Emerging Role of Eruca sativa Mill. in Male Reproductive Health
Abstract
1. Introduction
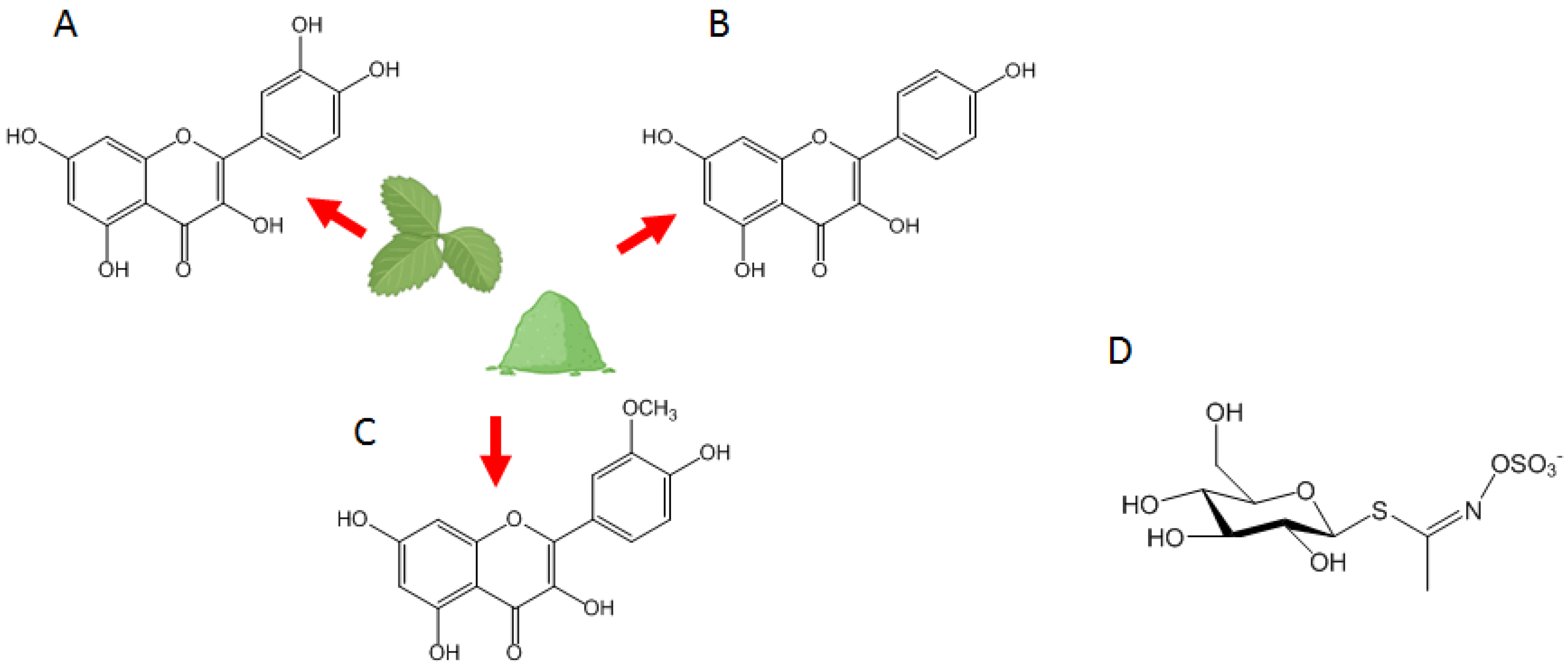
2. Eruca sativa: Taxonomic Framework and Phytochemical Characterization
3. Traditional Uses, and Pharmacological and Toxicological Profiles of Eruca sativa
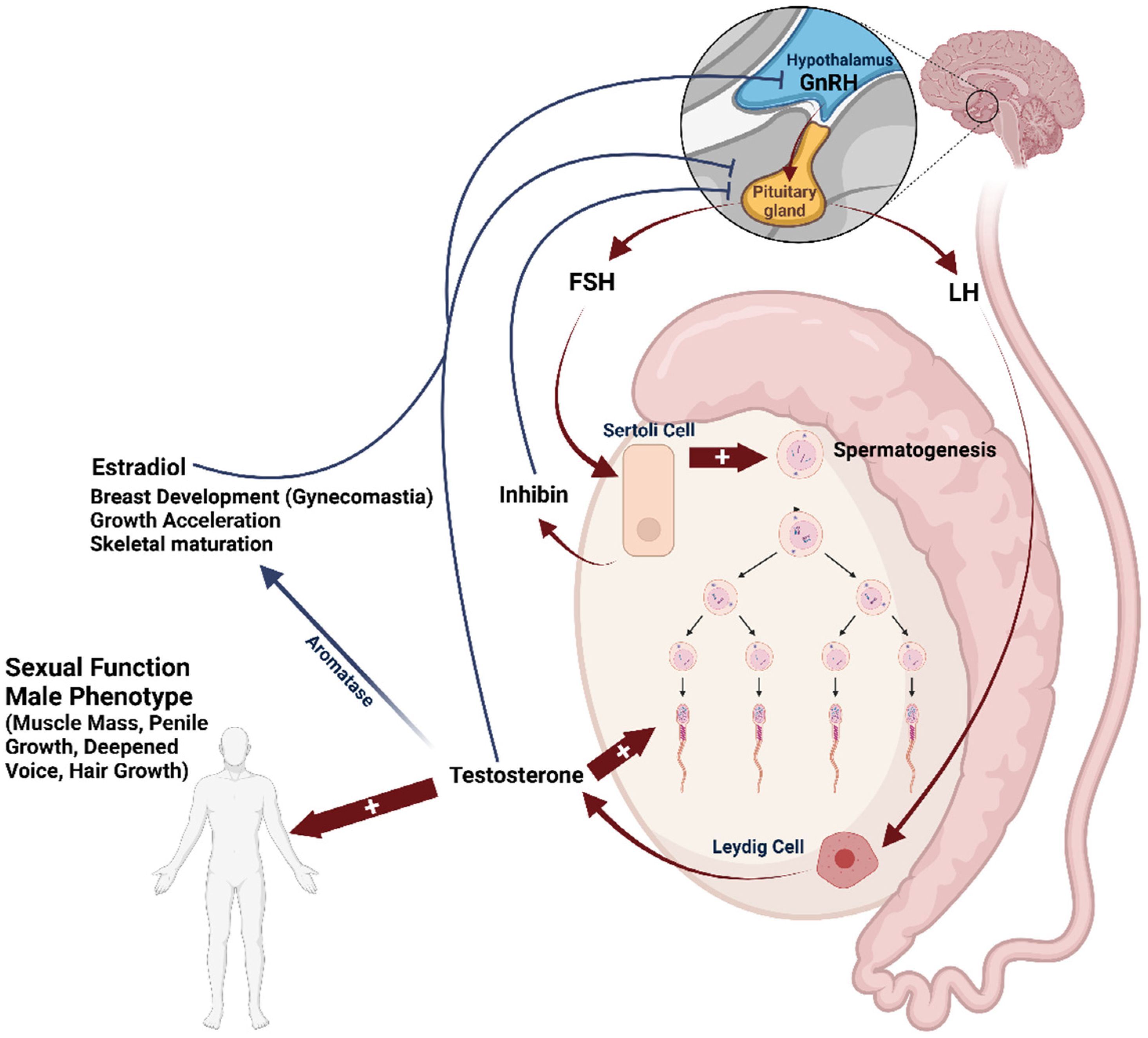
4. Regulation of Spermatogenesis and Risk Factors of Male Infertility
5. Effects of Eruca sativa on Male Fertility: Data from Animal Studies
6. Effects of Eruca sativa on Male Fertility: Data from Human Models
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic Regulation Is Important for Spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Gulfraz, M.; Sadiq, A.; Tariq, H.; Imran, M.; Qureshi, R.; Zeenat, A. Phytochemical Analysis and Antibacterial Activity of Eruca Sativa Seed. Pak. J. Bot. 2011, 43, 1351–1359. [Google Scholar]
- Alqasoumi, S.; Al-Sohaibani, M.; AlHowiriny, T.; AlYahya, M.; Rafatullah, S. Rocket “Eruca sativa”: A Salad Herb with Potential Gastric Antiulcer Activity. World J. Gastroenterol. 2009, 15, 1958. [Google Scholar] [CrossRef]
- Morales, M.; Janick, J. Arugula: A Promising Specialty Leaf Vegetable; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Hall, M.; Jobling, J.; Rogers, G. Some Perspectives on Rocket as a Vegetable Crop: A Review. J. Fruit. Ornam. Plant Res. 2012, 76, 21–41. [Google Scholar] [CrossRef]
- Cullis, C. Linum. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 177–189. [Google Scholar]
- Bozokalfa, K.; Eşiyok, D.; Yağmur, B. Use of Multivariate Analysis in Mineral Accumulation of Rocket (Eruca sativa) Accessions. Genetika 2011, 43, 437–448. [Google Scholar] [CrossRef]
- Bukhsh, E.; Akbar Malik, S.; Sheikh, A.; Ahmad, S. Estimation of Nutritional Value and Trace Elements Content of Carthamus oxyacantha, Eruca sativa and Plantago ovata. Pak. J. Bot. 2007, 39, 1181. [Google Scholar]
- Degl’innoocenti, E.; Pardossi, A.; Tattini, M.; Guidi, L. Phenolic compounds and antioxidant power in minimally processed salad. J. Food Biochem. 2008, 32, 642–653. [Google Scholar] [CrossRef]
- Jirovetz, L.; Smith, D.; Buchbauer, G. Aroma Compound Analysis of Eruca sativa (Brassicaceae) SPME Headspace Leaf Samples Using GC, GC−MS, and Olfactometry. J. Agric. Food Chem. 2002, 50, 4643–4646. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Kartal, M.; Sekeroglu, N.; Esiyok, D.; Sener, B.; Ugur, A.; Süntar, I.; Aslan, S. Variations in Fatty Acid Compositions of the Seed Oil of Eruca sativa Mill. Caused by Different Sowing Periods and Nitrogen Forms. Pharmacogn. Mag. 2010, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica Species for Glucosinolate Content. J. Environ. Sci. Health Part. B 2009, 44, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Helana Naguib, M.; Reham Ezzat, S.; George Emad, R. Studies on the Chemical Constituents of Fresh Leaf of Eruca sativa Extract and Its Biological Activity as Anticancer Agent in Vitro. J. Med. Plants Res. 2011, 5, 1184–1191. [Google Scholar]
- Taviano, M.; Melchini, A.; Filocamo, A.; Costa, C.; Catania, S.; Raciti, R.; Saha, S.; Needs, P.; Bisignano, G.; Miceli, N. Contribution of the Glucosinolate Fraction to the Overall Antioxidant Potential, Cytoprotection against Oxidative Insult and Antimicrobial Activity of Eruca sativa Mill. Leaves Extract. Pharmacogn. Mag. 2017, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, E.M.; Hegazy, E.M.; Riad, R.M.; Amer, H.A. Bio-Protective Effect of Eruca Sativa Seed Oil Against the Hazardus Effect of Aflatoxin B1 in Male-Rabbits. Int. J. Acad. Res. 2010, 2. Available online: https://www.researchgate.net/profile/Emtenan-Hanafi/publication/267196100_BIO-PROTECTIVE_EFFECT_OF_ERUCA_SATIVA_SEED_OIL_AGAINST_THE_HAZARDUS_EFFECT_OF_AFLATOXIN_B1_IN_MALE_-_RABBITS/links/55c8d74908aea2d9bdc91e64/BIO-PROTECTIVE-EFFECT-OF-ERUCA-SATIVA-SEED-OIL-AGAINST-THE-HAZARDUS-EFFECT-OF-AFLATOXIN-B1-IN-MALE-RABBITS.pdf (accessed on 27 December 2023).
- Villatoro-Pulido, M.; Priego-Capote, F.; Álvarez-Sánchez, B.; Saha, S.; Philo, M.; Obregón-Cano, S.; De Haro-Bailón, A.; Font, R.; Del Río-Celestino, M. An Approach to the Phytochemical Profiling of Rocket [Eruca sativa (Mill.) Thell]. J. Sci. Food Agric. 2013, 93, 3809–3819. [Google Scholar] [CrossRef]
- Kim, B.; Choi, Y.; Kim, H. Eruca Sativa and Its Flavonoid Components, Quercetin and Isorhamnetin, Improve Skin Barrier Function by Activation of Peroxisome Proliferator-Activated Receptor (PPAR)-α and Suppression of Inflammatory Cytokines. Phytother. Res. 2014, 28, 1359–1366. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Ganesh, N.; Gabbanini, S.; Valgimigli, L.; Srivastava, M.M. Phytochemical Potential of Eruca Sativa for Inhibition of Melanoma Tumor Growth. Fitoterapia 2011, 82, 647–653. [Google Scholar] [CrossRef]
- Rocket Genetic Resources Network; Padulosi, S. (Stefano); Project on Underutilized Mediterranean Species; International Plant Genetic Resources Institute. Rocket Genetic Resources Network: Report of the First Meeting, Lisbon, Portugal, 13–15 November 1994; IPGRI: Rome, Italy, 1995; ISBN 9290432551. [Google Scholar]
- Padulosi, S.; Pignone, D.; International Plant Genetic Resources Institute. Underutilized Mediterranean Species. In Proceedings of the Rocket Genetic Resources Network (Meeting) (2nd), Rocket: A Mediterranean Crop for the World: Report of a Workshop, Legnaro (Padova), Italy, 13–14 December 1996; ISBN 929043337X. [Google Scholar]
- Chun, J.-H.; Arasu, M.V.; Lim, Y.-P.; Kim, S.-J. Variation of Major Glucosinolates in Different Varieties and Lines of Rocket Salad. Hortic. Environ. Biotechnol. 2013, 54, 206–213. [Google Scholar] [CrossRef]
- Falk, K. Glucosinolate Biosynthesis: Demonstration and Characterization of the Condensing Enzyme of the Chain Elongation Cycle in Eruca sativa. Phytochemistry 2004, 65, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.M.; Aburjai, T.A. Effect of Ethnomedicinal Plants Used in Folklore Medicine in Jordan as Antibiotic Resistant Inhibitors on Escherichia Coli. BMC Complement. Altern. Med. 2010, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Sarwar Alam, M.; Kaur, G.; Jabbar, Z.; Javed, K.; Athar, M. Eruca sativa Seeds Possess Antioxidant Activity and Exert a Protective Effect on Mercuric Chloride Induced Renal Toxicity. Food Chem. Toxicol. 2007, 45, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Mahran, G.H.; Kadry, H.A.; Isaac, Z.G.; Thabet, C.K.; Al-Azizi, M.M.; El-Olemy, M.M. Investigation of Diuretic Drug Plants. 1. Phytochemical Screening and Pharmacological Evaluation of Anethum graveolens L., Apium graveolens L., Daucus carota L. and Eruca sativa Mill. Phytother. Res. 1991, 5, 169–172. [Google Scholar] [CrossRef]
- Rafatullah, S.; Al-Sheikh, A.; Alqsoumi, S.; Al-Yahya, M.; El-Tahir, K.; Galal, A. Protective Effect of Fresh Radish Juice (Raphanus Sativus L.) Against Carbon Tetrachloride-Induced Hepatotoxicity. Int. J. Pharmacol. 2008, 4, 130–134. [Google Scholar] [CrossRef]
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Magan, J.; Zhang, Y.; Rowland, I.R.; Wagstaff, C. Analysis of Phytochemical Composition and Chemoprotective Capacity of Rocket (Eruca sativa and Diplotaxis tenuifolia) Leafy Salad Following Cultivation in Different Environments. J. Agric. Food Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef]
- Heimler, D.; Isolani, L.; Vignolini, P.; Tombelli, S.; Romani, A. Polyphenol Content and Antioxidative Activity in Some Species of Freshly Consumed Salads. J. Agric. Food Chem. 2007, 55, 1724–1729. [Google Scholar] [CrossRef]
- Fuentes, E.; Alarcón, M.; Fuentes, M.; Carrasco, G.; Palomo, I. A Novel Role of Eruca sativa Mill. (Rocket) Extract: Antiplatelet (NF-ΚB Inhibition) and Antithrombotic Activities. Nutrients 2014, 6, 5839–5852. [Google Scholar] [CrossRef]
- Hamid, S.; Muhammad, G.; Farnaz, M.; Umara, A.; Kazi, M.A.; Abbas, H.; Asif, H.C. Anti-Inflammatory and Anti-Allergic Activity of Eruca Sativa Seed. Int. J. Curr. Pharm. Res. 2015, 7, 71–73. [Google Scholar]
- Al-Enazi, M.M.; Ahamd, R.; Rahiman, S. Antinociceptive and Anti-inflammatory Activities of Eruca sativa L. Leaves Extract. Adv. Biores. 2014, 5, 145–150. [Google Scholar] [CrossRef]
- Salih, R.A.; Abdulkareem, A. Dawah LD50 and Affective Dose of Eruca sativa Mill (Gergeer) Ethanolic Extract. Univ. Thi-Qar J. Agric. Res. 2022, 11, 11–24. [Google Scholar] [CrossRef]
- Bruce, R. An Up-and-down Procedure for Acute Toxicity Testing. Fundam. Appl. Toxicol. 1985, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Gromoll, J.; Nieschlag, E. The Follicle-Stimulating Hormone Receptor: Biochemistry, Molecular Biology, Physiology, and Pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef]
- Kangasniemi, M.; Kaipia, A.; Toppari, J.; Perheentupa, A.; Huhtaniemi, I.; Parvinen, M. Cellular Regulation of Follicle-Stimulating Hormone (FSH) Binding in Rat Seminiferous Tubules. J. Androl. 1990, 11, 336–343. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J. Hormonal Control of Germ Cell Development and Spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 55–65. [Google Scholar] [CrossRef]
- McLachlan, R.I. The Endocrine Control of Spermatogenesis. Best. Pr. Res. Clin. Endocrinol. Metab. 2000, 14, 345–362. [Google Scholar] [CrossRef]
- Huhtaniemi, I. A Hormonal Contraceptive for Men: How Close Are We. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; pp. 273–288. [Google Scholar]
- McLachlan, R.I. Identification of Specific Sites of Hormonal Regulation in Spermatogenesis in Rats, Monkeys, and Man. Recent. Prog. Horm. Res. 2002, 57, 149–179. [Google Scholar] [CrossRef]
- Meachem, S.J.; Mclachlan, R.I.; Stanton, P.G.; Robertson, D.M.; Wreford, N.G. FSH Immunoneutralization Acutely Impairs Spermatogonial Development in Normal Adult Rats. J. Androl. 1999, 20, 756–762. [Google Scholar] [CrossRef]
- Mclachlan, R.I.; Wreford, N.G.; Meachem, S.J.; De Kretser, D.M.; Robertson, D.M. Effects of Testosterone on Spermatogenic Cell Populations in the Adult Rat1. Biol. Reprod. 1994, 51, 945–955. [Google Scholar] [CrossRef][Green Version]
- Sharpe, R.; McKinnell, C.; Kivlin, C.; Fisher, J. Proliferation and Functional Maturation of Sertoli Cells, and Their Relevance to Disorders of Testis Function in Adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Ruwanpura, S.M.; McLachlan, R.I.; Meachem, S.J. Hormonal Regulation of Male Germ Cell Development. J. Endocrinol. 2010, 205, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Manetti, G.J.; Honig, S.C. Update on Male Hormonal Contraception: Is the Vasectomy in Jeopardy? Int. J. Impot. Res. 2010, 22, 159–170. [Google Scholar] [CrossRef]
- Hayes, F.J. Differential Regulation of Gonadotropin Secretion by Testosterone in the Human Male: Absence of a Negative Feedback Effect of Testosterone on Follicle-Stimulating Hormone Secretion. J. Clin. Endocrinol. Metab. 2001, 86, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant Systems and Oxidative Stress in the Testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Gagnon, C. Reactive Oxygen Species and Human Spermatozoa. J. Androl. 1992, 13, 379–386. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Storey, B.T. Role of Glutathione Peroxidase in Protecting Mammalian Spermatozoa from Loss of Motility Caused by Spontaneous Lipid Peroxidation. Gamete Res. 1989, 23, 77–90. [Google Scholar] [CrossRef]
- Sakkas, D.; Seli, E.; Bizzaro, D.; Tarozzi, N.; Manicardi, G.C. Abnormal Spermatozoa in the Ejaculate: Abortive Apoptosis and Faulty Nuclear Remodelling during Spermatogenesis. Reprod. Biomed. Online 2003, 7, 428–432. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Aitken, R.J. The Role of Sperm Oxidative Stress in Male Infertility and the Significance of Oral Antioxidant Therapy. Hum. Reprod. 2011, 26, 1628–1640. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, H.W.G. Andrology: Seminal Leukocytes: Passengers, Terrorists or Good Samaritans? Hum. Reprod. 1995, 10, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.C.L. Regulation of Sperm Function by Reactive Oxygen Species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Hcld, A.A. Oxidative Stress and Male Infertility: From Research Bench to Clinical Practice. J. Androl. 2002, 23, 737–752. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and Oxidative Stress: Dilemma for Sperm Function and Male Fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Trussell, J. Optimal Diagnosis and Medical Treatment of Male Infertility. Semin. Reprod. Med. 2013, 31, 235–236. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Mens. Health 2014, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of Mitochondrial Reactive Oxygen Species in the Generation of Oxidative Stress in Spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of Oxidative Stress and Antioxidants on Semen Functions. Vet. Med. Int. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Chen, S.; Allam, J.-P.; Duan, Y.; Haidl, G. Influence of Reactive Oxygen Species on Human Sperm Functions and Fertilizing Capacity Including Therapeutical Approaches. Arch. Gynecol. Obs. 2013, 288, 191–199. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J. Effect of Cigarette Smoking on Levels of Seminal Oxidative Stress in Infertile Men: A Prospective Study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Sh, R. Protective Effects of Quercetin on Spermatogenesis in Streptozotocin-Induced Diabetic Rat. J. Med. Plants 2009, 8, 57–64. [Google Scholar]
- Feng, S.L.; Li, S.H.; Wang, Y.; Chen, C.C.; Gao, B. Effect of Ligustrum Fruit Extract on Reproduction in Experimental Diabetic Rats. Asian J. Androl. 2001, 3, 71–73. [Google Scholar] [PubMed]
- Hassan, A.A.; Hassouna, M.M.; Taketo, T.; Gagnon, C.; Elhilali, M.M. The Effect of Diabetes on Sexual Behavior and Reproductive Tract Function in Male Rats. J. Urol. 1993, 149, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Rolo, A.P.; Palmeira, C.M. Diabetes and Mitochondrial Function: Role of Hyperglycemia and Oxidative Stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Pinto Provenzano, S.; Montagna, D.D.; Coppola, L.; Zara, V. Oxidative Stress Negatively Affects Human Sperm Mitochondrial Respiration. Urology 2013, 82, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative Stress and Male Infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA Damage Caused by Oxidative Stress: Modifiable Clinical, Lifestyle and Nutritional Factors in Male Infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- de Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for Male Subfertility. Cochrane Database Syst. Rev. 2022, 2022, CD007411. [Google Scholar] [CrossRef]
- Gharagozloo, P.; Gutiérrez-Adán, A.; Champroux, A.; Noblanc, A.; Kocer, A.; Calle, A.; Pérez-Cerezales, S.; Pericuesta, E.; Polhemus, A.; Moazamian, A.; et al. A Novel Antioxidant Formulation Designed to Treat Male Infertility Associated with Oxidative Stress: Promising Preclinical Evidence from Animal Models. Hum. Reprod. 2016, 31, 252–262. [Google Scholar] [CrossRef]
- Patel, S.R.; Sigman, M. Antioxidant Therapy in Male Infertility. Urol. Clin. N. Am. 2008, 35, 319–330. [Google Scholar] [CrossRef]
- Chang, H.; Yeung, T.C.; Yang, X.; Gao, J.; Wu, X.; Wang, C.C. Chinese Herbal Medicines as Complementary Therapy to in Vitro Fertilization-Embryo Transfer in Women with Infertility: Protocols and Applications. Hum. Fertil. 2023, 26, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.R.; Moustafa, N.A. Histological and Quantitative Study of the Effect of Eruca Sativa Seed Oil on The Testis of Albino Rat. Egypt. J. Hosp. Med. 2001, 2, 148–162. [Google Scholar] [CrossRef]
- Hussein, Z.F. Study the Effect of Eruca Sativa Leaves Extract on Male Fertility in Albino Mice. Al-Nahrain J. Sci. 2013, 16, 143–146. [Google Scholar] [CrossRef]
- Ansari, M.N.; Ganaie, M.A. Ameliorative Effect of Rocket Leaves on Fertility in Streptozotocin-Induced Diabetic Rats. Int. Res. J. Biol. Sci. 2014, 3, 89–97. [Google Scholar]
- Hassan, H.F.; Meligi, N.M. Effects of Sublethal Abamectin Exposure on Some Hormonal Profiles and Testicular Histopathology in Male Albino Rats and the Possible Ameliorative Role of Eruca Sativa. Environ. Sci. Pollut. Res. 2017, 24, 24690–24697. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, G.S.; El-Fark, M.O.; Hamdy, R.M. Protective Effect of Eruca Sativa Seed Oil against Oral Nicotine Induced Testicular Damage in Rats. Tissue Cell 2016, 48, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, C.; Cosci, I.; Moro, G.; Stortini, A.M.; Sandon, A.; De Angelis, C.; Galdiero, G.; Trifuoggi, M.; Pivonello, R.; Pedrucci, F.; et al. Seminal Cadmium Affects Human Sperm Motility through Stable Binding to the Cell Membrane. Front. Cell Dev. Biol. 2023, 11, 1134304. [Google Scholar] [CrossRef]
- Al-Okaily, B.N.; Al-Shammari, Z.M. The Impact of Eruca Sativa Seeds on Leydig’s Cells Number and Hormonal Profile In Cadmium Exposed Rats. Kufa J. Vet. Med. Sci. 2016, 7, 241–253. [Google Scholar] [CrossRef]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Grami, D.; Rtibi, K.; Hammami, I.; Selmi, S.; De Toni, L.; Foresta, C.; Marzouki, L.; Sebai, H. Protective Action of Eruca Sativa Leaves Aqueous Extracts Against Bisphenol A-Caused In Vivo Testicular Damages. J. Med. Food 2020, 23, 600–610. [Google Scholar] [CrossRef]
- Weckhuysen, B.M. A Sustainable Alternative to Bisphenol A. Nat. Sustain. 2023, 6, 1516–1517. [Google Scholar] [CrossRef]
- Ji, H.; Miao, M.; Liang, H.; Shi, H.; Ruan, D.; Li, Y.; Wang, J.; Yuan, W. Exposure of Environmental Bisphenol A in Relation to Routine Sperm Parameters and Sperm Movement Characteristics among Fertile Men. Sci. Rep. 2018, 8, 17548. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.A.; Almamoori, A.M.J.; Al-Hassnawi, A.T.S.; Hameedi, E.H. Oxidative Response Associated with Treatment of Male Albino Rats with Eruca Sativa Mill Leaves Extract and Correlations with Complete Blood Picture. J. Pharm. Sci. Res. 2017, 9, 2278–2285. [Google Scholar]
- Aitken, R.J. Reactive Oxygen Species as Mediators of Sperm Capacitation and Pathological Damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Ciccone, V.; Piragine, E.; Gorica, E.; Citi, V.; Testai, L.; Pagnotta, E.; Matteo, R.; Pecchioni, N.; Montanaro, R.; Di Cesare Mannelli, L.; et al. Anti-Inflammatory Effect of the Natural H2S-Donor Erucin in Vascular Endothelium. Int. J. Mol. Sci. 2022, 23, 15593. [Google Scholar] [CrossRef]
- Grami, D.; Rtibi, K.; Selmi, S.; Jridi, M.; Sebai, H.; Marzouki, L.; Sabovic, I.; Foresta, C.; De Toni, L. Aqueous Extract of Eruca Sativa Protects Human Spermatozoa from Mitochondrial Failure Due to Bisphenol A Exposure. Reprod. Toxicol. 2018, 82, 103–110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grami, D.; Selmi, S.; Rtibi, K.; Sebai, H.; De Toni, L. Emerging Role of Eruca sativa Mill. in Male Reproductive Health. Nutrients 2024, 16, 253. https://doi.org/10.3390/nu16020253
Grami D, Selmi S, Rtibi K, Sebai H, De Toni L. Emerging Role of Eruca sativa Mill. in Male Reproductive Health. Nutrients. 2024; 16(2):253. https://doi.org/10.3390/nu16020253
Chicago/Turabian StyleGrami, Dhekra, Slimen Selmi, Kais Rtibi, Hichem Sebai, and Luca De Toni. 2024. "Emerging Role of Eruca sativa Mill. in Male Reproductive Health" Nutrients 16, no. 2: 253. https://doi.org/10.3390/nu16020253
APA StyleGrami, D., Selmi, S., Rtibi, K., Sebai, H., & De Toni, L. (2024). Emerging Role of Eruca sativa Mill. in Male Reproductive Health. Nutrients, 16(2), 253. https://doi.org/10.3390/nu16020253




