High-Density Lipoprotein Is Located Alongside Insulin in the Islets of Langerhans of Normal and Rodent Models of Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models Utilized in This Study
2.1.1. Groups of Experimental Animal Models
Wistar Rats
Goto–Kakizaki Rats
Non-Diabetic Lean, Diabetic and Fatty Zucker Rats
2.2. Rodent Representation of Diabetes Mellitus Type 1 (T1DM)
2.3. Effect of Diet on the Cellular Density of HDL
Whole-Body Weight, and Glycemic Status
2.4. Paraffin Embedding of Tissue Samples
2.5. Immunohistochemistry of HDL in Islet Cells Using the Avidin–Biotin Complex Staining Method
2.6. The Double Labelling Immunofluorescence (IF) Method
2.7. Immunoelectron Microscopy
2.8. Morphometry of HDL, INS- and GLU-Immunoreactive Cells
2.9. HDL-Containing Beta Cell Granules
2.10. Statistical Evaluation of Data
3. Results
3.1. Comparison of Total Body Weight and Glycemic Levels of Rodent Models of Diabetes Mellitus
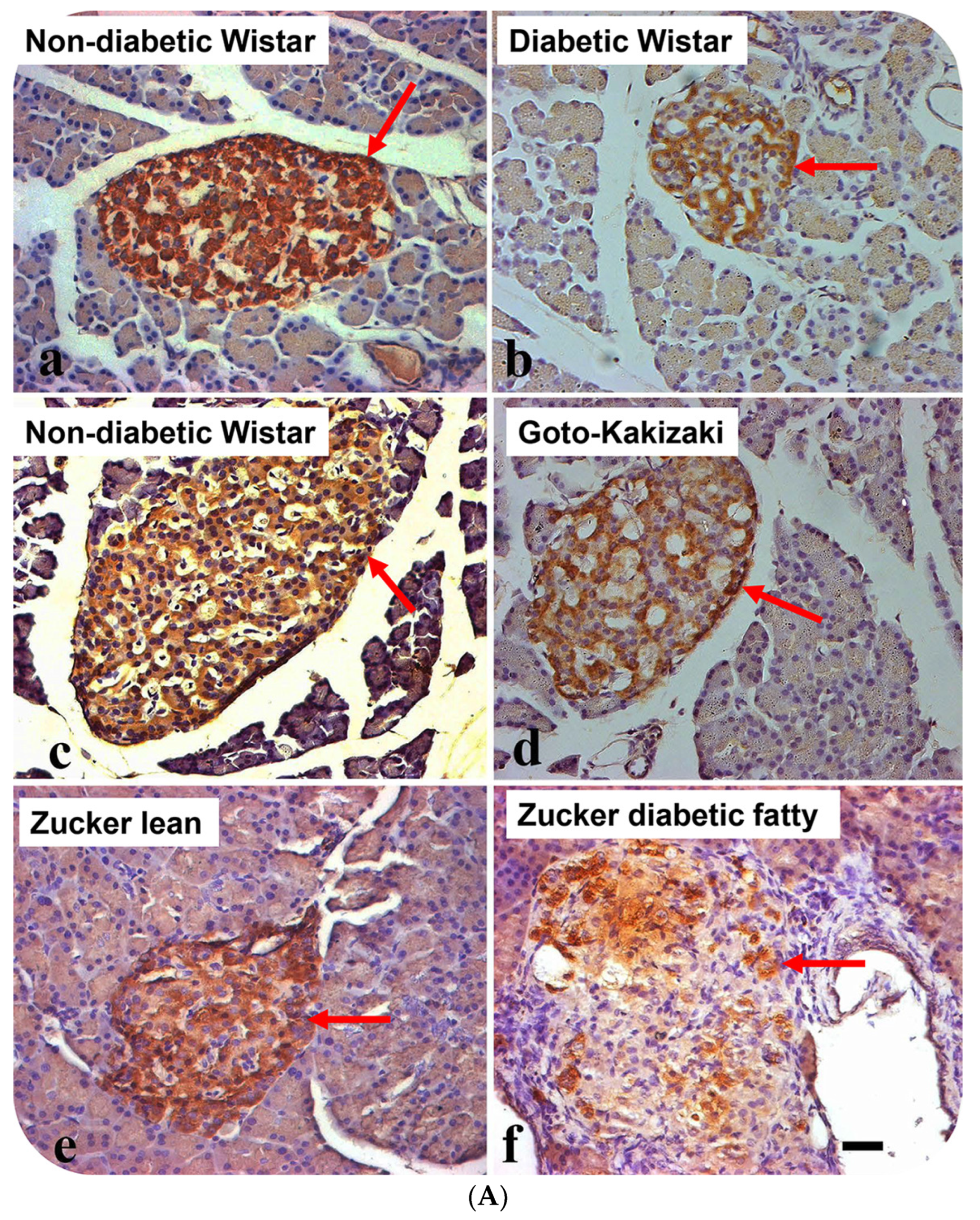
3.2. Localization of Alpha Lipoprotein (HDL) in Pancreatic Islet Cells
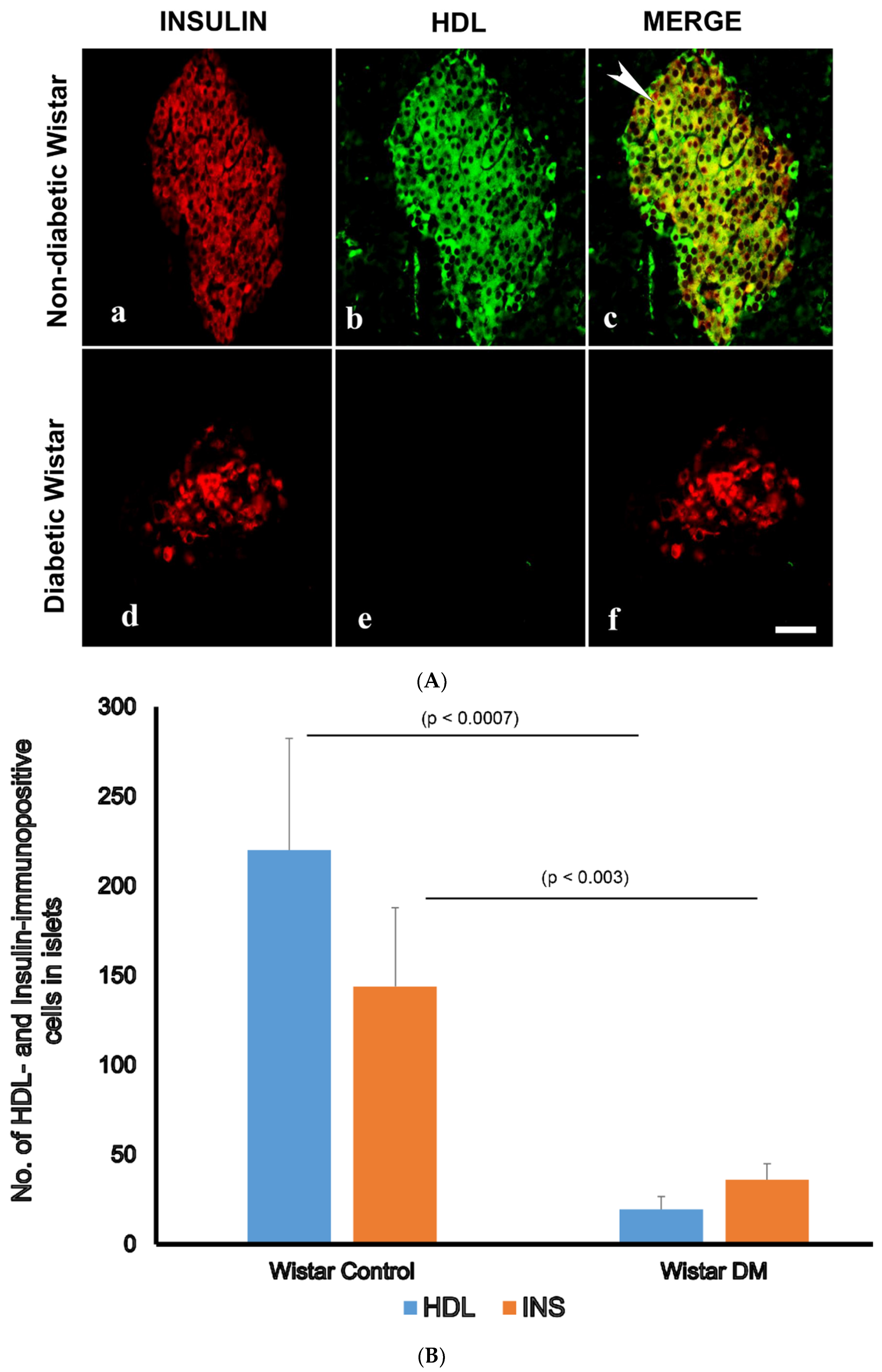
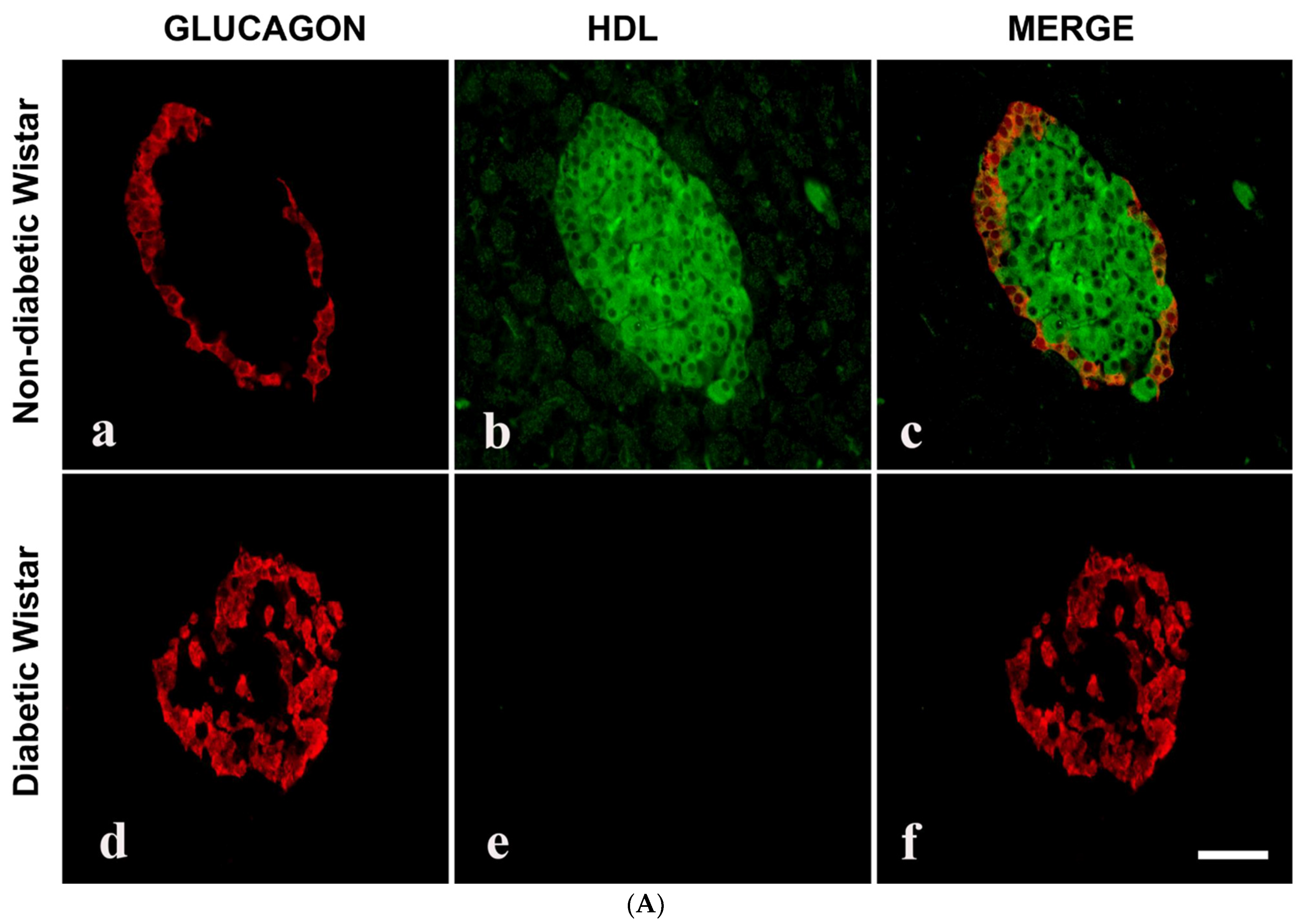
3.3. Double Labelling Immunofluorescence (IF) of HDL and Islet Hormones
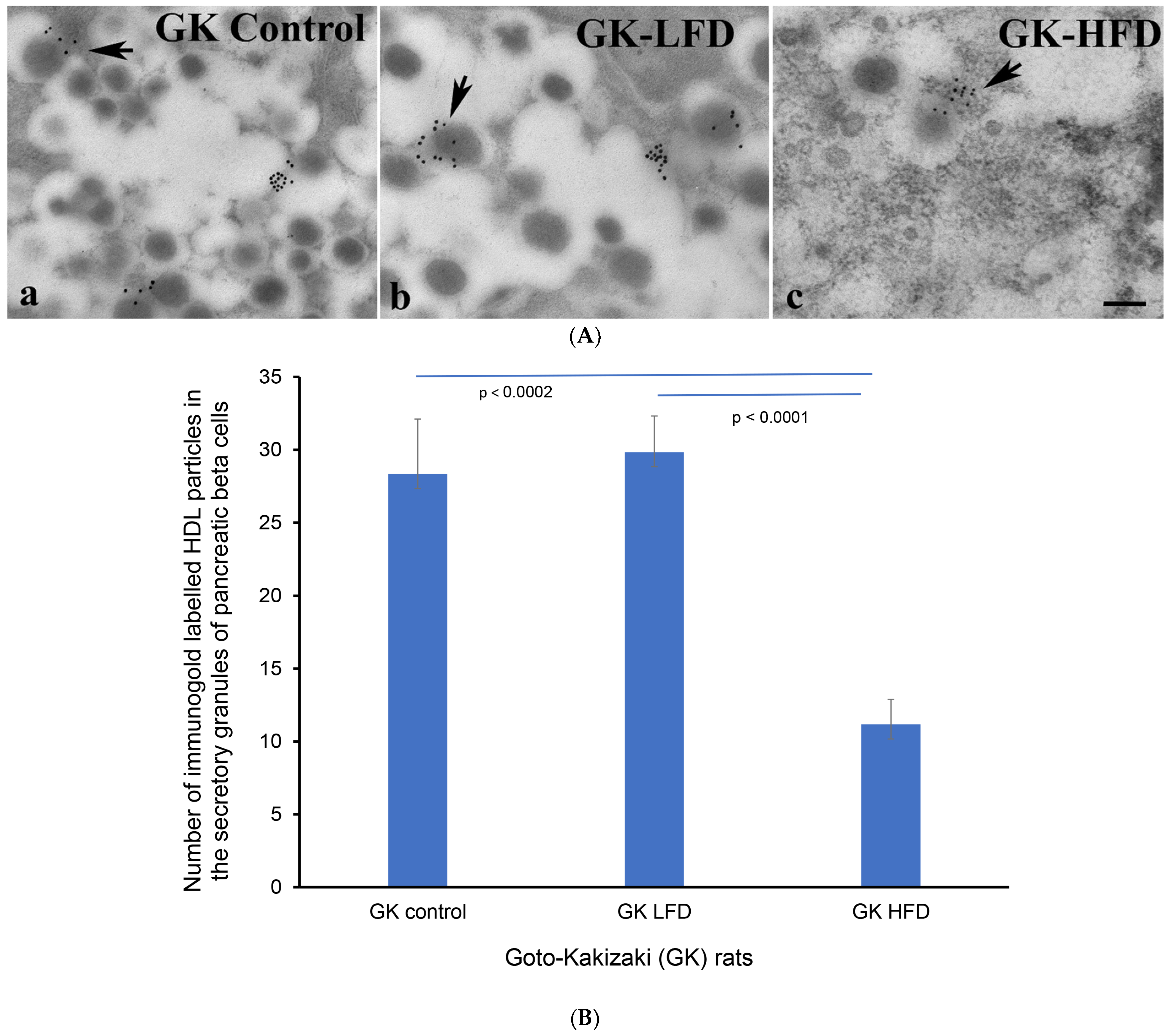
3.4. Transmission Immunoelectron Microscopy of HDL in β-Cells of GK Rats Fed on a High-Fat Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF. International Diabetes Federation Diabetes Atlas 2021; IDF: Brussels, Belgium, 2021. [Google Scholar]
- Adeghate, E.; Schattner, P.; Dunn, E. An Update on the Etiology and Epidemiology of Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2006, 1084, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Al Jaberi, S.; Cohen, A.; Saeed, Z.; Ojha, S.; Singh, J.; Adeghate, E. Obesity: Molecular Mechanisms, Epidemiology, Complications and Pharmacotherapy. Cell. Biochem. Mech. Obes. 2021, 23, 249–266. [Google Scholar]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism Linking Diabetes Mellitus and Obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Giacchetti, G.; Novello, M.; Colussi, G.; Cavarape, A.; Sechi, L.A. Cellular Mechanisms of Insulin Resistance in Rats with Fructose-Induced Hypertension. Am. J. Hypertens. 2003, 16, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Onodera, T.; Scherer, P.E. Lipotoxicity and β Cell Maintenance in Obesity and Type 2 Diabetes. J. Endocr. Soc. 2019, 3, 617–631. [Google Scholar] [CrossRef]
- Turner, R.C.; Millns, H.; Neil, H.A.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk Factors for Coronary Artery Disease in Non-Insulin Dependent Diabetes Mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef]
- Bie, J.; Wang, J.; Yuan, Q.; Kakiyama, G.; Ghosh, S.S.; Ghosh, S. Liver-Specific Transgenic Expression of Cholesteryl Ester Hydrolase Reduces Atherosclerosis in Ldlr−/− Mice. J. Lipid Res. 2014, 55, 729–738. [Google Scholar] [CrossRef]
- Shepherd, J. Dyslipidaemia in Diabetic Patients: Time for a Rethink. Diabetes Obes. Metab. 2007, 9, 609–616. [Google Scholar] [CrossRef]
- Rütti, S.; Ehses, J.A.; Sibler, R.A.; Prazak, R.; Rohrer, L.; Georgopoulos, S.; Meier, D.T.; Niclauss, N.; Berney, T.; Donath, M.Y.; et al. Low- and High-Density Lipoproteins Modulate Function, Apoptosis, and Proliferation of Primary Human and Murine Pancreatic Beta-Cells. Endocrinology 2009, 150, 4521–4530. [Google Scholar] [CrossRef] [PubMed]
- Fryirs, M.A.; Barter, P.J.; Appavoo, M.; Tuch, B.E.; Tabet, F.; Heather, A.K.; Rye, K.A. Effects of High-Density Lipoproteins on Pancreatic Beta-Cell Insulin Secretion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Brunham, L.R.; Verchere, C.B.; Hayden, M.R. HDL and LDL Cholesterol Significantly Influence Beta-Cell Function in Type 2 Diabetes Mellitus. Curr. Opin. Lipidol. 2010, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A.; Sibler, R.A. Possible Contributions of Lipoproteins and Cholesterol to the Pathogenesis of Diabetes Mellitus Type 2. Curr. Opin. Lipidol. 2011, 22, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Luquain-Costaz, C.; Delton, I. Oxysterols in Vascular Cells and Role in Atherosclerosis. Adv. Exp. Med. Biol. 2024, 1440, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Andraski, A.B.; Sacks, F.M.; Aikawa, M.; Singh, S.A. Understanding HDL Metabolism and Biology Through In Vivo Tracer Kinetics. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Cai, X.; Yu, X.; Gong, L. Markers of Insulin Resistance Associated with Non-Alcoholic Fatty Liver Disease in Non-Diabetic Population. Sci. Rep. 2023, 13, 20470. [Google Scholar] [CrossRef]
- Hussein, S.R.M.; Sadiq, A.M.; Johar, S.A.; Nasrawi, A.J.M. Insulin Level, Lipid Profile, and HOMA Index in Lean and Obese Patients with Polycystic Ovary Syndrome. J. Med. Life 2023, 16, 1258–1263. [Google Scholar]
- Bornfeldt, K.E. Apolipoprotein C3: Form Begets Function. J. Lipid Res. 2023, 65, 100475. [Google Scholar] [CrossRef]
- Howarth, F.C.; Qureshi, M.A.; Sobhy, Z.H.; Parekh, K.; Yammahi, S.R.; Adrian, T.E.; Adeghate, E. Structural Lesions and Changing Pattern of Expression of Genes Encoding Cardiac Muscle Proteins Are Associated with Ventricular Myocyte Dysfunction in Type 2 Diabetic Goto-Kakizaki Rats Fed a High-Fat Diet. Exp. Physiol. 2011, 96, 765–777. [Google Scholar] [CrossRef]
- Howarth, F.C.; Qureshi, M.A.; Hassan, Z.; Isaev, D.; Parekh, K.; John, A.; Oz, M.; Raza, H.; Adeghate, E.; Adrian, T.E. Contractility of Ventricular Myocytes Is Well Preserved despite Altered Mechanisms of Ca2+ Transport and a Changing Pattern of mRNA in Aged Type 2 Zucker Diabetic Fatty Rat Heart. Mol. Cell Biochem. 2012, 361, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Fernandez-Cabezudo, M.; Hameed, R.; El-Hasasna, H.; El Wasila, M.; Abbas, T.; Al-Ramadi, B. Orexin-1 Receptor Co-Localizes with Pancreatic Hormones in Islet Cells and Modulates the Outcome of Streptozotocin-Induced Diabetes Mellitus. PLoS ONE 2010, 5, e8587. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Adeghate, E.; Ponery, A.S.; Pallot, D.J.; Singh, J. Distribution of vasoactive intestinal polypeptide, neuropeptide-Y and substance P and their effects on insulin secretion from the in vitro pancreas of normal and diabetic rats. Peptides 2001, 22, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. Mechanism of the Beneficial and Protective Effects of Exenatide in Diabetic Rats. J. Endocrinol. 2014, 220, 291–304. [Google Scholar] [CrossRef]
- Elabadlah, H.; Hameed, R.; D’Souza, C.; Mohsin, S.; Adeghate, E.A. Exogenous Ghrelin Increases Plasma Insulin Level in Diabetic Rats. Biomolecules 2020, 10, 633. [Google Scholar] [CrossRef]
- Ogita, M.; Miyauchi, K.; Miyazaki, T.; Naito, R.; Konishi, H.; Tsuboi, S.; Dohi, T.; Kasai, T.; Yokoyama, T.; Okazaki, S.; et al. Low High-Density Lipoprotein Cholesterol Is a Residual Risk Factor Associated with Long-Term Clinical Outcomes in Diabetic Patients with Stable Coronary Artery Disease Who Achieve Optimal Control of Low-Density Lipoprotein Cholesterol. Heart Vessels 2014, 29, 35–41. [Google Scholar] [CrossRef]
- Eliasson, B.; Gudbjörnsdottir, S.; Zethelius, B.; Eeg-Olofsson, K.; Cederholm, J. LDL-Cholesterol versus Non-HDL-to-HDL-Cholesterol Ratio and Risk for Coronary Heart Disease in Type 2 Diabetes. Eur. J. Prev. Cardiol. 2014, 21, 1420–1428. [Google Scholar] [CrossRef]
- Yunke, Z.; Guoping, L.; Zhenyue, C. Triglyceride-to-HDL Cholesterol Ratio. Predictive Value for CHD Severity and New-Onset Heart Failure. Herz 2014, 39, 105–110. [Google Scholar] [CrossRef]
- Ngwasiri, C.; Kinoré, M.; Samadoulougou, S.; Kirakoya-Samadoulougou, F. Sex-Specific-Evaluation of Metabolic Syndrome Prevalence in Algeria: Insights from the 2016–2017 Non-Communicable Diseases Risk Factors Survey. Sci. Rep. 2023, 13, 18908. [Google Scholar] [CrossRef]
- Suresh, S.; Anand, A.; Singh, P.; Shahi, N.; Sharma, S.; Jethlia, A. Comparative Assessment of the Periodontal Findings in Child Subjects with a Normal Body Mass Index and in Obese Subjects. Cureus 2023, 15, e47897. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, K.A.; Goji, A.D.T.; Tanko, Y.; Muhammed, A.; Salisu, I.A. Protective Effects of Magnesium Chloride on Liver Enzymes and Biomarkers of Oxidative Stress in high fat diet fed Rats. Niger. J. Physiol. Sci. 2019, 34, 149–157. [Google Scholar] [PubMed]
- Tian, Y.; Xu, G.; Gao, H.; Xie, H.-Y.; Leng, Y.-L.; Fu, X.-X.; Xie, C.-G. The Mitigatory Effect of Shen-Qi Compound on the Diabetic Thoracic Aortic Complications through Inhibiting the Inflammatory Microenvironment by miR-223-3p/RBP-J/IRF8 Axis. Evid. Based Complement. Alternat Med. 2022, 2022, 6686931. [Google Scholar] [CrossRef]
- Sivgin, H.; Çetin, S. Effect of Empagliflozin Use on Monocyte High-Density Lipoprotein Ratio and Plasma Atherogenic Index in Obese and Non-Obese Type 2 Diabetic Patients. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8090–8100. [Google Scholar] [PubMed]
- Liu, L.; Wang, R.; Gao, J.; Yan, J.; Zhang, J.; Zhang, Z.; Liu, J.; Lin, H.; Rao, S.; Yao, X.; et al. Insulin Glargine Is More Suitable Than Exenatide in Preventing Muscle Loss in Non-Obese Type 2 Diabetic Patients with NAFLD. Exp. Clin. Endocrinol. Diabetes 2023, 131, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Loona, V. Comparison of Prevalence of Complications in Obese vs Non Obese Type-2 Diabetes Mellitus in B.R. Ambedkar Medical College And Hospital. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- Zakir, M.; Ahuja, N.; Surksha, M.A.; Sachdev, R.; Kalariya, Y.; Nasir, M.; Kashif, M.; Shahzeen, F.; Tayyab, A.; Khan, M.S.M.; et al. Cardiovascular Complications of Diabetes: From Microvascular to Macrovascular Pathways. Cureus 2023, 15, e45835. [Google Scholar] [CrossRef]
- Weng, J.; Ross, C.; Baker, J.; Alfuraih, S.; Shamloo, K.; Sharma, A. Diabetes-Associated Hyperglycemia Causes Rapid-Onset Ocular Surface Damage. Investig. Ophthalmol. Vis. Sci. 2023, 64, 11. [Google Scholar] [CrossRef]
- Syed, N.A.; Bhatti, A.; John, P. Molecular Link between Glo-1 Expression and Markers of Hyperglycemia and Oxidative Stress in Vascular Complications of Type 2 Diabetes Mellitus. Antioxidants 2023, 12, 1663. [Google Scholar] [CrossRef]
- Sheng, N.; Xing, F.; Wang, J.; Zhang, Q.-Y.; Nie, R.; Li-Ling, J.; Duan, X.; Xie, H.-Q. Recent Progress in Bone-Repair Strategies in Diabetic Conditions. Mater. Today Bio 2023, 23, 100835. [Google Scholar] [CrossRef] [PubMed]
- Poojari, A.S.; Wairkar, S.; Kulkarni, Y.A. Stem Cells as a Regenerative Medicine Approach in Treatment of Microvascular Diabetic Complications. Tissue Cell 2023, 85, 102225. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Roehrich, M.-E.; Mooser, V.; Lenain, V.; Herz, J.; Nimpf, J.; Azhar, S.; Bideau, M.; Capponi, A.; Nicod, P.; Haefliger, J.-A.; et al. Insulin-Secreting Beta-Cell Dysfunction Induced by Human Lipoproteins. J. Biol. Chem. 2003, 278, 18368–18375. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, T.; Xie, R.; Kelly, O.G.; Vale, W.W.; Sander, M.; Huising, M.O. Urocortin 3 Marks Mature Human Primary and Embryonic Stem Cell-Derived Pancreatic Alpha and Beta Cells. PLoS ONE 2012, 7, e52181. [Google Scholar] [CrossRef] [PubMed]
- Strutt, B.; Szlapinski, S.; Gnaneswaran, T.; Donegan, S.; Hill, J.; Bennett, J.; Hill, D.J. Ontology of the Apelinergic System in Mouse Pancreas during Pregnancy and Relationship with β-Cell Mass. Sci. Rep. 2021, 11, 15475. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, J.; Liang, A.; Strutt, B.; Hill, T.G.; Szlapinski, S.; Ramos-Álvarez, M.P.; Hill, D.J. Pleiotrophin Expression and Actions in Pancreatic β-Cells. Front. Endocrinol. 2022, 13, 777868. [Google Scholar] [CrossRef]
- Chapman, M.J. HDL Functionality in Type 1 and Type 2 Diabetes: New Insights. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 112–123. [Google Scholar] [CrossRef]
- Ochoa-Guzmán, A.; Guillén-Quintero, D.; Muñoz-Hernández, L.; García, A.; Díaz-Díaz, E.; Pérez-Méndez, O.; Rodríguez-Guillén, R.; Mitre-Aguilar, I.B.; Zentella-Dehesa, A.; Aguilar-Salinas, C.A.; et al. The Influence of High-Density Lipoprotein (HDL) and HDL Subfractions on Insulin Secretion and Cholesterol Efflux in Pancreatic Derived β-Cells. J. Endocrinol. Investig. 2021, 44, 1897–1904. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Widmann, C. High-Density Lipoprotein, Beta Cells, and Diabetes. Cardiovasc. Res. 2014, 103, 384–394. [Google Scholar] [CrossRef]
- Kurano, M.; Hara, M.; Tsuneyama, K.; Sakoda, H.; Shimizu, T.; Tsukamoto, K.; Ikeda, H.; Yatomi, Y. Induction of Insulin Secretion by Apolipoprotein M, a Carrier for Sphingosine 1-Phosphate. Biochim. Biophys. Acta 2014, 1841, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Del Giudice, R.; Nagao, M.; Grönberg, C.; Eliasson, L.; Lagerstedt, J.O. Apolipoprotein A-I Primes Beta Cells to Increase Glucose Stimulated Insulin Secretion. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165613. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Cochran, B.J.; Rye, K.A. Role of High-Density Lipoproteins in Cholesterol Homeostasis and Glycemic Control. J. Am. Heart Assoc. 2020, 9, e013531. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, X.; Wei, J.; Miao, R.; Wu, H.; Ma, K.; Tian, J. PDX-1: A Promising Therapeutic Target to Reverse Diabetes. Biomolecules 2022, 12, 1785. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, W.; Iwasa, H.; Tumurkhuu, M. Role of the Transcription Factor MAFA in the Maintenance of Pancreatic β-Cells. Int. J. Mol. Sci. 2022, 23, 4478. [Google Scholar] [CrossRef]
- Golson, M.L.; Kaestner, K.H. Fox transcription factors: From development to disease. Development 2016, 143, 4558–4570. [Google Scholar] [CrossRef]
- Mio, C.; Baldan, F.; Damante, G. NK2 homeobox gene cluster: Functions and roles in human diseases. Genes. Dis. 2022, 10, 2038–2048. [Google Scholar] [CrossRef]
- Munro, S.; Pelham, H.R. A C-terminal signal prevents secretion of luminal ER proteins. Cell 1987, 48, 899–907. [Google Scholar] [CrossRef]
- Steiner, D.F.K.W.; Clark, J.L.; Oyer, P.E.; Rubenstein, A. The biosynthesis of insulin. In Handbook of Physiology—Section 7 Endocrinology I; Steiner, D.F., Freinkel, N., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1972; pp. 175–198. [Google Scholar]
- Nishi, M.; Sanke, T.; Nagamatsu, S.; Bell, G.I.; Steiner, D.F. Islet amyloid polypeptide. A new beta cell secretory product related to islet amyloid deposits. J. Biol. Chem. 1990, 265, 4173–4176. [Google Scholar] [CrossRef]
- Sun, B.; Chen, H.; Xue, J.; Li, P.; Fu, X. The role of GLUT2 in glucose metabolism in multiple organs and tissues. Mol. Biol. Rep. 2023, 50, 6963–6974. [Google Scholar] [CrossRef] [PubMed]
- Holman, G.D. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflugers Arch. 2020, 472, 1155–1175. [Google Scholar] [CrossRef] [PubMed]
- Peppler, W.T.; Miotto, P.M.; Holloway, G.P.; Wright, D.C. CL 316, 243 mediated reductions in blood glucose are enhanced in RIP140−/− mice independent of alterations in lipolysis. Biochem. Biophys. Res. Commun. 2017, 486, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Leiss, V.; Flockerzie, K.; Novakovic, A.; Rath, M.; Schönsiegel, A.; Birnbaumer, L.; Schürmann, A.; Harteneck, C.; Nürnberg, B. Insulin secretion stimulated by L-arginine and its metabolite L-ornithine depends on Gα(i2). Am. J. Physiol. Endocrinol. Metab. 2014, 307, E800–E812. [Google Scholar] [CrossRef]
- Kemp, D.M.; Ubeda, M.; Habener, J.F. Identification and functional characterization of melatonin Mel 1a receptors in pancreatic beta cells: Potential role in incretin-mediated cell function by sensitization of cAMP signaling. Mol. Cell Endocrinol. 2002, 191, 157–166. [Google Scholar] [CrossRef]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. The effect of glucagon-like peptide-1 in the management of diabetes mellitus: Cellular and molecular mechanisms. Cell Tissue Res. 2014, 358, 343–358. [Google Scholar] [CrossRef]


| No | Rodent Model | Total wt. (g) | NF Blood Glucose Concentration (mg/dL) |
|---|---|---|---|
| 1 | Wistar (normal) rats | 310.8 ± 25.2 # | 38.0 ± 1.7 * |
| 2 | Wistar (diabetic, STZ-treated) rats | 241.7 ± 44.1 | 174.0 ± 3.6 |
| 3 | Goto–Kakizaki (GK) rats | 391.2 ± 28.1 ## | 124 ± 19.7 ** |
| 4 | Zucker lean (ZL) rats | 5 04.33 ± 63.36 | 117.8 ± 5.1 |
| 5 | Zucker diabetic (ZDF) rats | 738.5 ± 33.9 ♦♦♦ | 133.7 ± 7.5 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohsin, S.; Elabadlah, H.; Alotaiba, M.K.; AlAmry, S.; Almehairbi, S.J.; Harara, M.M.K.; Almuhsin, A.M.H.; Tariq, S.; Howarth, F.C.; Adeghate, E.A. High-Density Lipoprotein Is Located Alongside Insulin in the Islets of Langerhans of Normal and Rodent Models of Diabetes. Nutrients 2024, 16, 313. https://doi.org/10.3390/nu16020313
Mohsin S, Elabadlah H, Alotaiba MK, AlAmry S, Almehairbi SJ, Harara MMK, Almuhsin AMH, Tariq S, Howarth FC, Adeghate EA. High-Density Lipoprotein Is Located Alongside Insulin in the Islets of Langerhans of Normal and Rodent Models of Diabetes. Nutrients. 2024; 16(2):313. https://doi.org/10.3390/nu16020313
Chicago/Turabian StyleMohsin, Sahar, Haba Elabadlah, Mariam K. Alotaiba, Suhail AlAmry, Shamma J. Almehairbi, Maha M. K. Harara, Aisha M. H. Almuhsin, Saeed Tariq, Frank Christopher Howarth, and Ernest A. Adeghate. 2024. "High-Density Lipoprotein Is Located Alongside Insulin in the Islets of Langerhans of Normal and Rodent Models of Diabetes" Nutrients 16, no. 2: 313. https://doi.org/10.3390/nu16020313
APA StyleMohsin, S., Elabadlah, H., Alotaiba, M. K., AlAmry, S., Almehairbi, S. J., Harara, M. M. K., Almuhsin, A. M. H., Tariq, S., Howarth, F. C., & Adeghate, E. A. (2024). High-Density Lipoprotein Is Located Alongside Insulin in the Islets of Langerhans of Normal and Rodent Models of Diabetes. Nutrients, 16(2), 313. https://doi.org/10.3390/nu16020313







