Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients
Highlights
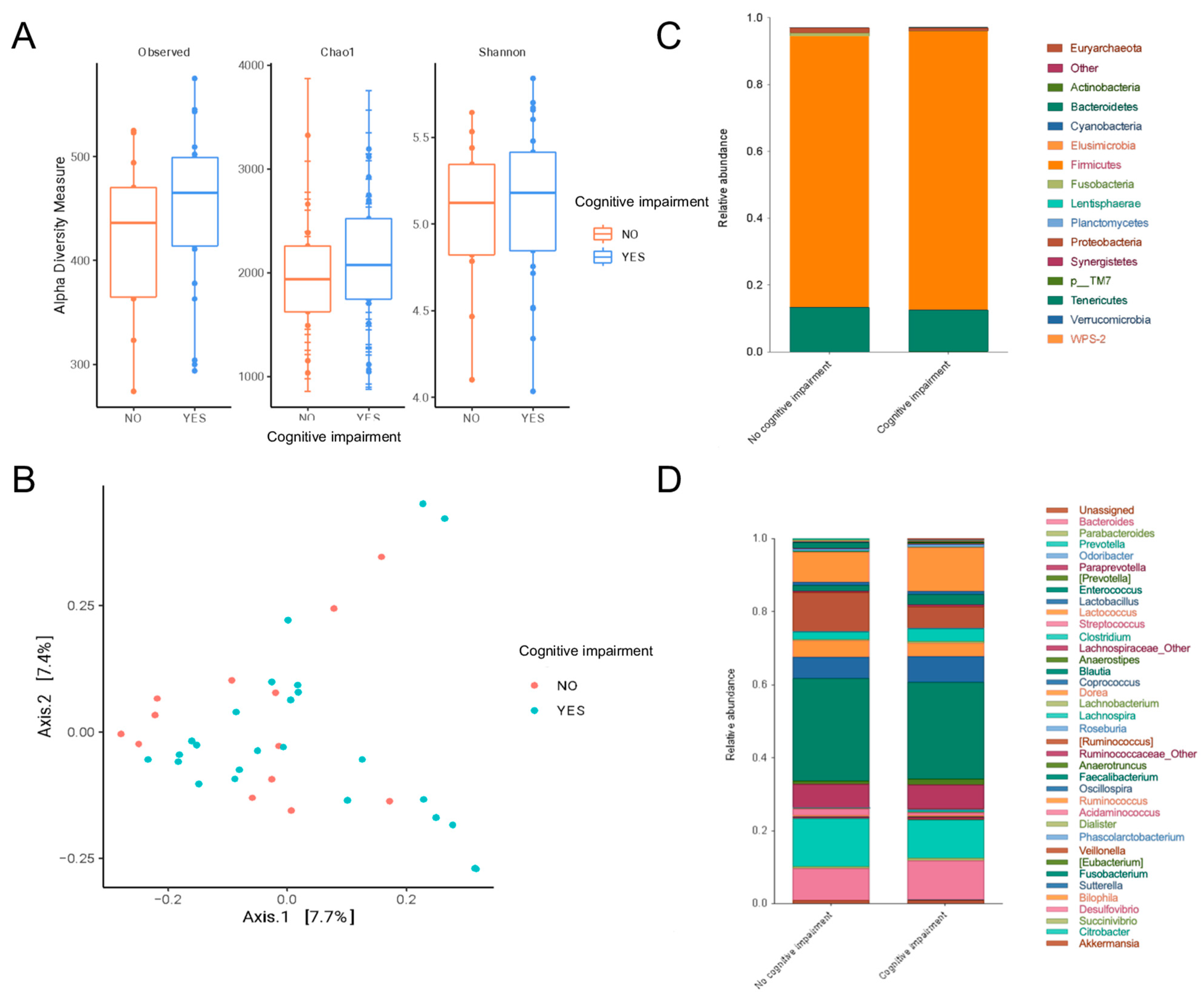
- Peritoneal dialysis patients with mild cognitive impairment had a gut microbiota enriched in Odoribacter, Anaerotruncus, S24_7 and Rikenellaceae.
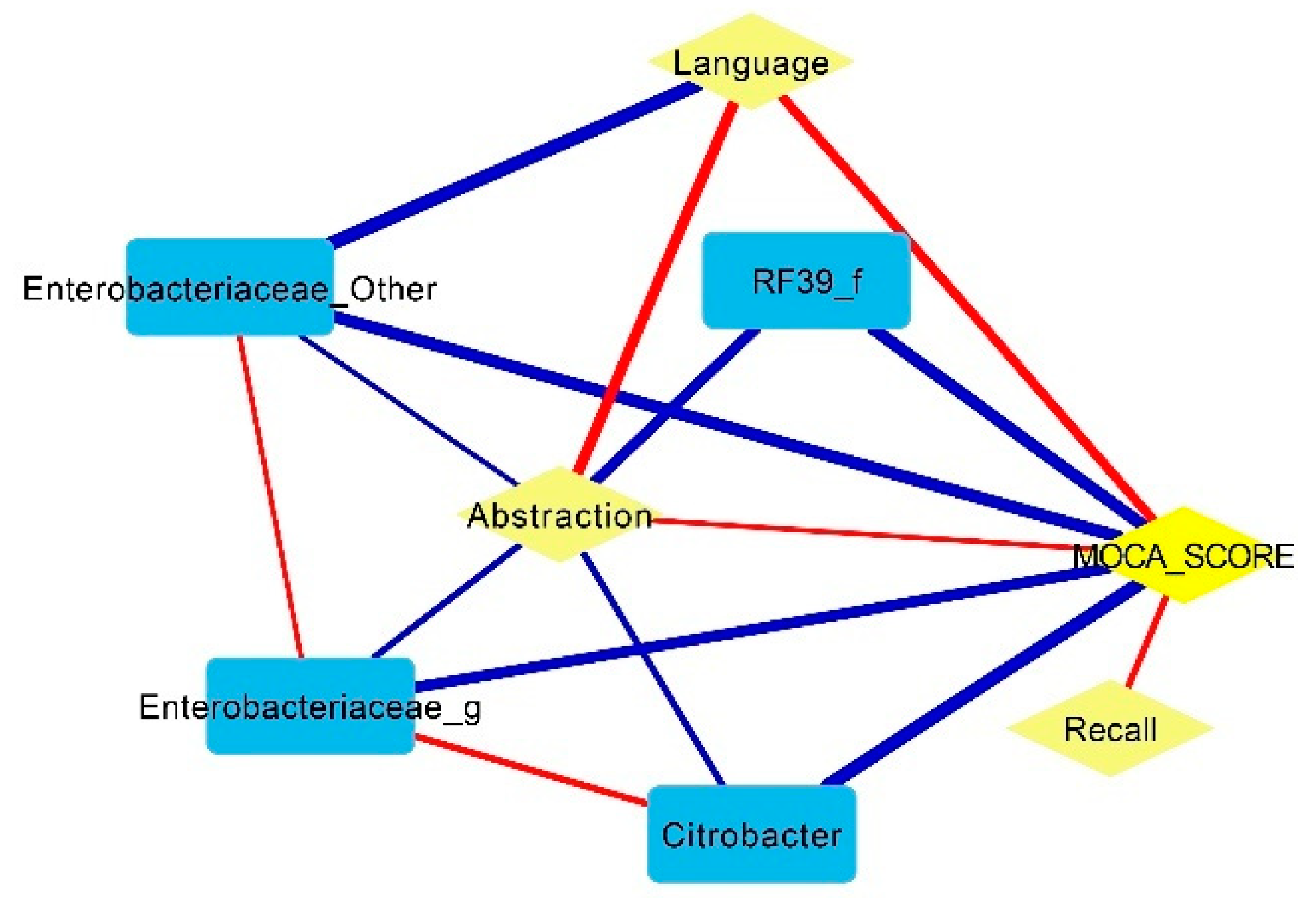
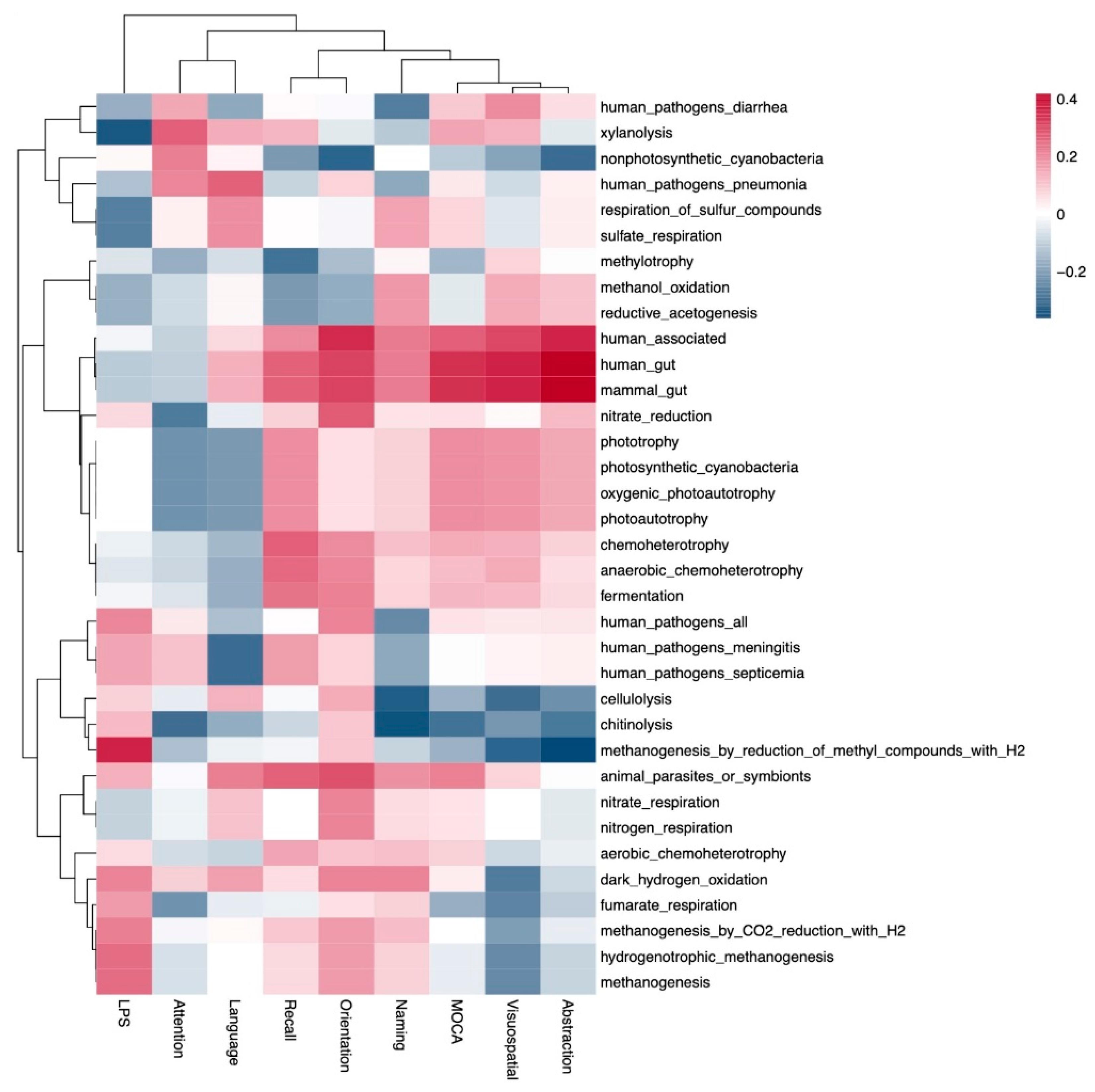
- Some pathobionts such as Enterobacteriaceae and Citrobacter had a negative correlation with cognitive function (abstraction and language of MoCA scores), while some short-chain-fatty-acid-producing bacteria such as Prevotella and Bifidobacterium were positively associated with cognitive function.
- Mucolytic bacteria such as Odoribacter, Intestinibacter and UBA1819 were associated with cognitive impairment after adjustments based on glucose levels and age.
- This study shows evidence linking gut metabolism with cognitive function in patients on peritoneal dialysis.
- Further studies on dietary interventions aimed at modifying the gut microbiota are needed to evaluate their impact on cognition in peritoneal dialysis patients.
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, I.; Wu, S.; Masson, P.; Kelly, P.J.; Duthie, F.A.; Whiteley, W.; Parker, D.; Gillespie, D.; Webster, A.C. Cognition in chronic kidney disease: A systematic review and meta-analysis. BMC Med. 2016, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; An, R.; Wang, Y.; Lei, J.; Liang, J.; Wan, Q. Risk factors and prevalence of cognitive impairment in maintenance haemodialysis patients: A systematic review and meta-analysis of observational studies. J. Adv. Nurs. 2023, 79, 3691–3706. [Google Scholar] [CrossRef] [PubMed]
- Shea, Y.F.; Lee, M.C.; Mok, M.M.; Chan, F.H.; Chan, T.M. Prevalence of cognitive impairment among peritoneal dialysis patients: A systematic review and meta-analysis. Clin. Exp. Nephrol. 2019, 23, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Félix, N.A.; Martin-del-Campo, F.; Cueto-Manzano, A.M.; Romo-Flores, M.L.; Velázquez-Vidaurri, A.L.; Sánchez-Soriano, A.; Ruvalcaba-Contreras, N.; Calderón-Fabian, A.; Rojas-Campos, E.; Cortés-Sanabria, L. Prevalence of mild cognitive impairment in automated peritoneal dialysis patients. Nephrol. Dial. Transpl. 2021, 36, 2106–2111. [Google Scholar] [CrossRef] [PubMed]
- Shea, Y.F.; Lee, M.S.; Mok, M.Y.; Lam, M.F.; Chu, L.W.; Chan, F.H.; Chan, T.M. Self-Care Peritoneal Dialysis Patients with Cognitive Impairment Have a Higher Risk of Peritonitis in the Second Year. Perit. Dial. Int. 2019, 39, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yi, C.; Wu, M.; Qiu, Y.; Wu, H.; Ye, H.; Peng, Y.; Xiao, X.; Lin, J.; Yu, X.; et al. Risk Factors and Clinical Outcomes of Cognitive Impairment in Diabetic Patients Undergoing Peritoneal Dialysis. Kidney Blood Press. Res. 2021, 46, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Tong, S.; Chu, X.; Feng, T.; Geng, M. Chronic Kidney Disease and Cognitive Impairment: The Kidney-Brain Axis. Kidney Dis. 2022, 8, 275–285. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Lun, H.; Yang, W.; Zhao, S.; Jiang, M.; Xu, M.; Liu, F.; Wang, Y. Altered gut microbiota and microbial biomarkers associated with chronic kidney disease. Microbiologyopen 2019, 8, e00678. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.I.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Meijers, B.; Evenepoel, P.; Anders, H.J. Intestinal microbiome and fitness in kidney disease. Nat. Rev. Nephrol. 2019, 15, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: Regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 9816–9842. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhao, W.; Lin, Z.; Wu, J.; Lin, H.; Li, Y.; Song, J.; Zhang, J.; Peng, H. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front. Cell. Infect. Microbiol. 2021, 11, 579386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Shen, J.; Jiang, R.; Jin, L.; Zhan, G.; Liu, J.; Sha, Q.; Xu, R.; Miao, L.; Yang, C. Abnormalities in gut microbiota and serum metabolites in hemodialysis patients with mild cognitive decline: A single-center observational study. Psychopharmacology 2020, 237, 2739–2752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Zheng, L.J.; Liu, Y.; Ye, Y.B.; Luo, S.; Lu, G.M.; Gong, D.; Zhang, L.J. The gut microbiota-inflammation-brain axis in end-stage renal disease: Perspectives from default mode network. Theranostics 2019, 9, 8171–8181. [Google Scholar] [CrossRef]
- Yang, Q.; Li, R.; Zhong, Z.; Mao, H.; Fan, J.; Lin, J.; Yang, X.; Wang, X.; Li, Z.; Yu, X. Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol. Dial. Transpl. 2011, 26, 3358–3365. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation, Inc. NKF-DOQI Clinical Practice Guidelines for Peritoneal Dialysis Adequacy. Am. J. Kidney Dis. 1997, 30 (Suppl. S2), S67–S136. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, C.; Feng, H.; Yuan, J.; Ding, L.; Fang, W.; Gu, A.; Huang, J.; Li, N.; Gu, L.; et al. Clinical characteristics associated with the properties of gut microbiota in peritoneal dialysis patients. Perit. Dial. Int. 2021, 41, 298–306. [Google Scholar] [CrossRef]
- Merino-Ribas, A.; Araujo, R.; Pereira, L.; Campos, J.; Barreiros, L.; Segundo, M.A.; Silva, N.; Costa, C.F.; Quelhas-Santos, J.; Trindade, F.; et al. Vascular Calcification and the Gut and Blood Microbiome in Chronic Kidney Disease Patients on Peritoneal Dialysis: A Pilot Study. Biomolecules 2022, 12, 867. [Google Scholar] [CrossRef]
- Gao, Q.; Li, D.; Wang, Y.; Zhao, C.; Li, M.; Xiao, J.; Kang, Y.; Lin, H.; Wang, N. Analysis of intestinal flora and cognitive function in maintenance hemodialysis patients using combined 16S ribosome DNA and shotgun metagenome sequencing. Aging Clin. Exp. Res. 2024, 36, 28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.; Zhang, J.; Li, Y.; Wu, Y.; Qi, X. Correlation between gut microbiome and cognitive impairment in patients undergoing peritoneal dialysis. BMC Nephrol. 2023, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, H.; Lin, W.; Yu, H.; Yang, B.; Jiang, C.; Zhang, J.; Wu, R.; Ding, F.; Pei, M.; et al. Specific gut microbiome and metabolome changes in patients with continuous ambulatory peritoneal dialysis and comparison between patients with different dialysis vintages. Front. Med. 2024, 10, 1302352. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, N.; Han, M.; Ban, M.; Sun, T.; Xu, J. Reviewing the role of gut microbiota in the pathogenesis of depression and exploring new therapeutic options. Front. Neurosci. 2022, 16, 1029495. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chang, C.C.; Huang, C.W.; Nouchi, R.; Cheng, C.H. Gut microbiota in patients with Alzheimer’s disease spectrum: A systematic review and meta-analysis. Aging 2022, 14, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Ho Do, M.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.H.; Diop, A.; Dubourg, G.; Khelaifia, S.; Richez, M.; Armstrong, N.; Maraninchi, M.; Fournier, P.E.; Raoult, D.; Million, M. Anaerotruncus massiliensis sp. nov., a succinate-producing bacterium isolated from human stool from an obese patient after bariatric surgery. New Microbes New Infect. 2019, 29, 100508. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS Neurosci. Ther. 2023, 29, 140–157. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mo, X.; Huang, H.; Chen, X.; Liu, H.; Peng, Z.; Chen, L.; Rong, S.; Yang, W.; Xu, S.; et al. Yeast β-glucan alleviates cognitive deficit by regulating gut microbiota and metabolites in Aβ1-42-induced AD-like mice. Int. J. Biol. Macromol. 2020, 161, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, M.; Liu, Z.; Zhang, Y.; Tu, H.; Lei, L.; Wu, P.; Zhang, A.; Yang, C.; Li, G.; et al. Gut Microbiome Composition Linked to Inflammatory Factors and Cognitive Functions in First-Episode, Drug-Naive Major Depressive Disorder Patients. Front. Neurosci. 2022, 15, 800764. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hang, Z.; Cai, S.; Lei, T.; Zhang, X.; Xiao, Z.; Wang, D.; Li, Y.; Bi, W.; Yang, Y.; Deng, S.; et al. Transfer of Tumor-Bearing Mice Intestinal Flora Can Ameliorate Cognition in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2022, 86, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, Y.; Cao, W.T.; Wang, Z.; Zhang, K.; Jiang, Z.; Jia, X.; Liu, C.Y.; Lin, H.R.; Zhong, H.; et al. Gut microbiome, cognitive function and brain structure: A multi-omics integration analysis. Transl. Neurodegener. 2022, 11, 49. [Google Scholar] [CrossRef]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.; Van Der Velden, S.; Ríos-Morales, M.; Van Faassen, M.J.; Loreti, M.G.; De Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef]
- Nagai, F.; Morotomi, M.; Watanabe, Y.; Sakon, H.; Tanaka, R. Alistipes indistinctus sp. nov. and Odoribacter laneus sp. nov., common members of the human intestinal microbiota isolated from faeces. Int. J. Syst. Evol. Microbiol. 2010, 60 Pt 6, 1296–1302. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wu, P.H.; Lee, H.H.; Mubanga, M.; Chen, C.S.; Kuo, M.C.; Chiu, Y.W.; Kuo, P.L.; Hwang, S.J. Indole-3 acid increased risk of impaired cognitive function in patients receiving hemodialysis. Neurotoxicology 2019, 73, 85–91. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Huang, M.F.; Liang, S.S.; Hwang, S.J.; Tsai, J.C.; Liu, T.L.; Wu, P.H.; Yang, Y.H.; Kuo, K.C.; Kuo, M.C.; et al. Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 2016, 53, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131. [Google Scholar] [CrossRef]
- Bang, S.J.; Kim, G.; Lim, M.Y.; Song, E.J.; Jung, D.H.; Kum, J.S.; Nam, Y.D.; Park, C.S.; Seo, D.H. The influence of in vitro pectin fermentation on the human fecal microbiome. AMB Express 2018, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qiu, W.; Zhu, X.; Li, X.; Xie, Z.; Carreras, I.; Dedeoglu, A.; Van Dyke, T.; Han, Y.W.; Karimbux, N.; et al. The Periodontal Pathogen Fusobacterium nucleatum Exacerbates Alzheimer’s Pathogenesis via Specific Pathways. Front. Aging Neurosci. 2022, 14, 912709. [Google Scholar] [CrossRef]
- Zhao, J.; Ning, X.; Liu, B.; Dong, R.; Bai, M.; Sun, S. Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren. Fail. 2021, 43, 102–112. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Zhao, Y.-Y.; Pahl, M.V. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol. Dial. Transpl. 2016, 31, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.; Aarts, E.; Arias Vasquez, A.; Bloemendaal, M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol. Psychiatry 2023, 28, 5037–5061. [Google Scholar] [CrossRef]
- Cooke, M.B.; Catchlove, S.; Tooley, K.L. Examining the Influence of the Human Gut Microbiota on Cognition and Stress: A Systematic Review of the Literature. Nutrients 2022, 14, 4623. [Google Scholar] [CrossRef]
- Kohn, N.; Szopinska-Tokov, J.; Llera Arenas, A.; Beckmann, C.F.; Arias-Vasquez, A.; Aarts, E. Multivariate associative patterns between the gut microbiota and large-scale brain network connectivity. Gut Microbes 2021, 13, 2006586. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, A.; Bastiaanssen, T.F.; Cryan, J.F.; Tinahones, F.J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; Gómez-Pérez, A.M.; et al. Taxonomic and Functional Fecal Microbiota Signatures Associated with Insulin Resistance in Non-Diabetic Subjects with Overweight/Obesity within the Frame of the PREDIMED-Plus Study. Front. Endocrinol. 2022, 13, 804455. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Lin, J.S.; Mao, Y.; Chen, G.D.; Zeng, F.F.; Dong, H.L.; Jiang, Z.; Wang, J.; Xiao, C.; Shuai, M.; et al. Erythrocyte n-6 Polyunsaturated Fatty Acids, Gut Microbiota, and Incident Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care 2020, 43, 2435–2443. [Google Scholar] [CrossRef]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized akkermansia muciniphila improves irritable bowel syndrome-like symptoms and related behavioral disorders in mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, H.; Wang, Y.; Cheng, R.; Pu, F.; Yang, Y.; Li, J.; Wu, S.; Shen, X.; He, F. Heat-inactivated Lacticaseibacillus paracasei N1115 alleviates the damage due to brain function caused by long-term antibiotic cocktail exposure in mice. BMC Neurosci. 2022, 23, 38. [Google Scholar] [CrossRef]
- Chao, C.T.; Lee, S.Y.; Yang, W.S.; Chen, H.W.; Fang, C.C.; Yen, C.J.; Chiang, C.K.; Hung, K.Y.; Huang, J.W. Citrobacter peritoneal dialysis peritonitis: Rare occurrence with poor outcomes. Int. J. Med. Sci. 2013, 10, 1092–1098. [Google Scholar] [CrossRef]
- Labarthe, S.; Plancade, S.; Raguideau, S.; Plaza Oñate, F.; Le Chatelier, E.; Leclerc, M.; Laroche, B. Four functional profiles for fibre and mucin metabolism in the human gut microbiome. Microbiome 2023, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Y.; Chen, Y.S.; Liu, Z. Relationship among Parkinson’s disease, constipation, microbes, and microbiological therapy. World J. Gastroenterol. 2024, 30, 225–237. [Google Scholar] [CrossRef]
- Campbell, A.; Gdanetz, K.; Schmidt, A.W.; Schmidt, T.M. H2 generated by fermentation in the human gut microbiome influences metabolism and competitive fitness of gut butyrate producers. Microbiome 2023, 11, 133. [Google Scholar] [CrossRef]


| Variable | Normal Cognitive Function (n = 14) | Mild Cognitive Impairment (n = 25) | p |
|---|---|---|---|
| Age (years) | 38 ± 14 | 53 ± 16 | 0.006 |
| Female sex, n (%) | 2 (14) | 5 (20) | 0.66 |
| Marital status, n (%) | 0.60 | ||
| Single | 5 (36) | 5 (20) | |
| Married | 7 (50) | 18 (72) | |
| Widowed/divorced | 2 (14) | 2 (8) | |
| Educational level, n (%) | 0.49 | ||
| Elementary/middle school | 7 (50) | 17 (68) | |
| High school/technical career | 5 (36) | 5 (20) | |
| Professional | 2 (14) | 3 (12) | |
| Diabetes mellitus, n (%) | 3 (21) | 14 (56) | 0.04 |
| Hypertension, n (%) | 11 (79) | 22 (88) | 0.65 |
| Cardiovascular disease, n (%) | 1 (7) | 3 (12) | 0.63 |
| Time on peritoneal dialysis (months) | 8 (6–20) | 14 (8–48) | 0.08 |
| Urine output (mL) | 70 (0–1000) | 500 (0–700) | 0.98 |
| Systolic blood pressure (mmHg) | 137 ± 25 | 133 ± 21 | 0.64 |
| Diastolic blood pressure (mmHg) | 89 ± 18 | 82 ± 14 | 0.22 |
| Body mass index (kg/m2) | 25.9 ± 4.0 | 27.0 ± 3.6 | 0.39 |
| Constipation, n (%) | 1 (7) | 12 (48) | 0.01 |
| Gastrointestinal symptoms (score) | 10 ± 2.1 | 12 ± 3.7 | 0.18 |
| Protein energy wasting, n (%) | 9 (64) | 15 (60) | 0.79 |
| Low muscle mass *, n (%) | 2 (14) | 8 (32) | 0.22 |
| Energy intake (kcal) | 1102 ± 355 | 1221 ± 397 | 0.36 |
| Protein intake (g) | 55 ± 28 | 57 ± 20 | 0.86 |
| Fiber intake (g) | 18 ± 6 | 18 ± 8 | 0.91 |
| Variable | Normal Cognitive Function (n = 14) | Mild Cognitive Impairment (n = 25) | p |
|---|---|---|---|
| Dialysis volume (L/day) | 10 (9.7–10.7) | 10 (9.6–10) | 0.77 |
| Ultrafiltration (mL/day) | 818 (534–1800) | 862 (371–1153) | 0.55 |
| Total Kt/Vurea (L/week) | 1.75 ± 0.59 | 1.89 ± 0.42 | 0.38 |
| Residual kidney function (mL/min) | 0.08 (0–3.1) | 1.3 (0–3.0) | 0.61 |
| nPNA (g/kg) | 0.82 ± 0.20 | 0.81 ± 0.13 | 0.89 |
| Hemoglobin (g/dL) | 11.3 ± 2.6 | 11.6 ± 2.44 | 0.73 |
| Glucose (mg/dL) | 98 ± 16 | 116 ± 48 | 0.10 |
| Urea (mg/dL) | 126 ± 31 | 120 ± 32 | 0.56 |
| Creatinine (mg/dL) | 14.9 ± 5.4 | 11.3 ± 3.7 | 0.02 |
| Phosphorus (mg/dL) | 5.9 ± 1.7 | 5.2 ± 1.2 | 0.15 |
| Calcium (mg/dL) | 8.3 ± 1.4 | 8.8 ± 0.7 | 0.40 |
| Potassium (mmol/L) | 4.6 ± 0.3 | 4.5 ± 0.6 | 0.58 |
| Sodium (mmol/L) | 141 ± 2.7 | 140 ± 3.1 | 0.19 |
| Total cholesterol (mg/dL) | 169 ± 53 | 176 ± 34 | 0.60 |
| Triglycerides (mg/dL) | 145 ± 95 | 132 ± 83 | 0.66 |
| Albumin (g/dL) | 4.03 ± 0.49 | 3.89 ± 0.41 | 0.38 |
| C-reactive protein (mg/L) | 0.85 (0.57–4.8) | 1.7 (0.50–7.3) | 0.50 |
| Lipopolysaccharides (ng/mL) | 58 (44–74) | 49 (33–87) | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-del-Campo, F.; Vega-Magaña, N.; Salazar-Félix, N.A.; Cueto-Manzano, A.M.; Peña-Rodríguez, M.; Cortés-Sanabria, L.; Romo-Flores, M.L.; Rojas-Campos, E. Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients. Nutrients 2024, 16, 2659. https://doi.org/10.3390/nu16162659
Martín-del-Campo F, Vega-Magaña N, Salazar-Félix NA, Cueto-Manzano AM, Peña-Rodríguez M, Cortés-Sanabria L, Romo-Flores ML, Rojas-Campos E. Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients. Nutrients. 2024; 16(16):2659. https://doi.org/10.3390/nu16162659
Chicago/Turabian StyleMartín-del-Campo, Fabiola, Natali Vega-Magaña, Noé A. Salazar-Félix, Alfonso M. Cueto-Manzano, Marcela Peña-Rodríguez, Laura Cortés-Sanabria, María L. Romo-Flores, and Enrique Rojas-Campos. 2024. "Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients" Nutrients 16, no. 16: 2659. https://doi.org/10.3390/nu16162659
APA StyleMartín-del-Campo, F., Vega-Magaña, N., Salazar-Félix, N. A., Cueto-Manzano, A. M., Peña-Rodríguez, M., Cortés-Sanabria, L., Romo-Flores, M. L., & Rojas-Campos, E. (2024). Gut Microbiome Is Related to Cognitive Impairment in Peritoneal Dialysis Patients. Nutrients, 16(16), 2659. https://doi.org/10.3390/nu16162659







