The Maternal Diet Index and Offspring Microbiota at 1 Month of Life: Insights from the Mediterranean Birth Cohort MAMI
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Clinical and Demographic Characteristics
2.2. Dietary Information
2.3. Infant Microbiota at 1 Month
2.4. Statistical Analysis
2.4.1. Cohort Description and Clinical Data
2.4.2. Alpha Diversity Analysis
2.4.3. Beta Diversity Analysis
2.4.4. Individual (MDI-med) Components and Infant Microbiota
3. Results
3.1. The Maternal Diet Index (MDI)
3.2. Clinical Characteristics and Descriptives
3.3. Infant Microbiota at 1 Month
3.3.1. Association between the Maternal Diet Index and Infant Alpha Diversity Indices (Shannon Index, Chao1 Index, and InvSimpson Index)
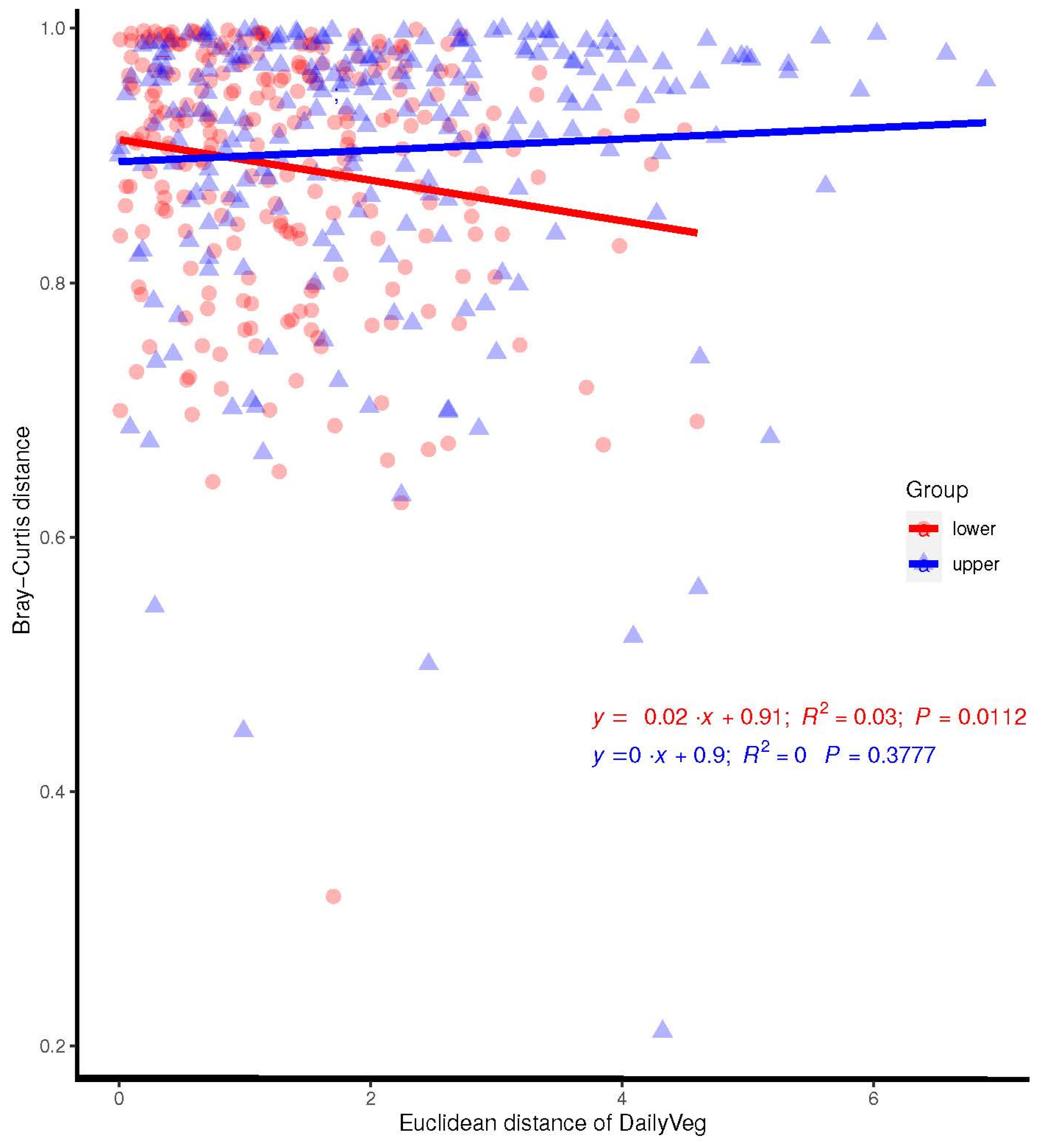
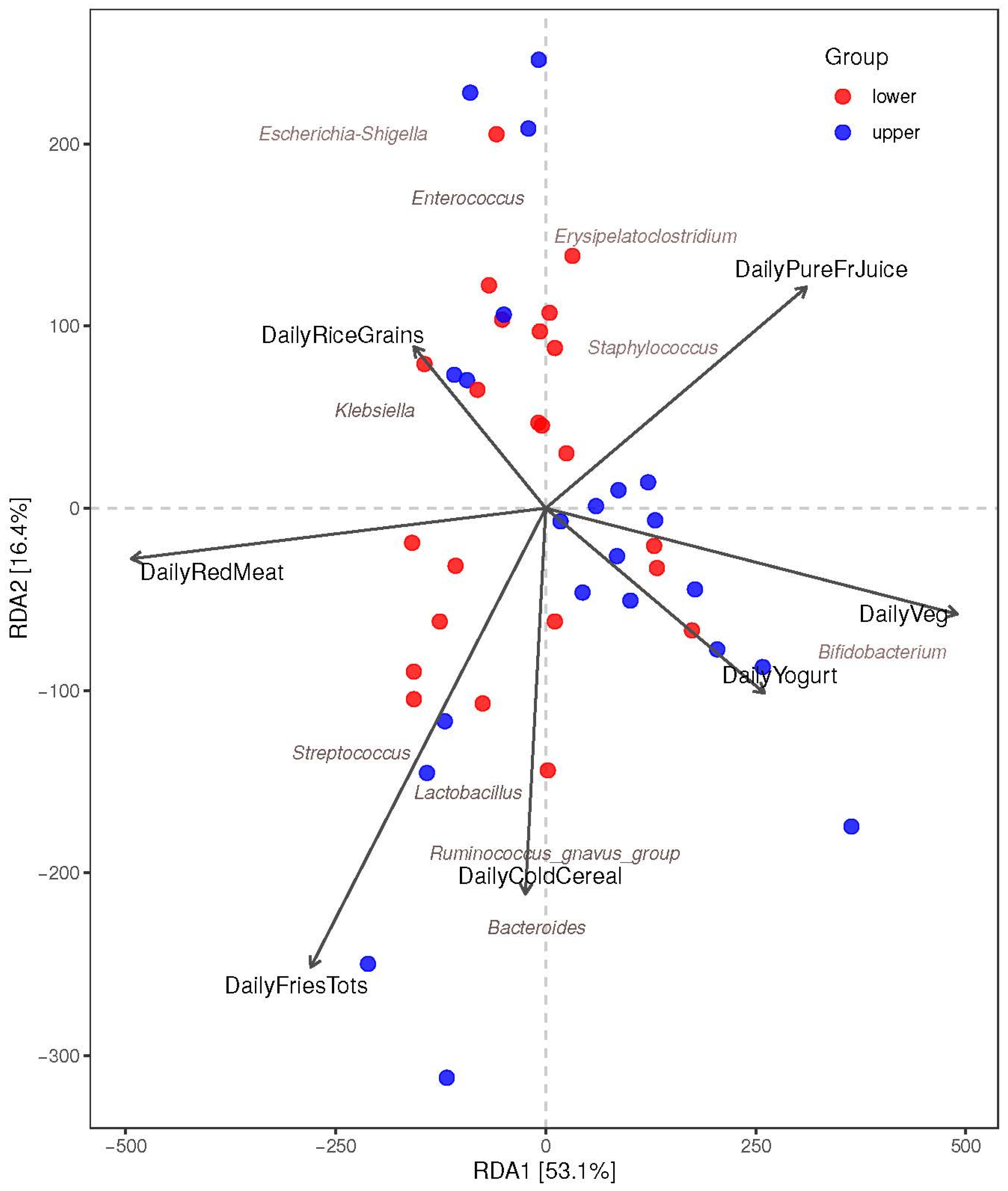
3.3.2. Association between the Maternal Diet Index and Beta Diversity (Bray–Curtis) of Infant Microbiomes at 1 Month
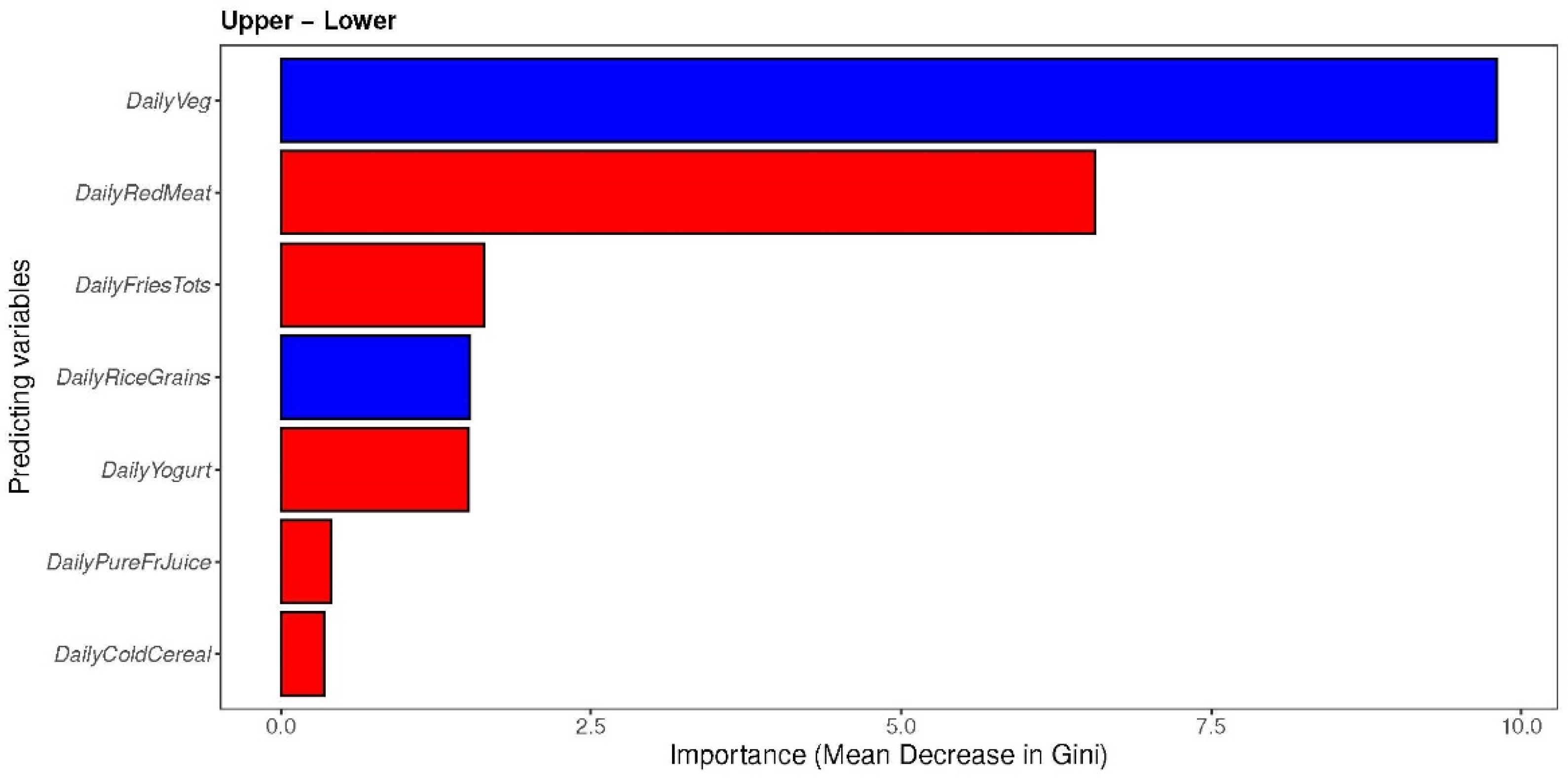
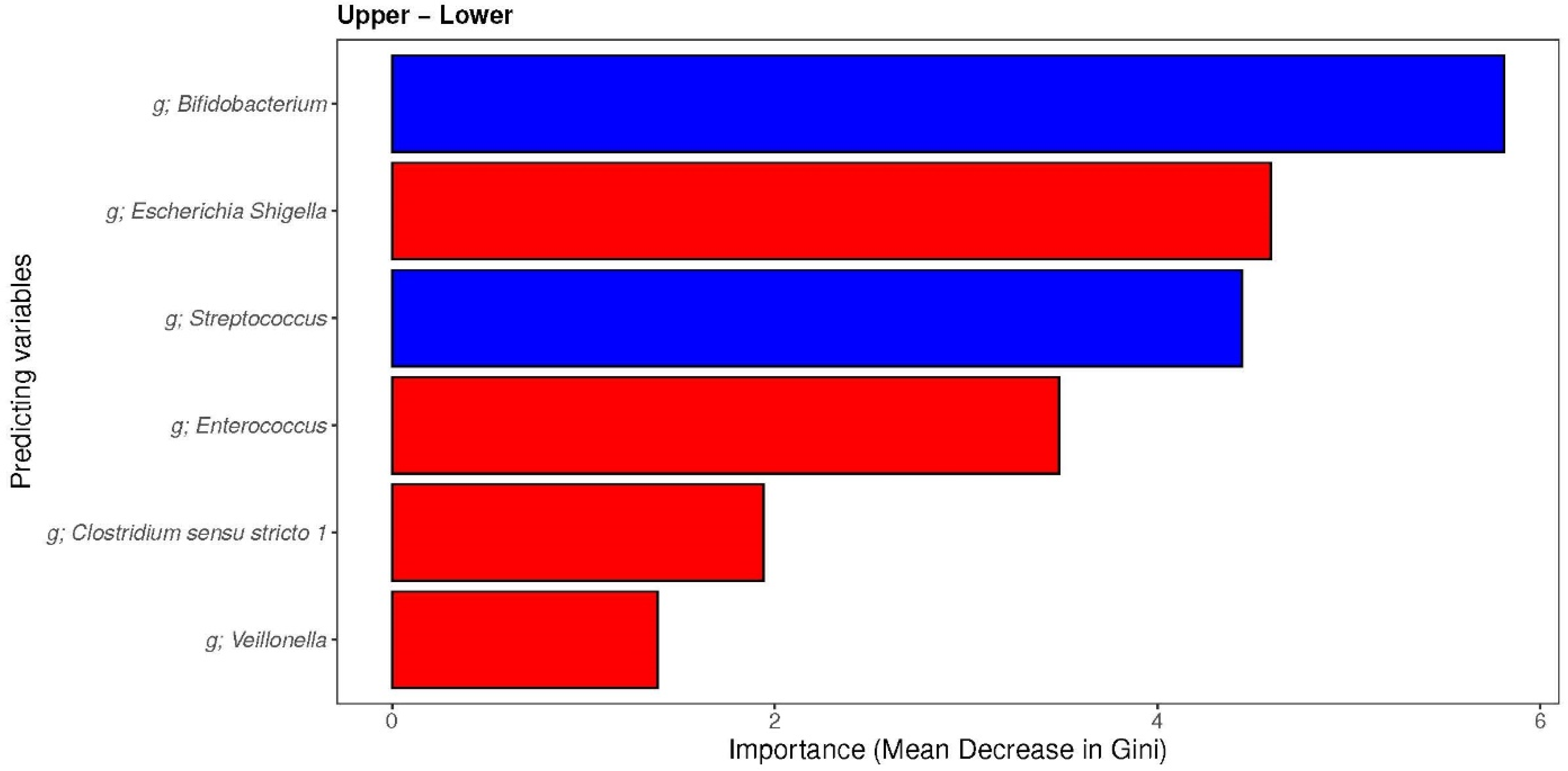
3.3.3. The Associations between Maternal Diet Index and Individual Infant-Specific Microbiota
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Index Components | Healthy Start | MAMI FFQ Responses Included | English Translation of MAMI FFQ Responses |
|---|---|---|---|
| Vegetables | Vegetables: How often did you eat other vegetables, NOT INCLUDING what you just told me about (lettuce salads, potatoes, or cooked dried beans) | Espinaca Brécol Lechuga Tomate Zanahorias Judía verde Calabacín Pimiento rojo Espárragos Alcachofa Cebolla Ajo Perejil Champiñón | Spinach Broccoli Lettuce Tomato Carrots Green bean Courgette Red pepper Asparagus Artichoke Onion Garlic Parsley Mushroom |
| Yogurt | Yogurt: How often did you eat yogurt (NOT including frozen yogurt)? | Yogurt entero Yogurt desnatado | Whole yogurt Skimmed yogurt |
| Fried potato | Fried potatoes: How often did you eat French fries, home fries, hash browned potatoes, or tater tots? | Patata frita casera | Fried potato |
| Fruit juice | Pure juice: How often did you drink 100% pure fruit juice such as orange, mango, apple, grape and pineapple juices? Do not include fruit-flavored drinks with added sugar or fruit juice you made at home and added sugar to. | Zumo envase | Commercial juice |
| Cereals | Cereal: How often did you eat cold cereal (box cereal such as Corn Flakes)? | Cereales desayuno | Breakfast cereals |
| Rice | Rice/grains: How often did you eat rice or other cooked grains (such as bulgur, cracked wheat, or millet)? | Arroz blanco | White rice |
| Meat | Red meat: How often did you eat red meat, such as beef, pork, ham, or sausage? Do not include chicken, turkey or seafood. | Ternera Cerdo Cordero Jamón serrano Jamón york Embutidos Carne picada Panceta | Beef Pig Lamb Serrano ham York ham Sausages Mince Bacon |
Appendix B
| Index Component | Diet 1: Average Intake for Participants with MDI < Median | Diet 2: Theoretical “Perfect” Diet | Diet 3: Theoretical “Better” Diet | Diet 4: Theoretical “ok” Diet |
|---|---|---|---|---|
| Components associated with allergy prevention | ← Intake (times/day) → | |||
| Vegetables | 4.9 | 3 | 3 | 1 |
| Yogurt | 0.38 | 1 | 4/7 | 4/7 |
| Components associated with increased allergy | ← Intake (times/day) → | |||
| Red meat | 1 | 1/7 | 3/7 | 3/7 |
| Cold cereals | 1.9 | 0 | 2/7 | 2/7 |
| Rice and grains | 1 | 0 | 2/7 | 2/7 |
| Fried potatoes | 1 | 1/14 | 1/7 | 1/7 |
| 100% pure fruit juice | 0.29 | 2/7 | 4/7 | 4/7 |
| Associated MDI value | 71.94 | 76.35 | 74.80 | 73.05 |
Appendix C
| Included (N = 45) | Excluded (N = 75) | Total (N = 120) | p Value | |
|---|---|---|---|---|
| Maternal Age | ||||
| Mean (sd) | 34.7 (3.99) | 35.0 (4.4) | 34.9 (4.2) | 0.718 |
| Weight Gain During Pregnancy (kg) | ||||
| Mean (sd) | 12.5 (3.7) | 12.4 (4.1) | 12.5 (3.95) | 0.873 |
| Pre-pregnancy BMI | ||||
| Median (Q1,Q3) | 23.7 (3.4) | 22.3 (3.3) | 22.9 (3.4) | 0.028 |
| Education | 0.695 | |||
| Basic school | 4 (8.9%) | 4 (5.3%) | 8 (6.7%) | |
| medium | 11 (24.4%) | 17 (22.7%) | 28 (23.3%) | |
| High university | 30 (66.7%) | 54 (72.0%) | 84 (70.0%) | |
| Antibiotic during pregnancy | 10 (22.2%) | 21 (28.0%) | 31 (25.8%) | 0.53 |
| Antibiotic Intrapartum | 18 (40.0%) | 18 (24.0%) | 36 (30.0%) | 0.10 |
| delivery_type | 0.117 | |||
| Vaginal delivery | 28 (62.2%) | 58 (77.3%) | 86 (71.7%) | |
| C-section | 17 (37.8%) | 17 (22.7%) | 34 (28.3%) | |
| Allergy/Atopy by 12 months | 0.474 | |||
| No | 32 (71.1%) | 59 (78.7%) | 91 (75.8%) | |
| Yes | 13 (28.9%) | 16 (21.3%) | 29 (24.2%) | |
| Sex | 0.636 | |||
| Female | 23 (51.1%) | 43 (57.3%) | 66 (55.0%) | |
| Male | 22 (48.9%) | 32 (42.7%) | 54 (45.0%) | |
| Breast feeding Practices at 1 month | 0.591 | |||
| breastmilk | 29 (64.4%) | 52 (69.3%) | 81 (67.5%) | |
| formula | 4 (8.9%) | 9 (12.0%) | 13 (10.8%) | |
| mixed | 12 (26.7%) | 14 (18.7%) | 26 (21.7%) | |
| Neonatal height at birth (cm) | 0.47 | |||
| Mean (sd) | 50.1 (2.2) | 49.9 (2.2) | 49.99 (2.2) | |
| Neonatal weight at birth (kg) | 0.78 | |||
| Mean (sd) | 3.4 (0.5) | 3.3 (0.4) | 3.3 (0.4) | |
| Gestational age (weeks) | 0.48 | |||
| Median (Q1,Q3) | 40 (39, 40) | 40 (39, 40) | 40 (39, 40) |
References
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The Maternal Diet Index in Pregnancy is associated with offspring allergic diseases: The Healthy Start Study. Allergy 2021, 77, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; Garcia-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martinez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2020, 60, 1429–1442. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, J. Maternal and infant microbiome: Next-generation indicators and targets for intergenerational health and nutrition care. Protein Cell 2023, 14, 807–823. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Li, Q.; Zheng, T.; Yang, S.; Chen, F.; Guan, W.; Zhang, S. Maternal microbe-specific modulation of the offspring microbiome and development during pregnancy and lactation. Gut Microbes 2023, 15, 2206505. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-Y.; Tung, Y.-T.; Yang, Y.-C.S.H.; Hsu, J.B.; Lee, C.-Y.; Chang, T.-H.; Su, E.C.-Y.; Hsieh, R.-H.; Chen, Y.-C. Maternal Vegetable and Fruit Consumption during Pregnancy and Its Effects on Infant Gut Microbiome. Nutrients 2021, 13, 1559. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, L.; Pecora, V.; Riccardi, C.; Fierro, V.; Valluzzi, R.; Mennini, M. How to predict and improve prognosis of food allergy. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Holt, P.G.; Inouye, M.; Logan, A.C.; Prescott, S.L.; Sly, P.D. An exposome perspective: Early-life events and immune development in a changing world. J. Allergy Clin. Immunol. 2017, 140, 24–40. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Alcántara, C.; Selma-Royo, M.; Boix-Amorós, A.; Dzidic, M.; Gimeno-Alcañiz, J.; Úbeda-Sansano, I.; Sorribes-Monrabal, I.; Escuriet, R.; Gil-Raga, F.; et al. MAMI: A birth cohort focused on maternal-infant microbiota during early life. BMC Pediatr. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Navarro, T.; Salazar, N.; Gutierrez-Diaz, I.; de Los Reyes-Gavilan, C.G.; Gueimonde, M.; Gonzalez, S. Different Intestinal Microbial Profile in Over-Weight and Obese Subjects Consuming a Diet with Low Content of Fiber and Antioxidants. Nutrients 2017, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Antony, K.M.; Ma, J.; Prince, A.L.; Showalter, L.; Moller, M.; Aagaard, K.M. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Al Khatib, H.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, H.J.; Wildeboer–Veloo, A.C.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67. Available online: https://www.ncbi.nlm.nih.gov/pubmed/10630441 (accessed on 4 December 2023). [CrossRef] [PubMed]
- Merter, Ö.S.; Altay, N. Effect of Feeding Fresh or Frozen Breast Milk on the Gut Microbiota of Premature Infants: A Prospective Observational Study. Biol. Res. Nurs. 2023, 26, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Yelverton, C.A.; Killeen, S.L.; Feehily, C.; Moore, R.L.; Callaghan, S.L.; Geraghty, A.A.; Byrne, D.F.; Walsh, C.J.; Lawton, E.M.; Murphy, E.F.; et al. Maternal breastfeeding is associated with offspring microbiome diversity; a secondary analysis of the MicrobeMom randomized control trial. Front. Microbiol. 2023, 14, 1154114. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.B.F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package. R Package Version 2.5-2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 4 December 2023).
- US Department of Agriculture. Dietary Guidelines for Americans: DGA 2020–2025. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 4 December 2023).
- Laursen, M.F.; Andersen, L.B.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant Gut Microbiota Development Is Driven by Transition to Family Foods Independent of Maternal Obesity. mSphere 2016, 1, e00069-15. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Tian, M.; Chen, J.; Chen, F.; Guan, W. Different Sources of High Fat Diet Induces Marked Changes in Gut Microbiota of Nursery Pigs. Front. Microbiol. 2020, 11, 859. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.N.; Madan, J.C.; Emond, J.A.; Morrison, H.G.; Christensen, B.C.; Karagas, M.R.; Hoen, A.G. Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]




| Total (N = 45) | Lower (N = 22) | Upper (N = 23) | p-Value * | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age mean (sd) | 34.71 (3.99) | 35.22 (3.98) | 34.18 (4.03) | 0.39 |
| Weight gain during pregnancy (kg) mean (sd) | 12.54 (3.70) | 13.39 (3.40) | 11.66 (3.87) | 0.12 |
| Pre-pregnancy BMI median (sd) | 23.73 (3.43) | 23.55 (3.29) | 23.92 (3.64) | 0.72 |
| Education | 1 | |||
| Basic education (mandatory school) | 4 (8.9%) | 2 (8.7%) | 2 (9.1%) | |
| Medium | 11 (24.4%) | 6 (26.1%) | 5 (22.7%) | |
| Higher education (university studies) | 30 (66.7%) | 15 (65.2%) | 15 (68.2%) | |
| Gestational age (weeks) median (IQR) | 40 (39–40) | 40 (38–40) | 40 (39–40) | 0.19 |
| Antibiotic during pregnancy prevalence (%) | 10 (22.2%) | 7 (30.4%) | 3 (13.6%) | 0.67 |
| Antibiotic intrapartum prevalence (%) | 18 (40.0%) | 12 (52.2%) | 6 (27.3%) | 0.16 |
| Mode of delivery prevalence (%) | 0.27 | |||
| Vaginal | 28 (62.2%) | 12 (52.2%) | 16 (72.7%) | |
| C-section | 17 (37.8%) | 11 (47.8%) | 6 (27.3%) | |
| Neonatal Characteristics | ||||
| Neonate sex prevalence (%) | 0.66 | |||
| Female | 23 (51.1%) | 13 (56.5%) | 10 (45.5%) | |
| Male | 22 (48.9%) | 10 (43.5%) | 12 (54.5%) | |
| Neonatal height at birth (cm) mean (sd) | 50.07 (2.25) | 49.09 (2.18) | 51.09 (1.86) | 0.002 |
| Neonatal weight at birth (kg) mean (sd) | 3.36 (0.48) | 3.24 (0.51) | 3.48 (0.43) | 0.10 |
| Breastfeeding practices at 1 month prevalence (%) | 0.17 | |||
| Exclusively breast milk | 29 (64.4%) | 13 (56.5%) | 16 (72.7%) | |
| Infant formula | 4 (8.9%) | 4 (17.4%) | 0 (0.0%) | |
| Mixed (breast milk and formula) | 12 (26.7%) | 6 (26.1%) | 6 (27.3%) |
| Unadjusted Model | Adjusted Model 1 + | Adjusted Model 2 ++ | ||||
|---|---|---|---|---|---|---|
| Outcome | Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value | Estimate (95% CI) | p-Value |
| Shannon Index | −0.35 (−0.66, −0.04) | 0.03 * | −0.33 (−0.66, −0.01) | 0.04 * | −0.33 (−0.68, 0.03) | 0.07 |
| Inverse Simpson | −1.99 (−3.8, −0.18) | 0.03 * | −1.78 (−3.62, 0.07) | 0.06 | −1.42 (−3.35, 0.52) | 0.15 |
| Chao1 Index | −3.9 (−18.02, 10.23) | 0.58 | −3.32 (−17.93, 11.29) | 0.65 | 1.56 (−12.91, 16.04) | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Rubio, R.; Pickett-Nairne, K.; González-Solares, S.; Collado, M.C.; Venter, C. The Maternal Diet Index and Offspring Microbiota at 1 Month of Life: Insights from the Mediterranean Birth Cohort MAMI. Nutrients 2024, 16, 314. https://doi.org/10.3390/nu16020314
Cabrera-Rubio R, Pickett-Nairne K, González-Solares S, Collado MC, Venter C. The Maternal Diet Index and Offspring Microbiota at 1 Month of Life: Insights from the Mediterranean Birth Cohort MAMI. Nutrients. 2024; 16(2):314. https://doi.org/10.3390/nu16020314
Chicago/Turabian StyleCabrera-Rubio, Raúl, Kaci Pickett-Nairne, Sonia González-Solares, Maria Carmen Collado, and Carina Venter. 2024. "The Maternal Diet Index and Offspring Microbiota at 1 Month of Life: Insights from the Mediterranean Birth Cohort MAMI" Nutrients 16, no. 2: 314. https://doi.org/10.3390/nu16020314
APA StyleCabrera-Rubio, R., Pickett-Nairne, K., González-Solares, S., Collado, M. C., & Venter, C. (2024). The Maternal Diet Index and Offspring Microbiota at 1 Month of Life: Insights from the Mediterranean Birth Cohort MAMI. Nutrients, 16(2), 314. https://doi.org/10.3390/nu16020314






