Effects of Supplementing Zinc Magnesium Aspartate on Sleep Quality and Submaximal Weightlifting Performance, following Two Consecutive Nights of Partial Sleep Deprivation
Abstract
1. Introduction
2. Materials and Method
2.1. Participants
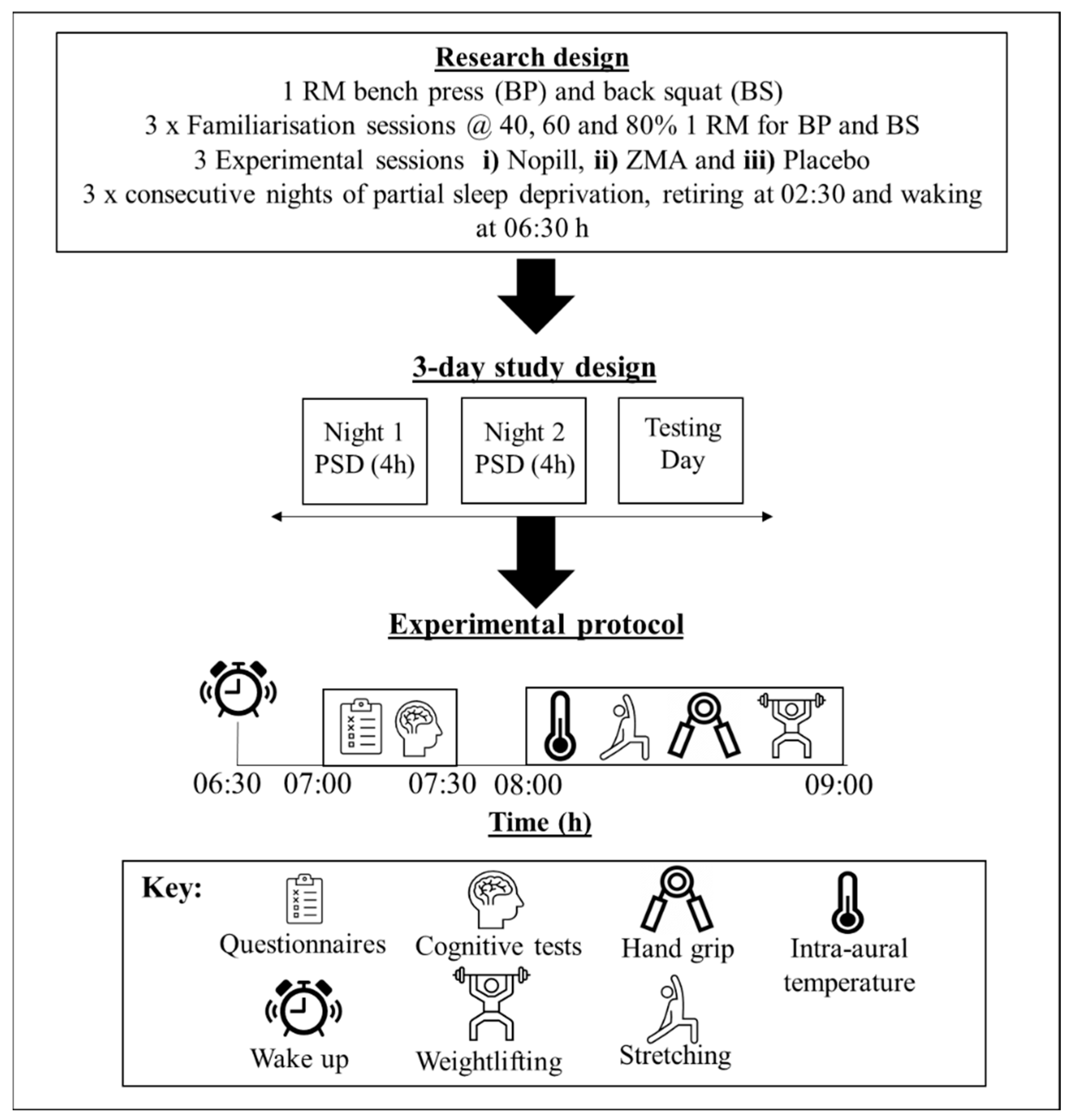
2.2. Research Design
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Performance Measures (Measured at 07:30 h)
3.2. Grip Strength (Left and Right Hand)
3.3. Bench Press
3.4. Back Squat
4. Physiological and Psychological Variables (Measured at 07:30 h)
4.1. Intra-Aural Temperature, Tiredness and Alertness
4.2. Profile of Mood State
4.3. Stroop (Word–Colour Interference Test)
5. Measures of Sleep
6. Discussion
Limitations
7. Conclusions
Practical Implications and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daher, J.; Mallick, M.; El Khoury, D. Prevalence of Dietary Supplement Use among Athletes Worldwide: A Scoping Review. Nutrients 2022, 14, 4109. [Google Scholar] [CrossRef]
- Binns, C.W.; Lee, M.K.; Lee, A.H. Problems and Prospects: Public Health Regulation of Dietary Supplements. Annu. Rev. Public Health 2018, 39, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Hys, K. Identification of the Reasons Why Individual Consumers Purchase Dietary Supplements. In Perspectives on Consumer Behaviour: Theoretical Aspects and Practical Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 193–209. [Google Scholar]
- Maughan, R.J.; Depiesse, F.; Geyer, H.; International Association of Athletics Federations. The use of dietary supplements by athletes. J. Sports Sci. 2007, 25 (Suppl. S1), S103–S113. [Google Scholar] [CrossRef]
- Clarkson, P.M. Minerals: Exercise performance and supplementation in athletes. J. Sports Sci. 1991, 9, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Cherasse, Y.; Urade, Y. Dietary Zinc Acts as a Sleep Modulator. Int. J. Mol. Sci. 2017, 18, 2334. [Google Scholar] [CrossRef]
- Moëzzi, N.; Peeri, M.; Martin Homaei, H. Effects of zinc, magnesium and vitamin B6 supplementation on hormones and performance in weightlifters. Ann. Biol. Res. 2013, 4, 163–168. [Google Scholar]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Nica, A.S.; Caramoci, A.; Vasilescu, M.; Ionescu, A.M.; Paduraru, D.; Mazilu, V. Magnesium supplementation in top athletes—Effects and recommendations. Sports Med. J. 2015, 11, 2482–2494. [Google Scholar]
- Brilla, L.R.; Conte, V. Effects of a Novel Zinc-Magnesium Formulation on Hormones and Strength. J. Exerc. Physiol. Online 2000, 3, 26–36. [Google Scholar]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- Wilborn, C.D.; Kerksick, C.M.; Campbell, B.I.; Taylor, L.W.; Marcello, B.M.; Rasmussen, C.J.; Greenwood, M.C.; Almada, A.; Kreider, R.B. Effects of Zinc Magnesium Aspartate (ZMA) Supplementation on Training Adaptations and Markers of Anabolism and Catabolism. J. Int. Soc. Sports Nutr. 2004, 1, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.L.; Albracht-Schulte, K.; Robert-McComb, J.J. Micronutrient deficiency in athletes and inefficiency of supplementation: Is low energy availability a culprit? PharmaNutrition 2020, 14, 100229. [Google Scholar] [CrossRef]
- Edwards, B.J.; Gallagher, C.; Pullinger, S.A.; de Mello, T.M.; Walsh, N.P. Athletic Performance; Effects of Sleep Loss. In The Encyclopedia of Sleep and Circadian Rhythms, 2nd ed.; Kushida, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 434–443. [Google Scholar]
- Gallagher, C.; Green, C.E.; Kenny, M.L.; Evans, J.R.; McCullagh, G.D.W.; Pullinger, S.A.; Edwards, B.J. Is implementing a post-lunch nap beneficial on evening performance, following two nights partial sleep restriction? Chronobiol. Int. 2023, 40, 1169–1186. [Google Scholar] [CrossRef]
- Thun, E.; Bjorvatn, B.; Flo, E.; Harris, A.; Pallesen, S. Sleep, circadian rhythms, and athletic performance. Sleep Med. Rev. 2015, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Blumert, P.A.; Crum, A.J.; Ernsting, M.; Volek, J.S.; Hollander, D.B.; Haff, E.E.; Haff, G.G. The acute effects of twenty-four hours of sleep loss on the performance of national-caliber male collegiate weightlifters. J. Strength Cond. Res. 2007, 21, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.; Waterhouse, J.; Reilly, T. The effects of circadian rhythmicity and time-awake on a simple motor task. Chronobiol. Int. 2007, 24, 1109–1124. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc deficiency in women, infants and children. J. Am. Coll. Nutr. 1996, 15, 113–120. [Google Scholar] [CrossRef]
- Antonio, J.; Stout, J.R. Supplements for Endurance Athletes; Human Kinetics: Champaign, IL, USA, 2002. [Google Scholar]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Training and Conditioning; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Robertson, C.M.; Pullinger, S.A.; Robinson, W.R.; Smith, M.E.; Burniston, J.G.; Waterhouse, J.M.; Edwards, B.J. Is the diurnal variation in muscle force output detected/detectable when multi-joint movements are analysed using the musclelab force-velocity encoder? Chronobiol. Int. 2018, 35, 1391–1401. [Google Scholar] [CrossRef]
- Brotherton, E.J.; Moseley, S.E.; Langan-Evans, C.; Pullinger, S.A.; Robertson, C.M.; Burniston, J.G.; Edwards, B.J. Effects of two nights partial sleep deprivation on an evening submaximal weightlifting performance; are 1 h powernaps useful on the day of competition? Chronobiol. Int. 2019, 36, 407–426. [Google Scholar] [CrossRef]
- Ikonte, C.J.; Mun, J.G.; Reider, C.A.; Grant, R.W.; Mitmesser, S.H. Micronutrient Inadequacy in Short Sleep: Analysis of the NHANES 2005–2016. Nutrients 2019, 11, 2335. [Google Scholar] [CrossRef]
- Deuster, P.A.; Hodgson, A.B.; Stear, S.J.; Burke, L.M.; Castell, L.M. A–Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance: Part 46. British J. Sports Med. 2013, 47, 809–810. [Google Scholar] [CrossRef][Green Version]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Chisholm, D.M.; Collis, M.L.; Kulak, L.L.; Davenport, W.; Gruber, N. Physical activity readiness. BC Med. J. 1975, 17, 375–378. [Google Scholar]
- Smith, S.; Chester, N.; Close, G. Supplement use in sport—A worrying game of Russian roulette for athletes today. Prof. Strength Cond. 2015, 38, 9–15. [Google Scholar]
- Waterhouse, J.; Reilly, T.; Atkinson, G.; Edwards, B. Jet lag: Trends and coping strategies. Lancet 2007, 369, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.H.; Leng, V.C. Time of day effects in simple repetitive tasks: Some possible mechanisms. Acta Psychol. 1982, 51, 207–221. [Google Scholar] [CrossRef]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Multi-Health Systems Inc.: Toronto, ON, Canada, 1992. [Google Scholar]
- Hoddes, E.; Zarcone, V.; Smythe, H.; Phillips, R.; Dement, W.C. Quantification of sleepiness: A new approach. Psychophysiology 1973, 10, 431–436. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Birk, T.J.; Birk, C.A. Use of ratings of perceived exertion for exercise prescription. Sports Med. 1987, 4, 1–8. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Walsh, N.P. Nutrition and Athlete Immune Health: New Perspectives on an Old Paradigm. Sports Med. 2019, 49 (Suppl. S2), 153–168. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. British J. Sports Med. 2020, 55, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Rafie, N.; Amani, R.; Shirani, F. The Role of Magnesium in Sleep Health: A Systematic Review of Available Literature. Biol. Trace Elem. Res. 2023, 201, 121–128. [Google Scholar] [CrossRef]
- Lin, H.H.; Tsai, P.S.; Fang, S.C.; Liu, J.F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174. [Google Scholar]
- Saito, H.; Cherasse, Y.; Suzuki, R.; Mitarai, M.; Ueda, F.; Urade, Y. Zinc-rich oysters as well as zinc-yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol. Nutr. Food Res. 2017, 61, 1600882. [Google Scholar] [CrossRef]
- Chapman, P.P.; Whitehead, J.R.; Binkert, R.H. The 225-lb Reps to Fatigue Test as a Submaximal Estimate of 1-RM Bench Press Performance in College Football Players. J. Strength Cond. Res. 1998, 12, 258–261. [Google Scholar] [CrossRef]
- Tonson, A.; Ratel, S.; Le Fur, Y.; Cozzone, P.; Bendahan, D. Effect of maturation on the relationship between muscle size and force production. Med. Sci. Sports Exerc. 2008, 40, 918–925. [Google Scholar] [CrossRef]
- Ammar, A.; Riemann, B.L.; Masmoudi, L.; Blaumann, M.; Abdelkarim, O.; Hökelmann, A. Kinetic and kinematic patterns during high intensity clean movement: Searching for optimal load. J. Sports Sci. 2018, 36, 1319–1330. [Google Scholar] [CrossRef]
- Bobilya, D.J.; Guerin, J.L.; Rowe, D.J. Zinc Transport Across an In Vitro Blood-Brain Barrier Model. J. Trace Elem. Exp. Med. 1997, 10, 9–18. [Google Scholar] [CrossRef]
- Prapavessis, H. The POMS and sports performance: A review. J. Appl. Sport Psychol. 2000, 12, 34–48. [Google Scholar] [CrossRef]
- Drust, B.; Waterhouse, J.; Atkinson, G.; Edwards, B.; Reilly, T. Circadian rhythms in sports performance—An update. Chronobiol. Int. 2005, 22, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Beaven, C.M.; Kilduff, L.P.; Drawer, S. Acute caffeine ingestion’s increase of voluntarily chosen resistance-training load after limited sleep. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Dudek, D.; Paul, I.A.; Sowa-Kućma, M.; Zieba, A.; Popik, P.; Pilc, A.; Nowak, G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009, 118, 187–195. [Google Scholar] [CrossRef]
- Sawada, T.; Yokoi, K. Effect of zinc supplementation on mood states in young women: A pilot study. Eur. J. Clin. Nutr. 2010, 64, 331–333. [Google Scholar] [CrossRef]
- Lai, J.; Moxey, A.; Nowak, G.; Vashum, K.; Bailey, K.; McEvoy, M. The efficacy of zinc supplementation in depression: Systematic review of randomised controlled trials. J. Affect. Disord. 2012, 136, e31–e39. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- Fekedulegn, D.; Andrew, M.E.; Shi, M.; Violanti, J.M.; Knox, S.; Innes, K.E. Actigraphy-Based Assessment of Sleep Parameters. Ann. Work Expo. Health 2020, 64, 350–367. [Google Scholar] [CrossRef]



| Habitual Average Intake | Mean ± SD |
|---|---|
| Energy (kcal) | 2256 ± 534 |
| Carbohydrate (g) | 238 ± 61 |
| Carbohydrate (g/kg) | 3 ± 1 |
| Fat (g) | 71 ± 20 |
| Fat (g/kg) | 1 ± 0 |
| Protein (g) | 146 ± 56 |
| Protein (g/kg) | 2 ± 1 |
| Zinc (mg) | 12 |
| Magnesium (mg) | 410 |
| Vitamin B6 (mg) | 3 |
| Habitual Sleep Variables | |
| Normative retiring (h:mm) | 23:50 |
| Normative waking (h:mm) | 08:02 |
| Variable | Significance Condition | Significance of Load | Interactions (COND and LOAD) |
|---|---|---|---|
| Grip Strength (N) | |||
| Left | F1.6, 23.4 = 0.07 (p = 0.887), ES = 0.007 | ||
| Right | F2.0, 30.0 = 0.08 (p = 0.928), ES = 0.009 | ||
| Bench Press | |||
| Average power (W) | F1.9, 28.6 = 0.43 (p = 0.645), ES = 0.042 | F1.3, 20.1 = 12.99 (p < 0.0005), ES = 0.397 | F2.2, 32.5 = 0.85 (p = 0.447) |
| Displacement (cm) | F2.0, 30.0 = 0.77 (p = 0.472), ES = 0.062 | F2.0, 30.0 = 7.46 (p = 0.002), ES = 0.418 | F3.3, 49.1 = 1.14 (p = 0.345) |
| Average velocity (ms−1) | F2.0, 30.0 = 0.45 (p = 0.644), ES = 0.007 | F1.1, 16.4 = 174.99 (p < 0.0005), ES = 0.928 | F2.4, 35.5 = 0.47 (p = 0.473) |
| Peak velocity (ms−1) | F1.6, 24.1 = 0.13 (p = 0.839), ES = 0.001 | F1.2, 17.2 = 210.92 (p < 0.0005), ES = 0.940 | F2.3, 34.4 = 2.35 (p = 0.104) |
| Time to peak velocity (s) | F2.0, 30.0 = 0.79 (p = 0.464), ES = 0.078 | F1.1, 16.1 = 31.60 (p < 0.0005), ES = 0.742 | F1.8, 27.1 = 2.81 (p = 0.083) |
| Perceived effort (VAS, 0–10 cm) | F1.9, 26.7 = 0.07 (p = 0.925) | F1.2, 17.9 = 244.28 (p < 0.0005) | F2.4, 33.8 = 0.46 (p = 0.670) |
| RPE (6–20) | F2.0, 30.0 = 0.68 (p = 0.514) | F1.3, 20.0 = 147.85 (p < 0.0005) | F2.2, 32.2 = 0.70 (p = 0.517) |
| RPE Breathing (6–20) | F1.8, 27.5 = 3.13 (p = 0.063) | F1.3, 20.0 = 63.07 (p < 0.0005) | F3.1, 46.5 = 1.00 (p = 0.401) |
| RPE Muscle fatigue (6–20) | F1.4, 20.8 = 1.68 (p = 0.203) | F1.5, 22.3 = 179.52 (p < 0.0005) | F2.6, 38.3 = 0.432 (p = 0.701) |
| Back Squat | |||
| Average power (W) | F1.3, 19.7 = 0.83 (p = 0.406), ES = 0.062 | F1.4, 21.0 = 32.29 (p < 0.0005), ES = 0.707 | F2.2, 32.4 = 1.18 (p = 0.324) |
| Displacement (cm) | F1.4, 21.4 = 1.51 (p = 0.241), ES = 0.094 | F1.4, 21.1 = 1.97 (p = 0.173), ES = 0.091 | F2.3, 34.9 = 0.45 (p = 0.671) |
| Average velocity (ms−1) | F2.0, 30.0 = 0.89 (p = 0.421), ES = 0.050 | F1.1, 16.4 = 86.64 (p < 0.0005), ES = 0.858 | F2.0, 29.9 = 0.74 (p = 0.485) |
| Peak velocity (ms−1) | F1.9, 28.7 = 0.13 (p = 0.872), ES = 0.003 | F1.2, 18.6 = 40.17 (p < 0.0005), ES = 0.766 | F2.2, 33.3 = 0.18 (p = 0.858) |
| Time to peak velocity (s) | F2.0, 30.0 = 0.50 (p = 0.609), ES = 0.059 | F1.2, 17.5 = 73.61 (p < 0.0005), ES = 0.839 | F2.2, 32.4 = 1.00 (p = 0.385) |
| Perceived effort (VAS, 0–10 cm) | F2.0, 28.0 = 0.11 (p = 0.893) | F1.3, 18.0 = 191.7 (p < 0.0005) | F2.1, 30.1 = 0.45 (p = 0.657) |
| RPE (6–20) | F2.0, 29.2 = 0.89 (p = 0.418) | F1.2, 18.4 = 192.70 (p < 0.0005) | F2.6, 38.7 = 2.20 (p = 0.112) |
| RPE Breathing (6–20) | F1.8, 27.6 = 3.33 (p = 0.054) | F1.2, 18.4 = 72.65 (p = 0.060) | F3.4, 51.4 = 1.65 (p = 0.184) |
| RPE Muscle fatigue (6–20) | F1.3, 19.1 = 2.71 (p = 0.109) | F1.3, 18.9 = 206.09 (p < 0.0005) | F4.0, 60.0 = 1.02 (p = 0.405) |
| Variables | NOPILL | ZMA | PLA | Significance Condition |
|---|---|---|---|---|
| Intra-aural temperature (°C) | 35.7 ± 1.1 | 35.6 ± 1.2 | 35.6 ± 1.1 | F2.0, 30.0 = 0.09 (p = 0.918) |
| Tiredness (0–10 VAS) | 8.0 ± 3.2 | 7.5 ± 3.4 | 7.2 ± 4.2 | F2.0, 30.0 = 0.51 (p = 0.604) |
| Alertness (0–10 VAS) | 3.2 ± 2.2 | 3.8 ± 2.3 | 3.8 ± 2.9 | F1.6, 23.9 = 0.56 (p = 0.542) |
| Stanford Sleepiness | 4.4 ± 1.4 | 4.1 ± 1.3 | 4.2 ± 1.4 | F1.5, 21.8 = 0.61 (p = 0.503) |
| Mood State–Vigour | 3.0 ± 2.7 | 3.6 ± 3.3 | 3.4 ± 3.1 | F1.5, 22.5 = 0.39 (p = 0.624) |
| Mood State–Anger | 1.8 ± 1.8 | 1.0 ± 2.3 | 1.9 ± 2.7 | F1.9, 28.5 = 1.31 (p = 0.284) |
| Mood State–Tension | 0.8 ± 1.3 | 0.6 ± 0.9 | 0.6 ± 0.7 | F1.5, 22.2 = 0.30 (p = 0.681) |
| Mood State–Calm | 5.8 ± 3.2 | 5.6 ± 4.1 | 6.6 ± 4.1 | F1.6, 24.3 = 0.95 (p = 0.390) |
| Mood State–Happiness | 4.1 ± 3.1 | 4.0 ± 4.2 | 4.3 ± 3.4 | F1.8, 26.6 = 0.04 (p = 0.948) |
| Mood State–Confusion | 1.9 ± 2.8 | 1.6 ± 1.5 | 1.9 ± 2.1 | F2.0, 30.0 = 0.19 (p = 0.831) |
| Mood State–Depression | 1.7 ± 1.9 | 1.5 ± 1.7 | 1.0 ± 1.4 | F2.0, 30.0 = 1.25 (p = 0.302) |
| Mood State–Fatigue | 8.8 ± 3.8 | 8.7 ± 3.8 | 8.3 ± 5.3 | F1.3, 19.9 = 0.08 (p = 0.842) |
| STROOP (Colours/NotW/TOTAL) | 58.1 ± 9.3 | 56.6 ± 11.2 | 60.6 ± 16.4 | F1.9, 28.2 = 0.80 (p = 0.454) |
| STROOP (Colours/NotW/ERROR) | 1.8 ± 1.6 | 2.4 ± 2.4 | 1.6 ± 1.2 | F1.4, 21.6 = 0.96 (p = 0.371) |
| STROOP (Words/NotC/TOTAL) | 98.1 ± 14.9 | 104.3 ± 12.5 | 107.5 ± 14.9 | F2.0, 30.0 = 4.28 (p = 0.023) |
| STROOP (Words/NotC/ERROR) | 0.9 ± 1.0 | 0.7 ± 0.9 | 0.8 ± 1.3 | F1.8, 27.0 = 0.13 (p = 0.863) |
| Actimetry Variables | NoPill | ZMA | PLA | Significance Condition | Significance Night | Significance Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N1 | N2 | N1 | N2 | ||||
| Actual sleep time (h:mm) | 3:55 ± 0:22 | 3:51 ± 0:21 | 3:52 ± 0:15 | 3:42 ± 0:58 | 3:53 ± 0:15 | 4:00 ± 0:17 | F2.0, 30.0 = 2.69 (p = 0.08) | F1.0, 15.0 = 0.11 (p = 0.742) | F1.4, 21.1 = 0.73 (p = 0.449) |
| Sleep latency (h:mm) | 0:14 ± 0:14 | 0:12 ± 0:11 | 0:12 ± 0:15 | 0:13 ± 0:11 | 0:08 ± 0:10 | 0:10 ± 0:10 | F2.0, 30.0 = 2.44 (p = 0.105) | F1.0, 15.0 = 0.00 (p = 0.985) | F1.6, 24.0 = 0.440 (p = 0.606) |
| Sleep efficiency (%) | 80.7 ± 9.2 | 78.3 ± 16.8 | 79.7 ± 12.4 | 79.2 ± 8.3 | 76.2 ± 12.2 | 77.3 ± 19.1 | F1.3, 19.7 = 0.45 (p = 0.559) | F1.0, 15.0 = 0.06 (p = 0.809) | F1.3, 19.4 = 0.31 (p = 0.644) |
| Fragmentation Index (%) | 23.5 ± 15.1 | 22.8 ± 12.0 | 20.6 ± 12.4 | 24.3 ± 14.2 | 31.3 ± 15.1 | 28.7 ± 14.0 | F1.5, 22.6 = 2.23 (p = 0.141) | F1.0, 15.0 = 0.00 (p = 0.969) | F2.0, 30.0 = 1.27 (p = 0.296) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, C.; Austin, V.; Dunlop, K.A.; Dally, J.; Taylor, K.; Pullinger, S.A.; Edwards, B.J. Effects of Supplementing Zinc Magnesium Aspartate on Sleep Quality and Submaximal Weightlifting Performance, following Two Consecutive Nights of Partial Sleep Deprivation. Nutrients 2024, 16, 251. https://doi.org/10.3390/nu16020251
Gallagher C, Austin V, Dunlop KA, Dally J, Taylor K, Pullinger SA, Edwards BJ. Effects of Supplementing Zinc Magnesium Aspartate on Sleep Quality and Submaximal Weightlifting Performance, following Two Consecutive Nights of Partial Sleep Deprivation. Nutrients. 2024; 16(2):251. https://doi.org/10.3390/nu16020251
Chicago/Turabian StyleGallagher, Chloe, Victoria Austin, Kyle A. Dunlop, Jasmine Dally, Kyle Taylor, Samuel A. Pullinger, and Ben J. Edwards. 2024. "Effects of Supplementing Zinc Magnesium Aspartate on Sleep Quality and Submaximal Weightlifting Performance, following Two Consecutive Nights of Partial Sleep Deprivation" Nutrients 16, no. 2: 251. https://doi.org/10.3390/nu16020251
APA StyleGallagher, C., Austin, V., Dunlop, K. A., Dally, J., Taylor, K., Pullinger, S. A., & Edwards, B. J. (2024). Effects of Supplementing Zinc Magnesium Aspartate on Sleep Quality and Submaximal Weightlifting Performance, following Two Consecutive Nights of Partial Sleep Deprivation. Nutrients, 16(2), 251. https://doi.org/10.3390/nu16020251








