Effectiveness of a Food Supplement Based on Glucomannan, D-Chiro-Inositol, Cinnamomum zeylanicum Blume and Inulin in Patients with Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Outcomes
- -
- Anthropometric measures, such as weight, height and waist circumference were measured by a trained dietician. Visceral adiposity index (VAI) was calculated using a combination of waist circumference, body mass index (BMI), triglycerides and high-density lipoproteins (LDL), using the equations proposed in [15].
- -
- Laboratory measures included markers of insulin-resistance or glucose metabolism, such as glycosylated haemoglobin (HbA1c), fasting plasma glucose and serum insulin. We also calculated the HOMA index [16] based on fasting plasma glucose and serum insulin levels at 8 am. HOMA uses fasting measurements of blood glucose and insulin concentrations to calculate indices of both insulin sensitivity and β-cell function. The principle of HOMA is that blood glucose and insulin concentrations are related by the feedback of glucose on β-cells to increase insulin secretion. Finally, we evaluated cholesterol metabolism, renal and hepatic function. All the laboratory measurements were performed using standard laboratory assessments.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- De la Iglesia, R.; Loria-Kohen, V.; Zulet, M.A.; Martinez, J.A.; Reglero, G.; Ramirez de Molina, A. Dietary strategies implicated in the prevention and treatment of metabolic syndrome. Int. J. Mol. Sci. 2016, 17, 1877. [Google Scholar] [CrossRef] [PubMed]
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. J. Am. Med. Assoc. 2001, 286, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Grave, R.D.; Suppini, A.; Marchesini, G.; Flin, F.L.I.N. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology 2008, 47, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef] [PubMed]
- Santini, A. Nutraceuticals: An Healthy Bet for the Future. J. Food Res. 2014, 3, 1–2. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Hosseinzadeh, H. Cinnamon effects on metabolic syndrome: A review based on its mechanisms. Iran. J. Basic Med. Sci. 2016, 19, 1258–1270. [Google Scholar]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef]
- Davinelli, S.; Nicolosi, D.; Di Cesare, C.; Scapagnini, G.; Di Marco, R. Targeting Meta-bolic Consequences of Insulin Resistance in Polycystic Ovary Syndrome by D-chiro-inositol and Emerging Nutraceuticals: A Focused Review. J. Clin. Med. 2020, 9, 987. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a Water-Soluble Cinnamon Extract on Body Composition and Features of the Metabolic Syndrome in Pre-Diabetic Men and Women. J. Int. Soc. Sports Nutr. 2006, 3, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Rahmani, J.; Kord-Varkaneh, H.; Sheikhi, A.; Larijani, B.; Esmaillzadeh, A. Cinnamon supplementation positively affects obesity: A systematic review and dose-response meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, T.; Peter, A.; Schulz, N.; Drescher, A.; Bergheim, I.; Machann, J.; Schick, F.; Siegel-Axel, D.; Schürmann, A.; Weigert, C.; et al. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS ONE 2014, 9, e92358. [Google Scholar] [CrossRef] [PubMed]
- Gargari, B.P.; Dehghan, P.; Aliasgharzadeh, A.; Jafar-Abadi, M.A. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab. J. 2013, 37, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Borghi, C.; Cicero, A.F. Nutraceuticals with a clinically detectable blood pressure-lowering effect: A review of available randomized clinical trials and their meta-analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171. [Google Scholar] [CrossRef]
- Fitó, M.; Konstantinidou, V. Nutritional Genomics and the Mediterranean Diet’s Effects on Human Cardiovascular Health. Nutrients 2016, 8, 218. [Google Scholar] [CrossRef]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Au-Yeung, F.; Jovanovski, E.; Jenkins, A.L.; Zurbau, A.; Ho, H.V.T.; Vuksan, V. The effects of gelled konjac glucomannan fibre on appetite and energy intake in healthy individuals: A randomised cross-over trial. Br. J. Nutr. 2018, 119, 109–116. [Google Scholar] [CrossRef]
- Kalra, B.; Kalra, S.; Sharma, J.B. The inositols and polycystic ovary syndrome. Indian J. Endocrinol. Metab. 2016, 20, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liang, W.; Wei, M.; Gou, X.; Han, S.; Bai, J. Effects of D-Chiro-Inositol on Glucose Metabolism in db/db Mice and the Associated Underlying Mechanisms. Front. Pharmacol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Lazarenko, R.; Geisler, J.; Bayliss, D.; Larner, J.; Li, C. D-chiro-inositol glycan stimulates insulin secretion in pancreatic β cells. Mol. Cell. Endocrinol. 2014, 387, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Law, B.Y.K.; Xu, Y. Effect of inulin-type carbohydrates on insulin resistance in patients with type 2 diabetes and obesity: A systematic review and meta-analysis. J. Diabetes Res. 2019, 2019, 5101423. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Jiao, Y.; Liu, Y.; Li, T.; Wang, Z.; Wang, D. Protective Effects of Konjac and Inulin Extracts on Type 1 and Type 2 Diabetes. J. Diabetes Res. 2019, 7, 3872182. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Sievenpiper, J.L.; Xu, Z.; Wong, E.Y.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Leiter, L.A.; Josse, R.G.; Stavro, M.P. Konjac-Mannan and American ginseng: Emerging al-ternative therapies for type 2 diabetes mellitus. J. Am. Coll. Nutr. 2001, 20, 370S–380S. [Google Scholar] [CrossRef]
- Ueno, H.; Haraguchi, N.; Azuma, M.; Shiiya, T.; Noda, T.; Ebihara, E.; Uehira, Y.; Uchida, T.; Sasaba, K.; Nakamura, M.; et al. Active Consumption of Konjac and Konjac Products Improves Blood Glucose Control in Patients with Type 2 Diabetes Mellitus. J. Am. Coll. Nutr. 2023, 42, 123–129. [Google Scholar] [CrossRef]
- Montt-Guevara, M.M.; Finiguerra, M.; Marzi, I.; Fidecicchi, T.; Ferrari, A.; Genazzani, A.D.; Simoncini, T. D-Chiro-Inositol Regulates Insulin Signaling in Human Adipocytes. Front. Endocrinol. 2021, 12, 660815. [Google Scholar] [CrossRef]
- Filippello, A.; Scamporrino, A.; Di Mauro, S.; Malaguarnera, R.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules 2020, 10, 1404. [Google Scholar] [CrossRef]
- Cheng, F.; Han, L.; Xiao, Y.; Pan, C.; Li, Y.; Ge, X.; Zhang, Y.; Yan, S.; Wang, M. D-Chiro-inositol Ameliorates High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance via PKCε-PI3K/AKT Path-way. J. Agric. Food Chem. 2019, 67, 5957–5967. [Google Scholar] [CrossRef]


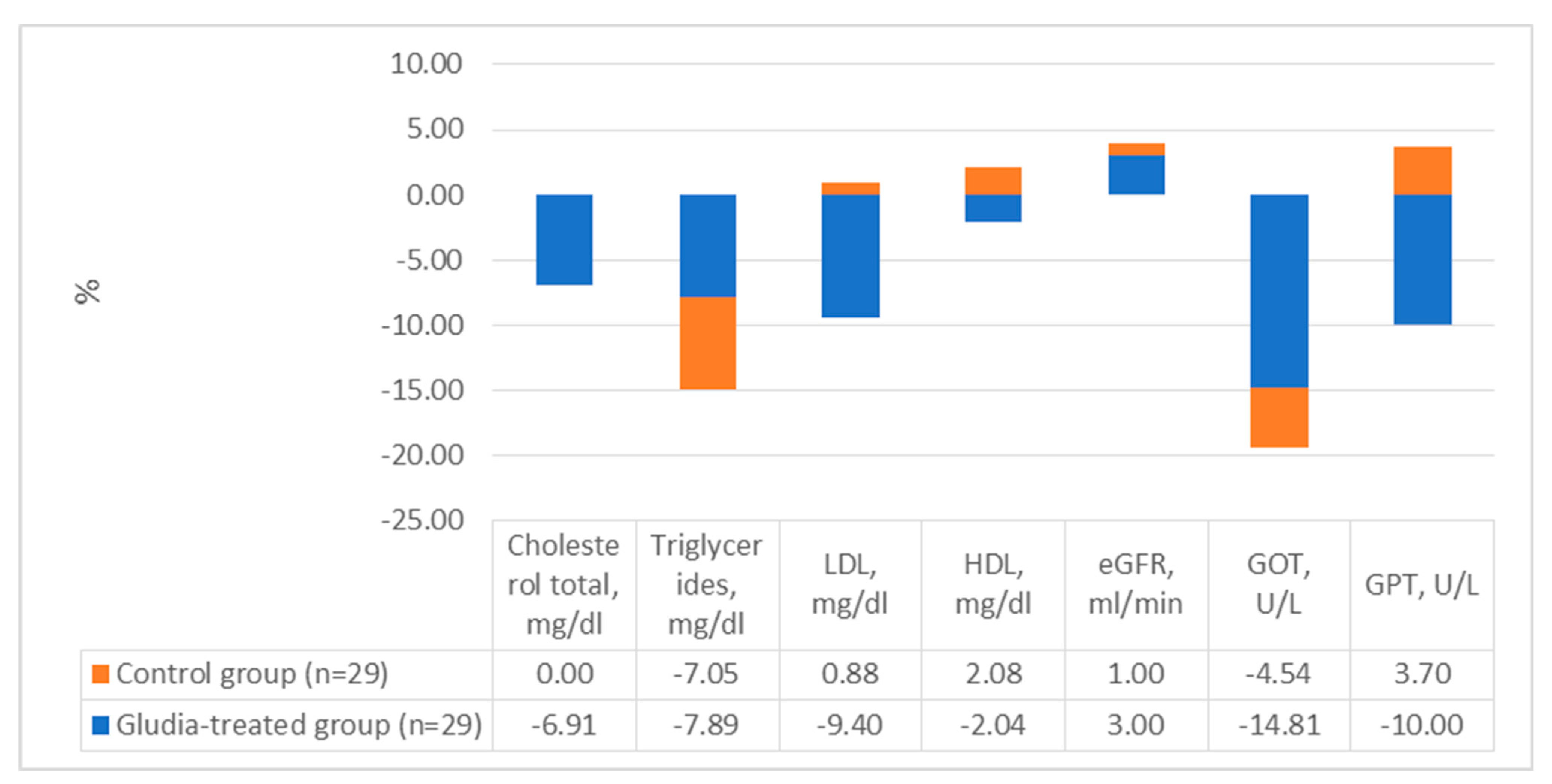
| Gludia-Treated Group (n = 29) | Control Group (n = 29) | Between Groups (Change 16 Weeks vs. Baseline) | p Value between Groups a | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 16 Weeks | Δ | Baseline | 16 Weeks | Δ | Δ | ||
| Measure | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean (95%CI) | |
| Age, years | 50 (19) | - | - | 62 (11) | - | - | - | 0.006 |
| Females, % | 51.7 | - | - | 69 | - | - | - | 0.29 |
| Weight, kg | 92.9 (24) | 89.6 (24) | −3.4 ± 3.9 ^^^ | 82.9 (1.4) | 82.6 (15.1) | −0.3 ± 2.0 | −2.7 (−4.4 to −1.1) | 0.001 |
| Body mass index, kg/m2 | 34.5 (7.2) | 32.4 (6.6) | −2.0 ± 3.4 ^^^ | 30.9 (5.6) | 30.7 (5.9) | −0.15 ± 0.78 | −0.98 (−1.59 to −0.37) | 0.002 |
| Waist circumference, cm | 108 (18) | 106 (16) | −2 ± 6 | 106 (16) | 106 (15) | 0 ± 3 | −0.99 (−3.45 to 1.47) | 0.42 |
| VAI | 4.28 (1.69) * | 4.01 (1.50) | −0.26 ± 1.03 | 6.22 (3.95) | 5.74 (3.84) | −0.47 ± 1.51 | −0.10 (−0.79 to 0.59) | 0.34 |
| HbA1c, % | 6.22 (1.06) | 5.96 (0.73) | −0.26 ± 0.77 | 6.47 (0.59) | 6.31 (0.58) | −0.17 ± 0.20 ^^^ | −0.20 (−0.43 to 0.03) | 0.09 |
| Fasting plasma glucose, mg/dL | 111 (32) | 102 (23) | −8 ± 31 | 120 (21) | 114 (19) | −6 ± 18 | −8.19 (−18.23 to 1.85) | 0.11 |
| Serum insulin, mIU/L | 19.0 (6.5) | 17.4 (5.8) | −1.7 ± 4.4 ^ | 17.0 (5.7) | 15.4 (5.4) | −1.6 ± 1.9 ^^ | 0.13 (−1.52 to 1.78) | 0.82 |
| HOMA index | 5.35 (2.77) | 4.35 (1.52) | −1.00 ± 2.00 ^^ | 5.24 (2.20) | 4.37 (1.72) | −0.89 ± 1.21 ^^ | −0.05 (−0.73 to 0.64) | 0.88 |
| Cholesterol total, mg/dL | 188 (36) | 175 (31) | −13 ± 22 ^^ | 192 (37) | 192 (43) | 0 ± 23 | −13 (−25 to −1) | 0.03 |
| Triglycerides, mg/dL | 114 (33) ** | 105 (30) | −9 ± 21 ^^ | 156 (62) | 146 (61) | −11 ± 28 | −4 (−19 to 9) | 0.50 |
| LDL, mg/dL | 117 (35) | 105 (30) | −11 ± 23 ^^ | 113 (34) | 114 (39) | 1 ± 25 | −11 (−23 to 1) | 0.08 |
| HDL, mg/dL | 49 (10) | 48 (9) | −1 ± 5 | 48 (11) | 49 (11) | 1 ± 5 | −1 (−4 to 2) | 0.42 |
| eGFR, ml/min | 100 (22) | 103 (21) | 3 ± 11 | 102 (26) | 101 (25) | −1 ± 10 | −4 (−23 to 15) | 0.69 |
| GOT, U/L | 27 (19) | 23 (13) | −4 ± 9 ^ | 22 (9) | 21 (9) | −1 ± 6 | −1 (−5 to 2) | 0.39 |
| GPT, U/L | 30 (19) | 27 (18) | −3 ± 7 ^ | 27 (15) | 28 (16) | 1 ± 9 | −2 (−8 to 4) | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citarrella, R.; Chianetta, R.; Amodeo, S.; Mirarchi, L.; Licata, A.; Soresi, M.; Veronese, N.; Barbagallo, M.; Giannitrapani, L. Effectiveness of a Food Supplement Based on Glucomannan, D-Chiro-Inositol, Cinnamomum zeylanicum Blume and Inulin in Patients with Metabolic Syndrome. Nutrients 2024, 16, 249. https://doi.org/10.3390/nu16020249
Citarrella R, Chianetta R, Amodeo S, Mirarchi L, Licata A, Soresi M, Veronese N, Barbagallo M, Giannitrapani L. Effectiveness of a Food Supplement Based on Glucomannan, D-Chiro-Inositol, Cinnamomum zeylanicum Blume and Inulin in Patients with Metabolic Syndrome. Nutrients. 2024; 16(2):249. https://doi.org/10.3390/nu16020249
Chicago/Turabian StyleCitarrella, Roberto, Roberta Chianetta, Simona Amodeo, Luigi Mirarchi, Anna Licata, Maurizio Soresi, Nicola Veronese, Mario Barbagallo, and Lydia Giannitrapani. 2024. "Effectiveness of a Food Supplement Based on Glucomannan, D-Chiro-Inositol, Cinnamomum zeylanicum Blume and Inulin in Patients with Metabolic Syndrome" Nutrients 16, no. 2: 249. https://doi.org/10.3390/nu16020249
APA StyleCitarrella, R., Chianetta, R., Amodeo, S., Mirarchi, L., Licata, A., Soresi, M., Veronese, N., Barbagallo, M., & Giannitrapani, L. (2024). Effectiveness of a Food Supplement Based on Glucomannan, D-Chiro-Inositol, Cinnamomum zeylanicum Blume and Inulin in Patients with Metabolic Syndrome. Nutrients, 16(2), 249. https://doi.org/10.3390/nu16020249






