal cell death. Toxicol. Appl. Pharmacol. 2015, 288, 387–398. [Google Scholar] [CrossRef]
- Virgili, M.; Contestabile, A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci. Lett. 2000, 281, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.-B.; Chen, X.-Q.; Hu, G.-Y. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006, 1111, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Malinowski, B.; Węclewicz, M.M.; Grześk, E.; Grześk, G. Resveratrol Increases Serum BDNF Concentrations and Reduces Vascular Smooth Muscle Cells Contractility via a NOS-3-Independent Mechanism. BioMed Res. Int. 2017, 2017, 9202954. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, S.; Li, K.; Li, X.; Yin, X.; Pang, Z. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61, 1600587. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Kang, J.-B.; Park, D.-J.; Shah, M.-A.; Koh, P.-O. Quercetin ameliorates glutamate toxicity-induced neuronal cell death by controlling calcium-binding protein parvalbumin. J. Vet. Sci. 2022, 23, e26. [Google Scholar] [CrossRef]
- Karagac, M.S.; Ceylan, H. Neuroprotective Potential of Tannic Acid Against Neurotoxic Outputs of Monosodium Glutamate in Rat Cerebral Cortex. Neurotox. Res. 2023, 41, 670–680. [Google Scholar] [CrossRef]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef]
- Shindyapina, A.V.; Petrunia, I.V.; Komarova, T.V.; Sheshukova, E.V.; Kosorukov, V.S.; Kiryanov, G.I.; Dorokhov, Y.L. Dietary Methanol Regulates Human Gene Activity. PLoS ONE 2014, 9, e102837. [Google Scholar] [CrossRef]
- Crook, T.; Bahar, H.; Sudilovsky, A. Age-associated memory impairment: Diagnostic criteria and treatment strategies. Int. J. Neurol. 1987, 21–22, 73–82. [Google Scholar]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Yan, H.; Bai, L.; Dai, R.; Wei, W.; Zhong, C.; Xue, T.; Wang, H.; Feng, Y.; et al. Altered topological patterns of brain networks in mild cognitive impairment and Alzheimer’s disease: A resting-state fMRI study. Psychiatry Res. Neuroimaging 2012, 202, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Vislocky, L.M.; Fernandez, M.L. Biomedical effects of grape products: Nutrition Reviews©, Vol. 68, No. 11. Nutr. Rev. 2010, 68, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Keevil, J.G.; Wiebe, D.A.; Aeschlimann, S.; Folts, J.D. Purple Grape Juice Improves Endothelial Function and Reduces the Susceptibility of LDL Cholesterol to Oxidation in Patients With Coronary Artery Disease. Circulation 1999, 100, 1050–1055. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.-E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols From Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. Ser. A 2019, 74, 996–1007. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a polyphenol-rich grape and blueberry extract (MemophenolTM) on cognitive function in older adults with mild cognitive impairment: A randomized, double-blind, placebo-controlled study. Front. Psychol. 2023, 14, 1144231. [Google Scholar] [CrossRef]
- Campane, L.Z.; Nucci, M.P.; Nishiyama, M.; Von Zuben, M.; Amaro, E., Jr.; Da Luz, P.L. Long term effects of red wine consumption in brain: An MRI, fMRI and neuropsychological evaluation study. Nutr. Neurosci. 2023, 26, 901–912. [Google Scholar] [CrossRef]
- Khachaturian, Z.S. Diagnosis of Alzheimer’s Disease. Arch. Neurol. 1985, 42, 1097–1105. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Muñoz, J.P.; Barbeito, L. The Molecular Bases of Alzheimer’s Disease and Other Neurodegenerative Disorders. Arch. Med. Res. 2001, 32, 367–381. [Google Scholar] [CrossRef]
- Bretsky, P.; Guralnik, J.M.; Launer, L.; Albert, M.; Seeman, T.E. The role of APOE-ε4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology 2003, 60, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.R.; Schiehser, D.M.; Weissberger, G.H.; Salmon, D.P.; Delis, D.C.; Bondi, M.W. Specific Measures of Executive Function Predict Cognitive Decline in Older Adults. J. Int. Neuropsychol. Soc. 2012, 18, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Maria Pasinetti, G. Moderate consumption of Cabernet Sauvignon attenuates A neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; et al. Heterogeneity in Red Wine Polyphenolic Contents Differentially Influences Alzheimer’s Disease-type Neuropathology and Cognitive Deterioration. J. Alzheimer's Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Hayden, E.Y.; Yamin, G.; Beroukhim, S.; Chen, B.; Kibalchenko, M.; Jiang, L.; Ho, L.; Wang, J.; Pasinetti, G.M.; Teplow, D.B. Inhibiting amyloid β-protein assembly: Size–activity relationships among grape seed-derived polyphenols. J. Neurochem. 2015, 135, 416–430. [Google Scholar] [CrossRef]
- Ksiezak-Reding, H.; Ho, L.; Santa-Maria, I.; Diaz-Ruiz, C.; Wang, J.; Pasinetti, G.M. Ultrastructural alterations of Alzheimer’s disease paired helical filaments by grape seed-derived polyphenols. Neurobiol. Aging 2012, 33, 1427–1439. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of Grape Seed-derived Polyphenols on Amyloid β-Protein Self-assembly and Cytotoxicity*. J. Biol. Chem. 2008, 283, 32176–32187. [Google Scholar] [CrossRef]
- Rivière, C.; Richard, T.; Quentin, L.; Krisa, S.; Mérillon, J.-M.; Monti, J.-P. Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorganic Med. Chem. 2007, 15, 1160–1167. [Google Scholar] [CrossRef]
- Li, M.; Jang, J.; Sun, B.; Surh, Y. Protective Effects of Oligomers of Grape Seed Polyphenols Against β-Amyloid-Induced Oxidative Cell Death. Ann. N. Y. Acad. Sci. 2004, 1030, 317–329. [Google Scholar] [CrossRef]
- Serdar, B.S.; Erkmen, T.; Koçtürk, S. Combinations of polyphenols disaggregate Aβ1–42 by passing through in vitro blood brain barrier developed by endothelium, astrocyte, and differentiated SH-SY5Y cells. Acta Neurobiol. Exp. 2021, 81, 335–349. [Google Scholar] [CrossRef]
- Wang, J.; Santa-Maria, I.; Ho, L.; Ksiezak-Reding, H.; Ono, K.; Teplow, D.B.; Pasinetti, G.M. Grape Derived Polyphenols Attenuate Tau Neuropathology in a Mouse Model of Alzheimer’s Disease. J. Alzheimer's Dis. 2010, 22, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s Disease Mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, W.; Cheng, A.; Freire, D.; Vempati, P.; Zhao, W.; Gong, B.; Janle, E.M.; Chen, T.-Y.; Ferruzzi, M.G.; et al. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 2014, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combined treatment with the phenolics (−)-epigallocatechin-3-gallate and ferulic acid improves cognition and reduces Alzheimer-like pathology in mice. J. Biol. Chem. 2019, 294, 2714–5444. [Google Scholar] [CrossRef] [PubMed]
- Churches, Q.I.; Caine, J.; Cavanagh, K.; Epa, V.C.; Waddington, L.; Tranberg, C.E.; Meyer, A.G.; Varghese, J.N.; Streltsov, V.; Duggan, P.J. Naturally occurring polyphenolic inhibitors of amyloid beta aggregation. Bioorganic Med. Chem. Lett. 2014, 24, 3108–3112. [Google Scholar] [CrossRef]
- Sanders, H.M.; Jovcevski, B.; Marty, M.T.; Pukala, T.L. Structural and mechanistic insights into amyloid-β and α-synuclein fibril formation and polyphenol inhibitor efficacy in phospholipid bilayers. FEBS J. 2022, 289, 215–230. [Google Scholar] [CrossRef]
- Ladiwala, A.R.A.; Mora-Pale, M.; Lin, J.C.; Bale, S.S.; Fishman, Z.S.; Dordick, J.S.; Tessier, P.M. Polyphenolic Glycosides and Aglycones Utilize Opposing Pathways To Selectively Remodel and Inactivate Toxic Oligomers of Amyloid β. ChemBioChem 2011, 12, 1749–1758. [Google Scholar] [CrossRef]
- on behalf of the Neurophenols Consortium; Dal-Pan, A.; Dudonné, S.; Bourassa, P.; Bourdoulous, M.; Tremblay, C.; Desjardins, Y.; Calon, F. Cognitive-Enhancing Effects of a Polyphenols-Rich Extract from Fruits without Changes in Neuropathology in an Animal Model of Alzheimer’s Disease. J. Alzheimer's Dis. 2016, 55, 115–135. [Google Scholar] [CrossRef]
- Hutton, C.P.; Lemon, J.A.; Sakic, B.; Rollo, C.D.; Boreham, D.R.; Fahnestock, M.; Wojtowicz, J.M.; Becker, S. Early Intervention with a Multi-Ingredient Dietary Supplement Improves Mood and Spatial Memory in a Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer's Dis. 2018, 64, 835–857. [Google Scholar] [CrossRef]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-β1–42 in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Yang, S.; Wang, Y.; Zhang, X.; Du, X.; Sun, X.; Zhao, M.; Huang, L.; Liu, R. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Jang, J. Protective effect of resveratrol on β-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med. 2003, 34, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Mi, M. Resveratrol Attenuates Aβ25–35 Caused Neurotoxicity by Inducing Autophagy Through the TyrRS-PARP1-SIRT1 Signaling Pathway. Neurochem. Res. 2016, 41, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-mediated cleavage of amyloid β1–42 peptide: Potential relevance to Alzheimer’s disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-L.; Yin, H.-H.; Xiao, C.; Bernards, M.T.; He, Y.; Guan, Y.-X. Understanding the Molecular Mechanisms of Polyphenol Inhibition of Amyloid β Aggregation. ACS Chem. Neurosci. 2023, 14, 4051–4061. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L. Resveratrol Suppresses Aβ-Induced Microglial Activation Through the TXNIP/TRX/NLRP3 Signaling Pathway. DNA Cell Biol. 2019, 38, 874–879. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Front. Neurosci. 2017, 10, 598. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, X.; Rui, Y.; Zhang, Z.; Qin, L.; Han, S.; Wan, Z. The combination of 1α,25dihydroxyvitaminD3 with resveratrol improves neuronal degeneration by regulating endoplasmic reticulum stress, insulin signaling and inhibiting tau hyperphosphorylation in SH-SY5Y cells. Food Chem. Toxicol. 2016, 93, 32–40. [Google Scholar] [CrossRef]
- Vion, E.; Page, G.; Bourdeaud, E.; Paccalin, M.; Guillard, J.; Rioux Bilan, A. Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 88, 1–6. [Google Scholar] [CrossRef]
- Richard, T.; Papastamoulis, Y.; Waffo-Teguo, P.; Monti, J.-P. 3D NMR structure of a complex between the amyloid beta peptide (1–40) and the polyphenol ε-viniferin glucoside: Implications in Alzheimer’s disease. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 5068–5074. [Google Scholar] [CrossRef]
- Qi, Y.; Shang, L.; Liao, Z.; Su, H.; Jing, H.; Wu, B.; Bi, K.; Jia, Y. Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metab. Brain Dis. 2019, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.-L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009, 54, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Sarroca, S.; Gatius, A.; Rodríguez-Farré, E.; Vilchez, D.; Pallàs, M.; Griñán-Ferré, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569. [Google Scholar] [CrossRef] [PubMed]
- Gacar, N.; Mutlu, O.; Utkan, T.; Komsuoglu Celikyurt, I.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. AGE 2013, 35, 1851–1865. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, G.; Liang, Z.; Sheng, S.; Shi, Y.; Peng, L.; Wang, Y.; Wang, F.; Zhang, X. Resveratrol improves cognition and decreases amyloid plaque formation in Tg6799 mice. Mol. Med. Rep. 2019, 19, 3783–3790. [Google Scholar] [CrossRef]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dußling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur. J. Nutr. 2016, 55, 741–747. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol Promotes Clearance of Alzheimer’s Disease Amyloid-β Peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. AMP-activated Protein Kinase Signaling Activation by Resveratrol Modulates Amyloid-β Peptide Metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Izquierdo-Ramírez, P.J.; Griñán-Ferré, C.; Pallàs, M.; Martín, M.; Albasanz, J.L. Neuroprotective Effects of Resveratrol by Modifying Cholesterol Metabolism and Aβ Processing in SAMP8 Mice. Int. J. Mol. Sci. 2022, 23, 7580. [Google Scholar] [CrossRef]
- Porquet, D.; Griñán-Ferré, C.; Ferrer, I.; Camins, A.; Sanfeliu, C.; Del Valle, J.; Pallàs, M. Neuroprotective Role of Trans-Resveratrol in a Murine Model of Familial Alzheimer’s Disease. J. Alzheimer's Dis. 2014, 42, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Capiralla, H.; Vingtdeux, V.; Zhao, H.; Sankowski, R.; Al-Abed, Y.; Davies, P.; Marambaud, P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J. Neurochem. 2012, 120, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Olivieri, G.; Meier, F.; Seifritz, E.; Wirz-Justice, A.; Müller-Spahn, F. Red Wine Ingredient Resveratrol Protects from β-Amyloid Neurotoxicity. Gerontology 2003, 49, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, L.; Pan, X.; Chen, J.; Wang, L.; Wang, W.; Cheng, R.; Wu, F.; Feng, X.; Yu, Y.; et al. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget 2016, 7, 17380–17392. [Google Scholar] [CrossRef]
- Umeda, T.; Sakai, A.; Shigemori, K.; Yokota, A.; Kumagai, T.; Tomiyama, T. Oligomer-Targeting Prevention of Neurodegenerative Dementia by Intranasal Rifampicin and Resveratrol Combination—A Preclinical Study in Model Mice. Front. Neurosci. 2021, 15, 763476. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Prediction of resveratrol target proteins: A bioinformatics analysis. J. Biomol. Struct. Dyn. 2024, 42, 1088–1097. [Google Scholar] [CrossRef]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2004, 1690, 193–202. [Google Scholar] [CrossRef]
- Yao, J.; Gao, X.; Sun, W.; Yao, T.; Shi, S.; Ji, L. Molecular Hairpin: A Possible Model for Inhibition of Tau Aggregation by Tannic Acid. Biochemistry 2013, 52, 1893–1902. [Google Scholar] [CrossRef]
- Baruah, P.; Moorthy, H.; Ramesh, M.; Padhi, D.; Govindaraju, T. A natural polyphenol activates and enhances GPX4 to mitigate amyloid-β induced ferroptosis in Alzheimer’s disease. Chem. Sci. 2023, 14, 9427–9438. [Google Scholar] [CrossRef]
- Gerzson, M.F.B.; Bona, N.P.; Soares, M.S.P.; Teixeira, F.C.; Rahmeier, F.L.; Carvalho, F.B.; Da Cruz Fernandes, M.; Onzi, G.; Lenz, G.; Gonçales, R.A.; et al. Tannic Acid Ameliorates STZ-Induced Alzheimer’s Disease-Like Impairment of Memory, Neuroinflammation, Neuronal Death and Modulates Akt Expression. Neurotox. Res. 2020, 37, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Regitz, C.; Marie Dußling, L.; Wenzel, U. Amyloid-beta (A β 1–42)-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Molaei, A.; Hatami, H.; Dehghan, G.; Sadeghian, R.; Khajehnasiri, N. Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in an animal model of Alzheimer’s disease. EXCLI J. 2020, 19, Doc596. [Google Scholar]
- Zeng, Y.-Q.; Wang, Y.-J.; Zhou, X.-F. Effects of (−) epicatechin on the pathology of APP/PS1 transgenic mice. Front. Neurol. 2014, 5, 69. [Google Scholar] [CrossRef]
- Hole, K.L.; Staniaszek, L.E.; Menon Balan, G.; Mason, J.M.; Brown, J.T.; Williams, R.J. Oral (−)-Epicatechin Inhibits Progressive Tau Pathology in rTg4510 Mice Independent of Direct Actions at GSK3β. Front. Neurosci. 2021, 15, 697319. [Google Scholar] [CrossRef]
- Tang, J.; Sun, R.; Wan, J.; Xu, Z.; Zou, Y.; Zhang, Q. Atomic insights into the inhibition of R3 domain of tau protein by epigallocatechin gallate, quercetin and gallic acid. Biophys. Chem. 2024, 305, 107142. [Google Scholar] [CrossRef]
- Shi, D.; Hao, Z.; Qi, W.; Jiang, F.; Liu, K.; Shi, X. Aerobic exercise combined with chlorogenic acid exerts neuroprotective effects and reverses cognitive decline in Alzheimer’s disease model mice (APP/PS1) via the SIRT1//PGC-1α/PPARγ signaling pathway. Front. Aging Neurosci. 2023, 15, 1269952. [Google Scholar] [CrossRef]
- Li, H.-L.; Zhang, S.-Y.; Ren, Y.-S.; Zhou, J.-C.; Zhou, Y.-X.; Huang, W.-Z.; Piao, X.-H.; Yang, Z.-Y.; Wang, S.-M.; Ge, Y.-W. Identification of ellagic acid and urolithins as natural inhibitors of Aβ25–35-induced neurotoxicity and the mechanism predication using network pharmacology analysis and molecular docking. Front. Nutr. 2022, 9, 966276. [Google Scholar] [CrossRef]
- Tan, F.H.P.; Najimudin, N.; Watanabe, N.; Shamsuddin, S.; Azzam, G. p-Coumaric acid attenuates the effects of Aβ42 in vitro and in a Drosophila Alzheimer’s disease model. Behav. Brain Res. 2023, 452, 114568. [Google Scholar] [CrossRef]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.Y.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin suppresses the toxicity of Aβ deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer’s disease. Nutr. Neurosci. 2016, 19, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin inhibits tau aggregation by interacting with the protein and preventing the formation of β-strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, S.; Kolandaivel, P. Study on the inter- and intra-peptide salt-bridge mechanism of Aβ 23–28 oligomer interaction with small molecules: QM/MM method. Mol. BioSyst. 2015, 11, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, J.; Lu, Y.; Deng, Y.; Zhao, R.; Xiao, S. Myricetin Restores Aβ-Induced Mitochondrial Impairments in N2a-SW Cells. ACS Chem. Neurosci. 2022, 13, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Delaunay, J.-C.; Immel, F.; Cullin, C.; Monti, J.-P. The Polyphenol Piceid Destabilizes Preformed Amyloid Fibrils and Oligomers In Vitro: Hypothesis on Possible Molecular Mechanisms. Neurochem. Res. 2009, 34, 1120–1128. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Yu, X.; Su, Y.; Wang, T.; Zhou, W.; Zhang, H.; Wang, Y.; Liu, R. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014, 264, 173–180. [Google Scholar] [CrossRef]
- Godos, J.; Micek, A.; Mena, P.; Del Rio, D.; Galvano, F.; Castellano, S.; Grosso, G. Dietary (Poly)phenols and Cognitive Decline: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Nutr. Food Res. 2024, 68, 2300472. [Google Scholar] [CrossRef]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.-F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.; Barberger-Gateau, P. Flavonoid Intake and Cognitive Decline over a 10-Year Period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Tosatti, J.A.G.; Fontes, A.F.D.S.; Caramelli, P.; Gomes, K.B. Effects of Resveratrol Supplementation on the Cognitive Function of Patients with Alzheimer’s Disease: A Systematic Review of Randomized Controlled Trials. Drugs Aging 2022, 39, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Pellegrini, S.; Tagliazucchi, D. Synergistic protection of PC12 cells from β-amyloid toxicity by resveratrol and catechin. Brain Res. Bull. 2003, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Stern, Y.; Cosentino, S.; Tatarina-Nulman, O.; Dorrejo, J.N.; Gu, Y. The Association Between Alcohol Use and the Progression of Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 1356–1362. [Google Scholar] [CrossRef]
- Mehlig, K.; Skoog, I.; Guo, X.; Schutze, M.; Gustafson, D.; Waern, M.; Ostling, S.; Bjorkelund, C.; Lissner, L. Alcoholic Beverages and Incidence of Dementia: 34-Year Follow-up of the Prospective Population Study of Women in Goteborg. Am. J. Epidemiol. 2007, 167, 684–691. [Google Scholar] [CrossRef]
- Handing, E.P.; Andel, R.; Kadlecova, P.; Gatz, M.; Pedersen, N.L. Midlife Alcohol Consumption and Risk of Dementia Over 43 Years of Follow-Up: A Population-Based Study From the Swedish Twin Registry. J. Gerontol. Ser. A 2015, 70, 1248–1254. [Google Scholar] [CrossRef]
- Peters, R.; Peters, J.; Warner, J.; Beckett, N.; Bulpitt, C. Alcohol, dementia and cognitive decline in the elderly: A systematic review. Age Ageing 2008, 37, 505–512. [Google Scholar] [CrossRef]
- Wojtowicz, J.S. Long-Term Health Outcomes of Regular, Moderate Red Wine Consumption. Cureus 2023, 15, e46786. [Google Scholar] [CrossRef]
- Klinedinst, B.S.; Le, S.T.; Larsen, B.; Pappas, C.; Hoth, N.J.; Pollpeter, A.; Wang, Q.; Wang, Y.; Yu, S.; Wang, L.; et al. Genetic Factors of Alzheimer’s Disease Modulate How Diet is Associated with Long-Term Cognitive Trajectories: A UK Biobank Study. J. Alzheimer's Dis. 2020, 78, 1245–1257. [Google Scholar] [CrossRef]
- Artero, S. Neuroanatomical localisation and clinical correlates of white matter lesions in the elderly. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1304–1308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuo, H.-K.; Lipsitz, L.A. Cerebral White Matter Changes and Geriatric Syndromes: Is There a Link? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, M818–M826. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Palumbo, M.; Aliano, C.; Lempereur, L.; Scoto, G.; Renis, M. Red wine micronutrients as protective agents in Alzheimer-like induced insult. Life Sci. 2003, 72, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Barker, W.W.; Luis, C.A.; Kashuba, A.; Luis, M.; Harwood, D.G.; Loewenstein, D.; Waters, C.; Jimison, P.; Shepherd, E.; Sevush, S.; et al. Relative Frequencies of Alzheimer Disease, Lewy Body, Vascular and Frontotemporal Dementia, and Hippocampal Sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 2002, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zheng, Y.; Wu, T.; Wu, C.; Cheng, X. Oral administration of grape seed polyphenol extract restores memory deficits in chronic cerebral hypoperfusion rats. Behav. Pharmacol. 2017, 28, 207–213. [Google Scholar] [CrossRef]
- Li, Z.; Fang, F.; Wang, Y.; Wang, L. Resveratrol protects CA1 neurons against focal cerebral ischemic reperfusion-induced damage via the ERK-CREB signaling pathway in rats. Pharmacol. Biochem. Behav. 2016, 146–147, 21–27. [Google Scholar] [CrossRef]
- Ozacmak, V.H.; Sayan-Ozacmak, H.; Barut, F. Chronic treatment with resveratrol, a natural polyphenol found in grapes, alleviates oxidative stress and apoptotic cell death in ovariectomized female rats subjected to chronic cerebral hypoperfusion. Nutr. Neurosci. 2016, 19, 176–186. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, P.; Rao, Y.; Chen, L. Resveratrol Reverses the Synaptic Plasticity Deficits in a Chronic Cerebral Hypoperfusion Rat Model. J. Stroke Cerebrovasc. Dis. 2016, 25, 122–128. [Google Scholar] [CrossRef]
- Sun, Z.-K.; Ma, X.-R.; Jia, Y.-J.; Liu, Y.-R.; Zhang, J.-W.; Zhang, B.-A. Effects of resveratrol on apoptosis in a rat model of vascular dementia. Exp. Ther. Med. 2014, 7, 843–848. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 377–387. [Google Scholar]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, M.; Miguel, Á.; Estruch, R.; Ros, E. Polyphenol-Rich Foods in the Mediterranean Diet are Associated with Better Cognitive Function in Elderly Subjects at High Cardiovascular Risk. J. Alzheimer’s Dis. 2012, 29, 773–782. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health—A comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Spindler, L.R.B.; Fryer, T.D.; Hong, Y.T.; Malpetti, M.; Aigbirhio, F.I.; White, S.R.; Camacho, M.; O’Brien, J.T.; Williams-Gray, C.H. Neuroinflammation is linked to dementia risk in Parkinson’s disease. Brain 2024, 147, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Tambe, M.A.; De Rus Jacquet, A.; Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Ferruzzi, M.G.; Wu, Q.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Protective effects of polyphenol-rich extracts against neurotoxicity elicited by paraquat or rotenone in cellular models of Parkinson’s disease. Antioxidants 2023, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Tikhonova, M.A.; Tikhonova, N.G.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Effects of Grape Polyphenols on the Life Span and Neuroinflammatory Alterations Related to Neurodegenerative Parkinson Disease-Like Disturbances in Mice. Molecules 2020, 25, 5339. [Google Scholar] [CrossRef]
- Pogačnik, L.; Pirc, K.; Palmela, I.; Skrt, M.; Kim, K.S.; Brites, D.; Brito, M.A.; Ulrih, N.P.; Silva, R.F.M. Potential for brain accessibility and analysis of stability of selected flavonoids in relation to neuroprotection in vitro. Brain Res. 2016, 1651, 17–26. [Google Scholar] [CrossRef]
- Bournival, J.; Quessy, P.; Martinoli, M.-G. Protective Effects of Resveratrol and Quercetin Against MPP+ -Induced Oxidative Stress Act by Modulating Markers of Apoptotic Death in Dopaminergic Neurons. Cell. Mol. Neurobiol. 2009, 29, 1169–1180. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.-S.; Zhou, H.; Wilson, B.; Hong, J.-S.; Gao, H.-M. Resveratrol Protects Dopamine Neurons Against Lipopolysaccharide-Induced Neurotoxicity through Its Anti-Inflammatory Actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Ji, M.; Liu, S.; Wu, X.; Wang, Y.; Liu, R. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef]
- Lu, K.-T.; Ko, M.-C.; Chen, B.-Y.; Huang, J.-C.; Hsieh, C.-W.; Lee, M.-C.; Chiou, R.Y.Y.; Wung, B.-S.; Peng, C.-H.; Yang, Y.-L. Neuroprotective Effects of Resveratrol on MPTP-Induced Neuron Loss Mediated by Free Radical Scavenging. J. Agric. Food Chem. 2008, 56, 6910–6913. [Google Scholar] [CrossRef]
- Ardah, M.T.; Eid, N.; Kitada, T.; Haque, M.E. Ellagic Acid Prevents α-Synuclein Aggregation and Protects SH-SY5Y Cells from Aggregated α-Synuclein-Induced Toxicity via Suppression of Apoptosis and Activation of Autophagy. Int. J. Mol. Sci. 2021, 22, 13398. [Google Scholar] [CrossRef]
- Dimpfel, W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine 2009, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Naidu, P.S.; Kulkarni, S.K. Quercetin Potentiates L-Dopa Reversal of Drug-Induced Catalepsy in Rats: Possible COMT/MAO Inhibition. Pharmacology 2003, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, F.; Hajizadeh Moghaddam, A.; Zare, M. Research Paper: Neuroprotective Effect of Quercetin Nanocrystal in a 6-Hydroxydopamine Model of Parkinson Disease: Biochemical and Behavioral Evidence. Basic. Clin. Neurosci. J. 2018, 9, 317–324. [Google Scholar] [CrossRef]
- Farag, S.; Tsang, C.; Murphy, P.N. Polyphenol supplementation and executive functioning in overweight and obese adults at risk of cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0286143. [Google Scholar] [CrossRef]
- Wang, J.; Tang, C.; Ferruzzi, M.G.; Gong, B.; Song, B.J.; Janle, E.M.; Chen, T.; Cooper, B.; Varghese, M.; Cheng, A.; et al. Role of standardized grape polyphenol preparation as a novel treatment to improve synaptic plasticity through attenuation of features of metabolic syndrome in a mouse model. Mol. Nutr. Food Res. 2013, 57, 2091–2102. [Google Scholar] [CrossRef]
- Liu, M.-H.; Yuan, C.; He, J.; Tan, T.-P.; Wu, S.-J.; Fu, H.-Y.; Liu, J.; Yu, S.; Chen, Y.-D.; Le, Q.-F.; et al. Resveratrol Protects PC12 Cells from High Glucose-Induced Neurotoxicity Via PI3K/Akt/FoxO3a Pathway. Cell. Mol. Neurobiol. 2015, 35, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, R.; Oh, A.; Hou, L.; Lee, E.; Aguilar, J.; Li, A.; Mack, W.J. Flavonoid quercetin and its glucuronide and sulfate conjugates bind to 67-kDa laminin receptor and prevent neuronal cell death induced by serum starvation. Biochem. Biophys. Res. Commun. 2023, 671, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Francoeur, M.-A.; Renaud, J.; Martinoli, M.-G. Quercetin and Sesamin Protect Neuronal PC12 Cells from High-Glucose-Induced Oxidation, Nitrosative Stress, and Apoptosis. Rejuvenation Res. 2012, 15, 322–333. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Ren, G.; Yin, L.; Liang, X.; Geng, T.; Dang, H.; An, R. Resveratrol limits diabetes-associated cognitive decline in rats by preventing oxidative stress and inflammation and modulating hippocampal structural synaptic plasticity. Brain Res. 2016, 1650, 1–9. [Google Scholar] [CrossRef]
- Thomas, J.; Garg, M.L.; Smith, D.W. Dietary resveratrol supplementation normalizes gene expression in the hippocampus of streptozotocin-induced diabetic C57Bl/6 mice. J. Nutr. Biochem. 2014, 25, 313–318. [Google Scholar] [CrossRef]
- Evans, H.; Howe, P.; Wong, R. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Huhn, S.; Beyer, F.; Zhang, R.; Lampe, L.; Grothe, J.; Kratzsch, J.; Willenberg, A.; Breitfeld, J.; Kovacs, P.; Stumvoll, M.; et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults—A randomized controlled trial. NeuroImage 2018, 174, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Ebner, N.; Dzierzewski, J.M.; Zlatar, Z.Z.; Gurka, M.J.; Dotson, V.M.; Kirton, J.; Mankowski, R.T.; Marsiske, M.; Manini, T.M. Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. J. Altern. Complement. Med. 2018, 24, 725–732. [Google Scholar] [CrossRef] [PubMed]
- De Vries, K.; Medawar, E.; Korosi, A.; Witte, A.V. The Effect of Polyphenols on Working and Episodic Memory in Non-pathological and Pathological Aging: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 8, 720756. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Asseburg, H.; Schäfer, C.; Müller, M.; Hagl, S.; Pohland, M.; Berressem, D.; Borchiellini, M.; Plank, C.; Eckert, G.P. Effects of Grape Skin Extract on Age-Related Mitochondrial Dysfunction, Memory and Life Span in C57BL/6J Mice. Neuromol. Med. 2016, 18, 378–395. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef]
- Fragua, V.; Lepoudère, A.; Leray, V.; Baron, C.; Araujo, J.A.; Nguyen, P.; Milgram, N.W. Effects of dietary supplementation with a mixed blueberry and grape extract on working memory in aged beagle dogs. J. Nutr. Sci. 2017, 6, e35. [Google Scholar] [CrossRef]
- Bensalem, J.; Servant, L.; Alfos, S.; Gaudout, D.; Layé, S.; Lafenetre, P.; Pallet, V. Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice. Front. Behav. Neurosci. 2016, 10, 9. [Google Scholar] [CrossRef]
- Jalloh, A.; Flowers, A.; Hudson, C.; Chaput, D.; Guergues, J.; Stevens, S.M.; Bickford, P. Polyphenol Supplementation Reverses Age-Related Changes in Microglial Signaling Cascades. Int. J. Mol. Sci. 2021, 22, 6373. [Google Scholar] [CrossRef]
- Chou, L.-M.; Lin, C.-I.; Chen, Y.-H.; Liao, H.; Lin, S.-H. A diet containing grape powder ameliorates the cognitive decline in aged rats with a long-term high-fructose-high-fat dietary pattern. J. Nutr. Biochem. 2016, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Juarez, D.; Arteaga, I.; Cortes, H.; Vazquez-Roque, R.; Lopez-Lopez, G.; Flores, G.; Treviño, S.; Guevara, J.; Diaz, A. Chronic resveratrol administration reduces oxidative stress and brain cell loss and improves memory of recognition in old rats. Synapse 2023, 77, e22271. [Google Scholar] [CrossRef] [PubMed]
- Gocmez, S.S.; Gacar, N.; Utkan, T.; Gacar, G.; Scarpace, P.J.; Tumer, N. Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol. Learn. Mem. 2016, 131, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Melgar, A.; Albasanz, J.L.; Palomera-Ávalos, V.; Pallàs, M.; Martín, M. Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol. Neurobiol. 2019, 56, 2881–2895. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Li, W.-F.; Li, F.; Zhang, Z.; Dai, Y.-D.; Xu, A.-L.; Qi, C.; Gao, J.-M.; Gao, J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem. Biophys. Res. Commun. 2013, 435, 597–602. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Albasanz, J.L.; Pallàs, M.; Martín, M. Resveratrol Differently Modulates Group I Metabotropic Glutamate Receptors Depending on Age in SAMP8 Mice. ACS Chem. Neurosci. 2020, 11, 1770–1780. [Google Scholar] [CrossRef]
- Sarubbo, F.; Ramis, M.R.; Aparicio, S.; Ruiz, L.; Esteban, S.; Miralles, A.; Moranta, D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. AGE 2015, 37, 37. [Google Scholar] [CrossRef]
- Frolinger, T.; Smith, C.; Cobo, C.F.; Sims, S.; Brathwaite, J.; Boer, S.; Huang, J.; Pasinetti, G.M. Dietary polyphenols promote resilience against sleep deprivation-induced cognitive impairment by activating protein translation. FASEB J. 2018, 32, 5390–5404. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, J.; Bi, W.; Ferruzzi, M.; Yemul, S.; Freire, D.; Mazzola, P.; Ho, L.; Dubner, L.; Pasinetti, G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015, 89, 191–197. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Bai, X.; Xie, Y.; Zhang, T.; Bo, S.; Chen, X. Resveratrol reversed chronic restraint stress-induced impaired cognitive function in rats. Mol. Med. Rep. 2017, 16, 2095–2100. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.-D.; Dong, C.M.; Ho, L.C.; Lam, C.T.W.; Zhou, X.-D.; Wu, E.X.; Zhou, Z.-J.; Wang, X.-M.; Zhang, Z.-J. Resveratrol, a natural polyphenol, prevents chemotherapy-induced cognitive impairment: Involvement of cytokine modulation and neuroprotection. Neurobiol. Dis. 2018, 114, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-D.; Chen, X.; Yang, L.-J.; Gao, X.-R.; Xia, Q.-R.; Qi, C.-C.; Ge, J.-F. Resveratrol ameliorates learning and memory impairments induced by bilateral hippocampal injection of streptozotocin in mice. Neurochem. Int. 2022, 159, 105385. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.-T. Alcohol consumption and dementia risk: A dose–response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Feng, Y. Alcohol consumption and risk of Alzheimer’s disease: A dose–response meta-analysis. Geriatr. Gerontol. Int 2022, 22, 278–285. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Mahmoudinezhad, M.; Faghfouri, A.H.; Mohammadzadeh Honarvar, N.; Regestein, Q.R.; Papatheodorou, S.I.; Mekary, R.A.; Willett, W.C. Alcohol consumption in relation to cognitive dysfunction and dementia: A systematic review and dose-response meta-analysis of comparative longitudinal studies. Ageing Res. Rev. 2024, 100, 102419. [Google Scholar] [CrossRef]
- Lao, Y.; Hou, L.; Li, J.; Hui, X.; Yan, P.; Yang, K. Association between alcohol intake, mild cognitive impairment and progression to dementia: A dose–response meta-analysis. Aging Clin. Exp. Res. 2021, 33, 1175–1185. [Google Scholar] [CrossRef]
- Lucerón-Lucas-Torres, M.; Saz-Lara, A.; Díez-Fernández, A.; Martínez-García, I.; Martínez-Vizcaíno, V.; Cavero-Redondo, I.; Álvarez-Bueno, C. Association between Wine Consumption with Cardiovascular Disease and Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2785. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- Pereira, Q.C.; Dos Santos, T.W.; Fortunato, I.M.; Ribeiro, M.L. The Molecular Mechanism of Polyphenols in the Regulation of Ageing Hallmarks. Int. J. Mol. Sci. 2023, 24, 5508. [Google Scholar] [CrossRef]
- Song, B.; Wang, H.; Xia, W.; Zheng, B.; Li, T.; Liu, R.H. Combination of apple peel and blueberry extracts synergistically induced lifespan extension via DAF-16 in Caenorhabditis elegans. Food Funct. 2020, 11, 6170–6185. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; ElSayed, A.; Beger, B.; Naidoo, P.; Shilton, T.; Jain, N.; Armstrong-Walenczak, K.; Mwangi, J.; Wang, Y.; Eiselé, J.-L.; et al. The Impact of Alcohol Consumption on Cardiovascular Health: Myths and Measures. Glob. Heart 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; Hayes, A.; Fonville, L.; Zafar, R.; Palmer, E.O.C.; Paterson, L.; Lingford-Hughes, A. Alcohol and the Brain. Nutrients 2021, 13, 3938. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Bouvard, V.; Nethan, S.T.; Freudenheim, J.L.; Abnet, C.C.; English, D.R.; Rehm, J.; Balbo, S.; Buykx, P.; Crabb, D.; et al. The IARC Perspective on Alcohol Reduction or Cessation and Cancer Risk. N. Engl. J. Med. 2023, 389, 2486–2494. [Google Scholar] [CrossRef]
- Anderson, B.O.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D.; et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Minzer, S.; Estruch, R.; Casas, R. Wine Intake in the Framework of a Mediterranean Diet and Chronic Non-Communicable Diseases: A Short Literature Review of the Last 5 Years. Molecules 2020, 25, 5045. [Google Scholar] [CrossRef]
- Godos, J.; Scazzina, F.; Paternò Castello, C.; Giampieri, F.; Quiles, J.L.; Briones Urbano, M.; Battino, M.; Galvano, F.; Iacoviello, L.; De Gaetano, G.; et al. Underrated aspects of a true Mediterranean diet: Understanding traditional features for worldwide application of a “Planeterranean” diet. J. Transl. Med. 2024, 22, 294. [Google Scholar] [CrossRef]
- Letenneur, L. Risk of Dementia and Alcohol and Wine Consumption: A Review of Recent Results. Biol. Res. 2004, 37, 189–193. [Google Scholar] [CrossRef]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of Wine Consumption with Alzheimer’s Disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vauzour, D. Wine Polyphenols and Neurodegenerative Diseases: An Update on the Molecular Mechanisms Underpinning Their Protective Effects. Beverages 2018, 4, 96. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2021, 8, 1. [Google Scholar] [CrossRef]
- Jin, S.; Guan, X.; Min, D. Evidence of Clinical Efficacy and Pharmacological Mechanisms of Resveratrol in the Treatment of Alzheimer’s Disease. Curr. Alzheimer Res. 2023, 20, 588–602. [Google Scholar] [CrossRef]
| Red Wine Polyphenols | Total Content (mg/100 mL) |
|---|---|
| Flavonoids | 82.50 |
| Anthocyanins | 22.34 |
| Dihydroflavonols | 5.44 |
| Flavanols | 53.86 |
| Flavanones | 0.86 |
| Phenolic acids | 17.15 |
| Hydroxybenzoic acids | 7.00 |
| Hydroxycinnamic acids | 9.99 |
| Hydroxyphenylacetic acids | 0.16 |
| Stilbenes | 4.36 |
| Other polyphenols | 4.37 |
| Hydroxybenzaldehydes | 0.71 |
| Tyrosols | 3.66 |
| All polyphenols | 108.38 |
| Polyphenol | Neurocognitive Disorders | Study Model | Study Period | Dosage | Molecular Effects |
|---|---|---|---|---|---|
| Resveratrol | Alzheimer’s disease | In vitro (SH-SY5Y) | 48 h | 1 μM | Effects on Aβ: disruption of Aβ1–42 aggregation by inducing its fragmentation into smaller peptides and interfering with the Aβ17–42 pentamer [156,157,167,168,169,170,171,172,173] |
| In vivo (mice models) | 2–10 months | 0.35–1% and 1 g/kg (diet or water) | |||
| In vitro (N2a and SH-SY5Y) | 30 min and 8 h | 10–25 μM | Effects on tau: reduced phosphorylation and increased activity of phosphatases [159,160,178,179] | ||
| In vivo (wild-type mice) | 2 weeks | 25 mg/kg (daily intraperitoneal injections) | |||
| In vitro (PC12 cells) | 2–24 h | 20–40 μM | Neuroprotective effects: a reduction in oxidative stress, microglial activation, apoptotic pathways activation, inhibition of neurotrophic pathways and mitochondrial dysfunction, and induction of autophagy [152,153,154,158,163,164,165,166,174,175,176,177] | ||
| In vivo (mice models) | 2–10 months | 0.35–1% and 1 g/kg (diet or water) | |||
| Vascular dementia | In vivo (rat models) | 1 h to 4 weeks | 30–40 mg/kg (intraperitoneal injections) and daily oral dose of 25 mg/kg | A reduction in lipid peroxidation and apoptotic pathways’ activation, restoration of reduced glutathione levels, and improvement in synaptic transmission and spinogenesis [218,219,220,221] | |
| Parkinson’s disease dementia | In vitro (PC12 cells, SH-SY5Y, and dopaminergic neurons) | 30 min to 72 h | 0.1–60 μM | Inhibition of α-synuclein aggregation and cytotoxicity, a reduction in oxidative status and apoptotic pathways’ activation, augmentation of the level of tyrosine hydroxylase, and attenuation of inflammation [229,230,231,232] | |
| In vivo (rat and mice models) | 1–5 weeks | 20 mg/kg intravenous and 10–50 mg/kg/day | |||
| Quercetin | Alzheimer’s disease | In vitro (primary neuron cultures) | 0.1–5 μM | Direct effects Aβ and tau pathology and a reduction in oxidative stress, microgliosis, and astrocytosis [184,185,186,189] | |
| In vivo (rat models) | 1 week | 80 mg/kg (intraperitoneal injections) | |||
| Vascular dementia | In vitro (rat vascular smooth muscle) | 20–90 min | 1–100 μM | Improvement in endothelial function and a reduction in blood pressure through inhibition of angiotensin-converting enzyme activity and by increasing NO bioavailability [222] | |
| In vivo (rat models) | 5–45 min | 14.7 µmol/kg intravenous and 88.7 µmol/kg oral administration | |||
| Parkinson’s disease dementia | In vitro (PC12 cells) | 3 h | 0.1 μM | A reduction in α-synuclein aggregation, oxidative stress, and apoptotic pathways’ activation and incrementation of autophagic clearance [229,234,235,236] | |
| In vivo (rat models) | 30–120 min to 4 weeks | 25–100 mg/kg oral administration | |||
| Ellagic acid | Alzheimer’s disease | In vitro (PC12 cells) | 24 h | 0.1–1 μM | A reduction in oxidative stress and apoptotic pathways’ activation and promotion of neurite outgrowth [191] |
| Parkinson’s disease dementia | In vitro (SH-SY5Y) | 1–48 h | 25–150 μM | Reducing α-synuclein aggregation and apoptotic pathways, and incrementation of autophagic clearance [233] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccardi, V.; Tagliafico, L.; Persia, A.; Page, E.; Ottaviani, S.; Cremonini, A.L.; Borgarelli, C.; Pisciotta, L.; Mecocci, P.; Nencioni, A.; et al. The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review. Nutrients 2024, 16, 3431. https://doi.org/10.3390/nu16203431
Boccardi V, Tagliafico L, Persia A, Page E, Ottaviani S, Cremonini AL, Borgarelli C, Pisciotta L, Mecocci P, Nencioni A, et al. The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review. Nutrients. 2024; 16(20):3431. https://doi.org/10.3390/nu16203431
Chicago/Turabian StyleBoccardi, Virginia, Luca Tagliafico, Angelica Persia, Elena Page, Silvia Ottaviani, Anna Laura Cremonini, Consuelo Borgarelli, Livia Pisciotta, Patrizia Mecocci, Alessio Nencioni, and et al. 2024. "The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review" Nutrients 16, no. 20: 3431. https://doi.org/10.3390/nu16203431
APA StyleBoccardi, V., Tagliafico, L., Persia, A., Page, E., Ottaviani, S., Cremonini, A. L., Borgarelli, C., Pisciotta, L., Mecocci, P., Nencioni, A., & Monacelli, F. (2024). The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review. Nutrients, 16(20), 3431. https://doi.org/10.3390/nu16203431










