Chemopreventive Effects of Polysaccharides and Flavonoids from Okra Flowers in Azomethane/Dextran Sulfate Sodium-Induced Murine Colitis-Associated Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AFE and APE
2.2. Cell Lines and Cell Culture
2.3. Mice and Animal Experiments
2.3.1. Mice
2.3.2. AOM/DSS Animal Study
2.3.3. Disease Activity Index
2.3.4. Enumeration of Lesions and Measurement of Colon Length
2.4. Gut Microbiota Analyses
2.5. Hematoxylin and Eosin (H&E) Staining and Immunohistochemistry
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. APE and AFE Attenuated the AOM/DSS-Induced Physiological Index
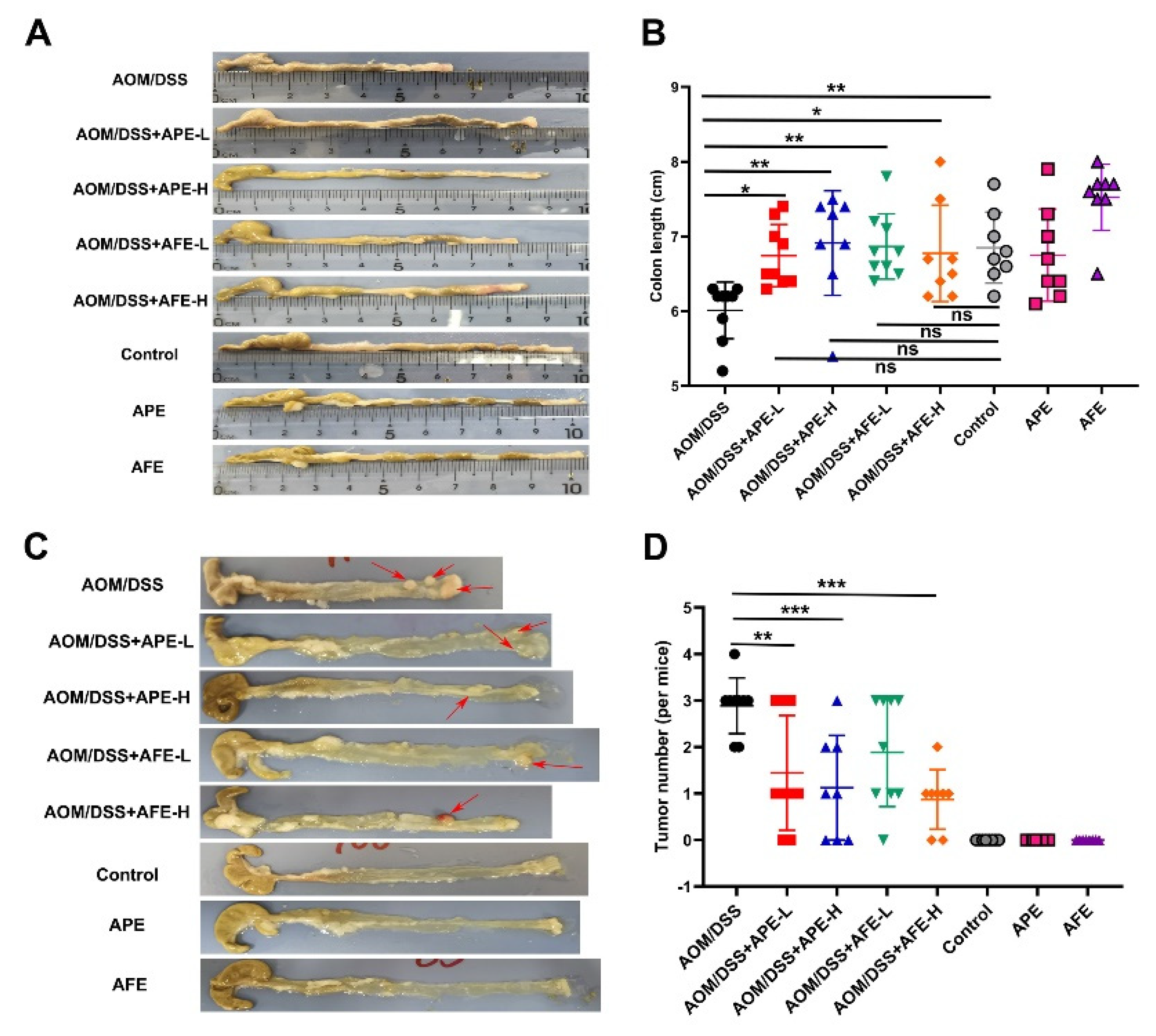
3.2. APE and AFE Attenuate AOM/DSS-Induced Colorectal Carcinogensis
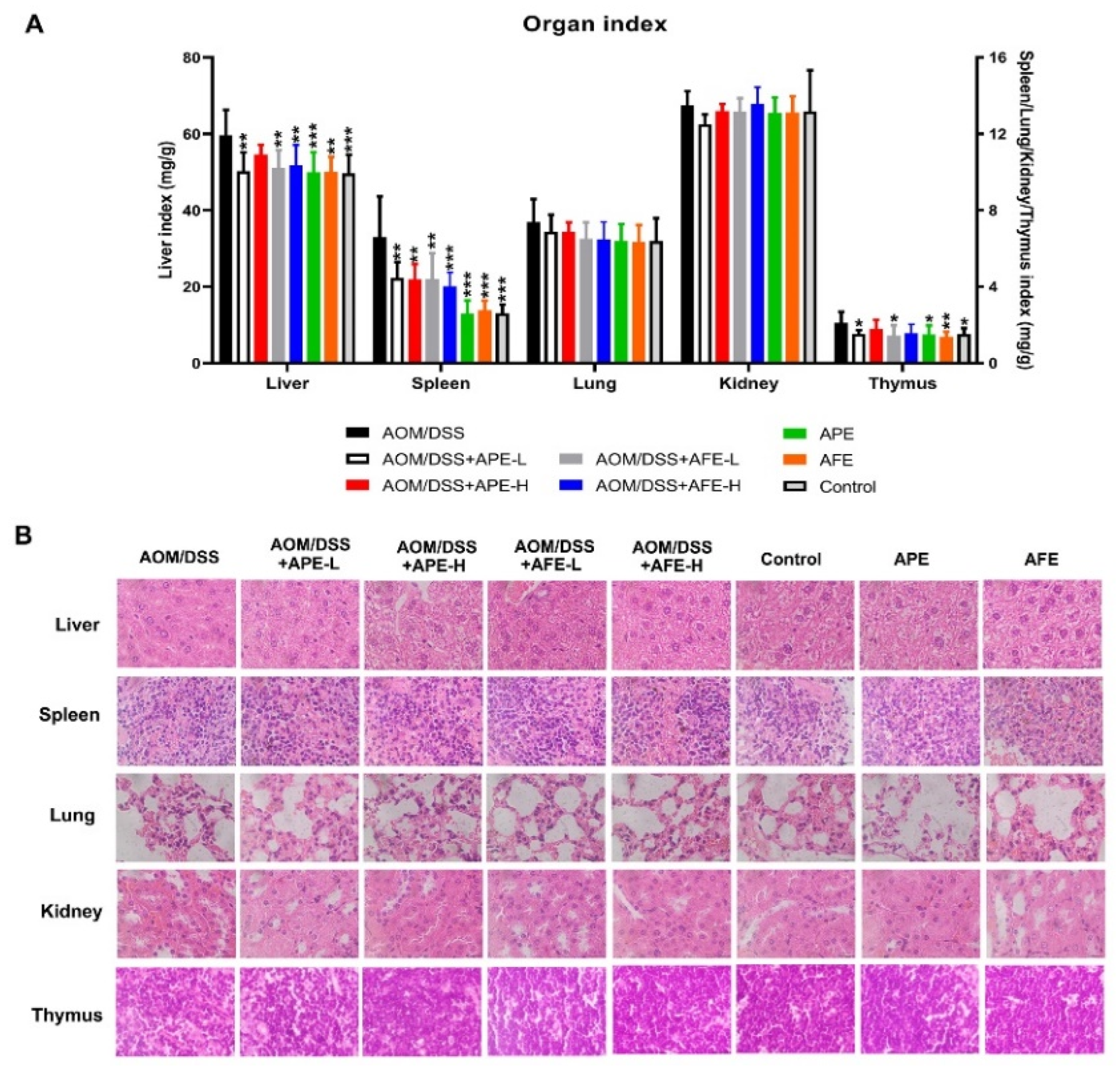
3.3. APE and AFE Protect Organs against AOM/DSS-Induced Injury
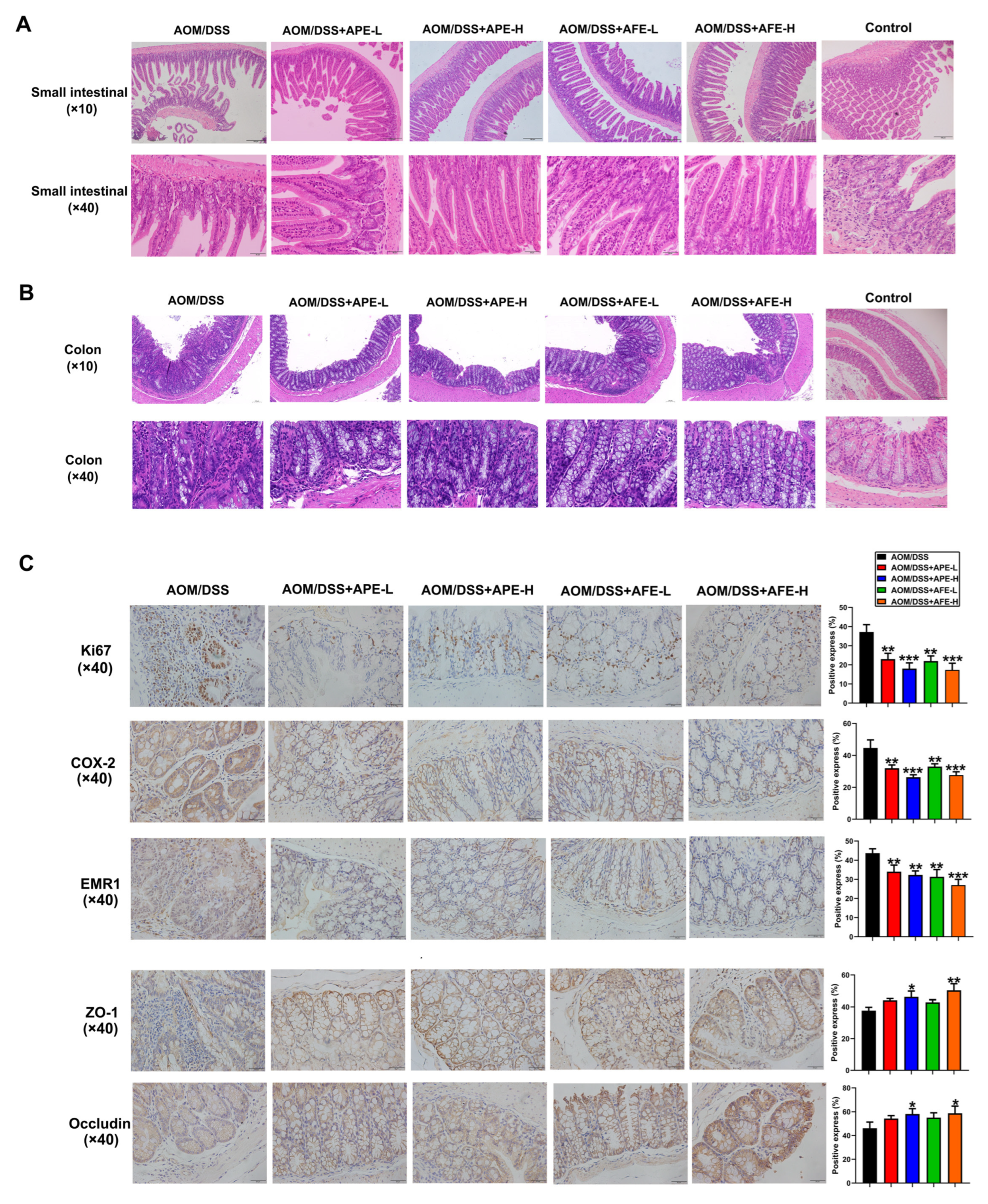
3.4. APE and AFE Protect the Small Intestine and Colon against AOM/DSS-Induced Injury
3.5. APE and AFE Inhibited Colonic Inflammation in the AOM/DSS Mice by Regulating the Inflammatory Signaling Pathway
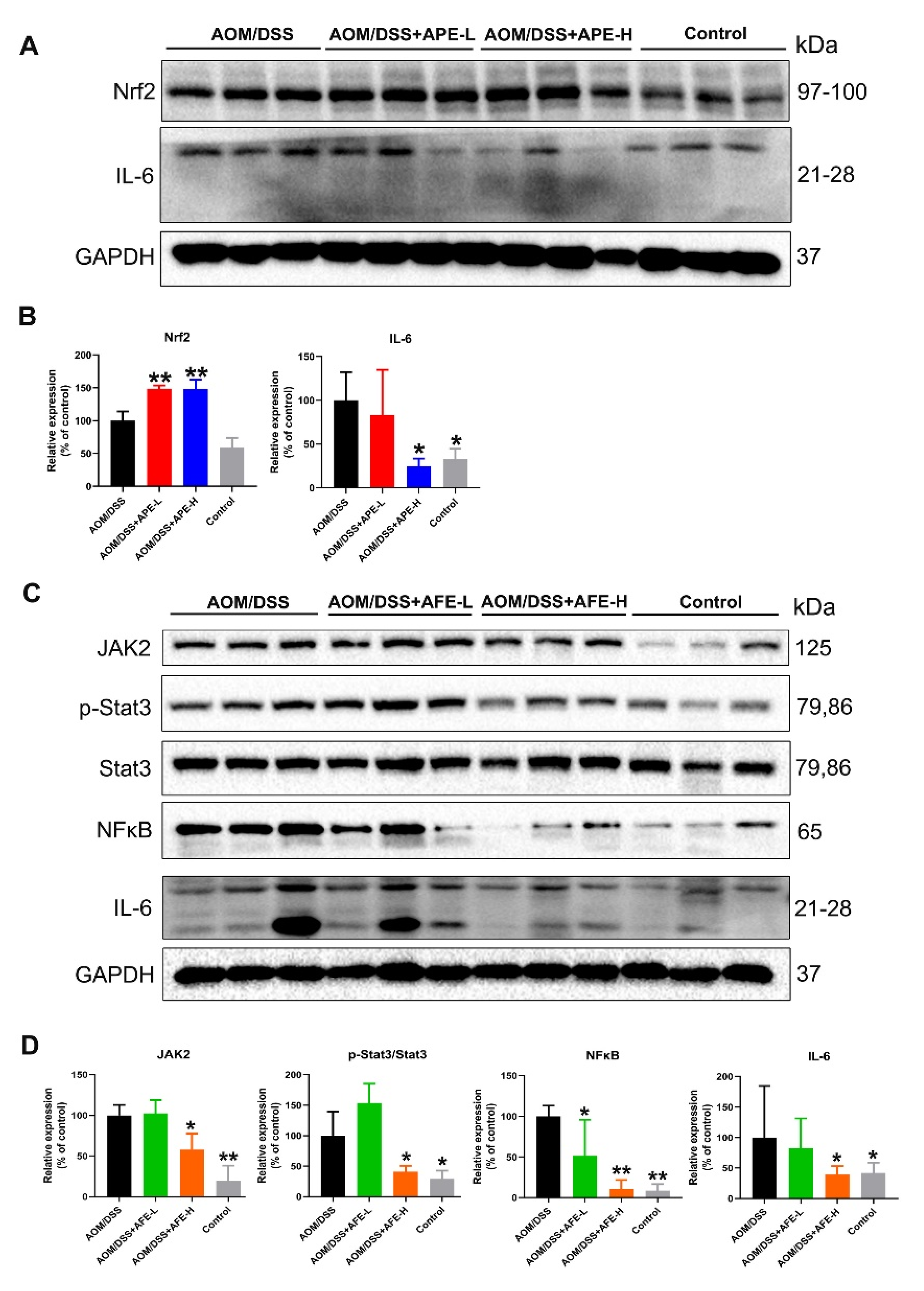
3.5.1. APE and AFE Regulated IL-6 and Related Signaling Pathways in the AOM/DSS Mice
3.5.2. APE and AFE Regulated the MAPKs Signaling Pathway in the AOM/DSS Mice
3.6. APE and AFE Inhibited the CRC-Associated Signaling Pathways
3.6.1. APE and AFE Inhibited PI3K/AKT/mTOR Signal Transduction in the AOM/DSS Mice
3.6.2. APE and AFE Partly Regulated the Wnt/β-Catenin Signaling Pathway in the AOM/DSS Mice
3.7. AFE Inhibited CT26 Colon Cancer Cell Growth and Metastasis through the Regulation of Multiple Pathways
3.8. APE and AFE Treatment Modulated the Diversity and Composition of the Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lu, Y.; Yu, Z.D.; Xu, L.; Liu, J.; Chen, K.S.; Zhang, P.Y. The inhibitory effect of polysaccharide from Rhizopus nigricans on colitis-associated colorectal cancer. Biomed. Pharmacother. 2019, 112, 108593. [Google Scholar] [CrossRef]
- Feagins, L.A.; Souza, R.F.; Spechler, S.J. Carcinogenesis in IBD: Potential targets for the prevention of colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Chumanevich, A.A.; Poudyal, D.; Cui, X.; Davis, T.; Wood, P.A.; Smith, C.D.; Hofseth, L.J. Suppression of colitis-driven colon cancer in mice by a novel small molecule inhibitor of Sphingosine kinase. Carcinogenesis 2010, 31, 1787–1793. [Google Scholar] [CrossRef]
- Gomez-Chou, S.B.; Swidnicka-Siergiejko, A.K.; Badi, N.; Chavez-Tomar, M.; Lesinski, G.B.; Bekaii-Saab, T.; Farren, M.R.; Mace, T.A.; Schmidt, C.; Liu, Y.; et al. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017, 77, 2647–2660. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.J.; Manna, P.P.; Daglia, M.; Atanasov, A.G.; et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar] [CrossRef]
- Lee, J.H.; Khor, T.O.; Shu, L.M.; Su, Z.Y.; Fuentes, F.; Kong, A.N.T. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol. Ther. 2013, 137, 153–171. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xiang, Q.F.; Zhao, J.; Mao, G.H.; Feng, W.W.; Chen, Y.; Li, Q.; Wu, X.Y.; Yang, L.Q.; Zhao, T. Purification, structural elucidation and physicochemical properties of a polysaccharide from Abelmoschus esculentus L. (okra) flowers. Int. J. Biol. Macromol. 2020, 155, 740–750. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, M.F.; Liao, H.B.; Li, Y.X.; Han, W.; Yuan, K. Content determination of the flavonoids in the different parts and different species of Abelmoschus esculentus L. by reversed phase-high performance liquid chromatograph and colorimetric method. Pharmacogn. Mag. 2014, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, T.; Feng, W.W.; Wang, W.; Zou, Y.; Zheng, D.H.; Takase, M.; Li, Q.; Wu, H.Y.; Yang, L.Q.; et al. Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus. Carbohydr. Polym. 2014, 106, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Wang, M.; Chen, X.; Tian, L.; Wang, L.; Yang, W.; Chen, L.; He, F.; Yin, W. Flavonoid-rich extracts from okra flowers exert antitumor activity in colorectal cancer through induction of mitochondrial dysfunction-associated apoptosis, senescence and autophagy. Food Funct. 2020, 11, 10448–10466. [Google Scholar] [CrossRef]
- Shan, S.; Wu, C.; Shi, J.; Zhang, X.; Niu, J.; Li, H.; Li, Z. Inhibitory Effects of Peroxidase from Foxtail Millet Bran on Colitis-Associated Colorectal Carcinogenesis by the Blockage of Glycerophospholipid Metabolism. J. Agric. Food Chem. 2020, 68, 8295–8307. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933–948. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Nakayama, M.; Han, T.S.; Naoi, K.; Ju, X.L.; Maeda, Y.; Robine, S.; Tsuchiya, K.; Sato, T.; Sato, H.; et al. Suppressing TGF beta Signaling in Regenerating Epithelia in an Inflammatory Microenvironment Is Sufficient to Cause Invasive Intestinal Cancer. Cancer Res. 2015, 75, 766–776. [Google Scholar] [CrossRef]
- Xu, Z.S.; Zhang, H.X.; Li, W.W.; Ran, Y.; Liu, T.T.; Xiong, M.G.; Li, Q.L.; Wang, S.Y.; Wu, M.; Shu, H.B.; et al. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 10447–10452. [Google Scholar] [CrossRef]
- Aguilar, E.; Esteves, P.; Sancerni, T.; Lenoir, V.; Aparicio, T.; Bouillaud, F.; Dentin, R.; Prip-Buus, C.; Ricquier, D.; Pecqueur, C.; et al. UCP2 Deficiency Increases Colon Tumorigenesis by Promoting Lipid Synthesis and Depleting NADPH for Antioxidant Defenses. Cell Rep. 2019, 28, 2306–2316. [Google Scholar] [CrossRef]
- Capuano, A.; Pivetta, E.; Sartori, G.; Bosisio, G.; Favero, A.; Cover, E.; Andreuzzi, E.; Colombatti, A.; Cannizzaro, R.; Scanziani, E.; et al. Abrogation of EMILIN1-beta 1 integrin interaction promotes experimental colitis and colon carcinogenesis. Matrix Biol. 2019, 83, 97–115. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A Novel Method in the Induction of Reliable Experimental Acute and Chronic Ulcerative-Colitis in Mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef]
- Yu, C.H.; Wen, X.D.; Zhang, Z.Y.; Zhang, C.F.; Wu, X.H.; Martin, A.; Du, W.; He, T.C.; Wang, C.Z.; Yuan, C.S. American ginseng attenuates azoxymethane/dextran sodium sulfate-induced colon carcinogenesis in mice. J. Ginseng Res. 2015, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alcoholado, L.; Laborda-Illanes, A.; Otero, A.; Ordonez, R.; Gonzalez-Gonzalez, A.; Plaza-Andrades, I.; Ramos-Molina, B.; Gomez-Millan, J.; Queipo-Ortuno, M.I. Relationships of Gut Microbiota Composition, Short-Chain Fatty Acids and Polyamines with the Pathological Response to Neoadjuvant Radiochemotherapy in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 9549. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Zackular, J.P.; Chen, G.Y.; Schloss, P.D. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2014, 2, 20. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Chassard, C.; Lacroix, C. Carbohydrates and the human gut microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 453–460. [Google Scholar] [CrossRef]
- Pan, H.W.; Du, L.T.; Li, W.; Yang, Y.M.; Zhang, Y.; Wang, C.X. Biodiversity and richness shifts of mucosa-associated gut microbiota with progression of colorectal cancer. Res. Microbiol. 2020, 171, 107–114. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Wei, X.; Tao, J.; Xiao, S.; Jiang, S.; Shang, E.; Zhu, Z.; Qian, D.; Duan, J. Xiexin Tang improves the symptom of type 2 diabetic rats by modulation of the gut microbiota. Sci. Rep. 2018, 8, 3685. [Google Scholar] [CrossRef] [PubMed]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front. Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chang, Y.T.; Tseng, T.H.; Wang, C.J. Mulberry leaf extract inhibit hepatocellular carcinoma cell proliferation via depressing IL-6 and TNF-alpha derived from adipocyte. J. Food Drug Anal. 2018, 26, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, M.J.; Cohen, P.; Spiegelman, B.M. Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 2011, 11, 886–895. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Chen, N.C.; Koh, Y.C.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Pterostilbene Inhibits Adipocyte Conditioned-Medium-Induced Colorectal Cancer Cell Migration through Targeting FABP5-Related Signaling Pathway. J. Agric. Food Chem. 2019, 67, 10321–10329. [Google Scholar] [CrossRef]
- Papadatos-Pastos, D.; Rabbie, R.; Ross, P.; Sarker, D. The role of the PI3K pathway in colorectal cancer. Crit. Rev. Oncol. Hematol. 2015, 94, 18–30. [Google Scholar] [CrossRef]
- Pandurangan, A.K. Potential targets for prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt pathways. Asian Pac. J. Cancer Prev. 2013, 14, 2201–2205. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, X.; Li, P.; Zhang, H.; Lin, T.; Miao, Z.; Ma, S. Isobavachalcone isolated from Psoralea corylifolia inhibits cell proliferation and induces apoptosis via inhibiting the AKT/GSK-3beta/beta-catenin pathway in colorectal cancer cells. Drug Des. Dev. Ther. 2019, 13, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; O’Donnell, K.A.; Zeller, K.I.; Nguyen, T.; Osthus, R.C.; Li, F. The c-Myc target gene network. Semin. Cancer Biol. 2006, 16, 253–264. [Google Scholar] [CrossRef] [PubMed]


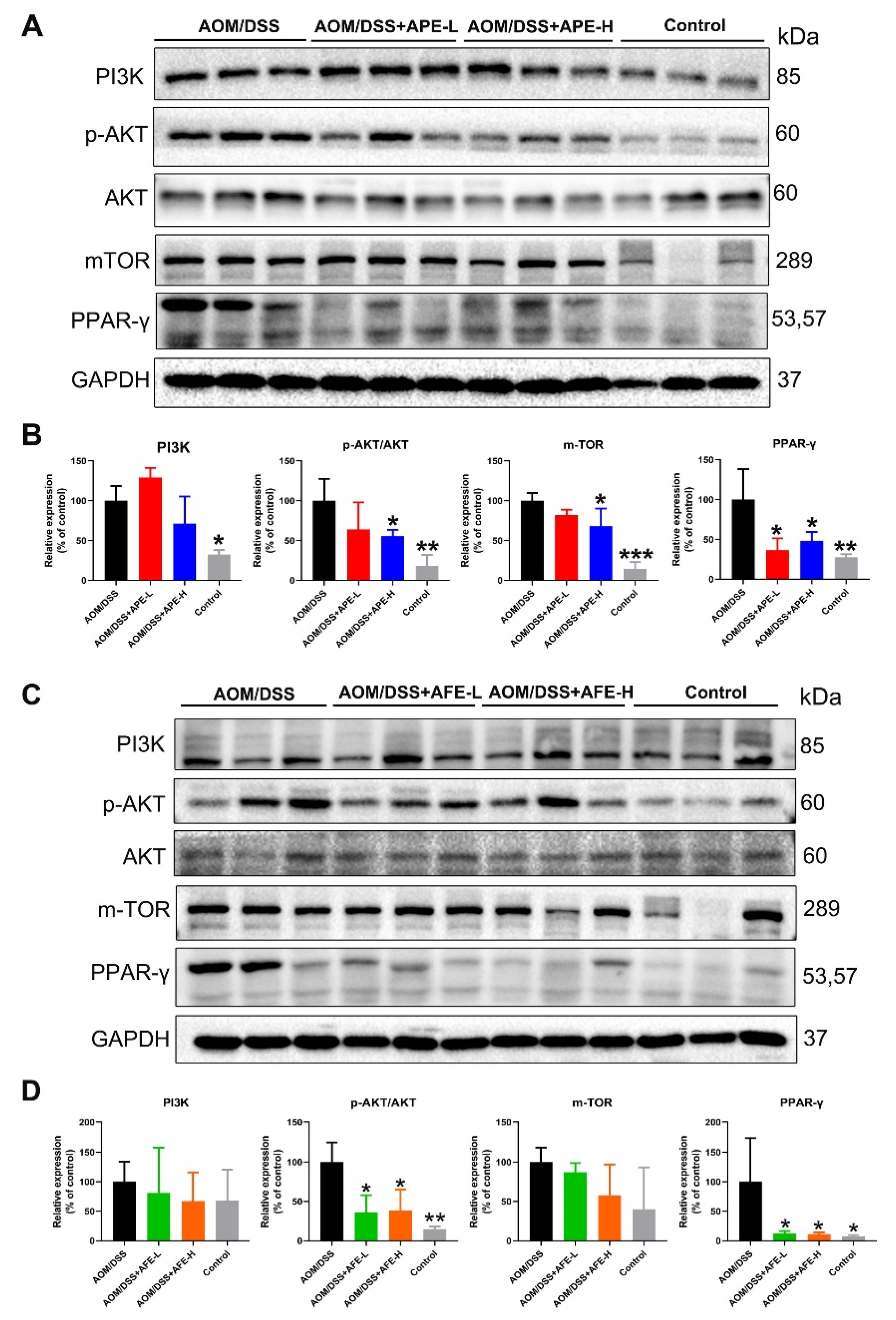
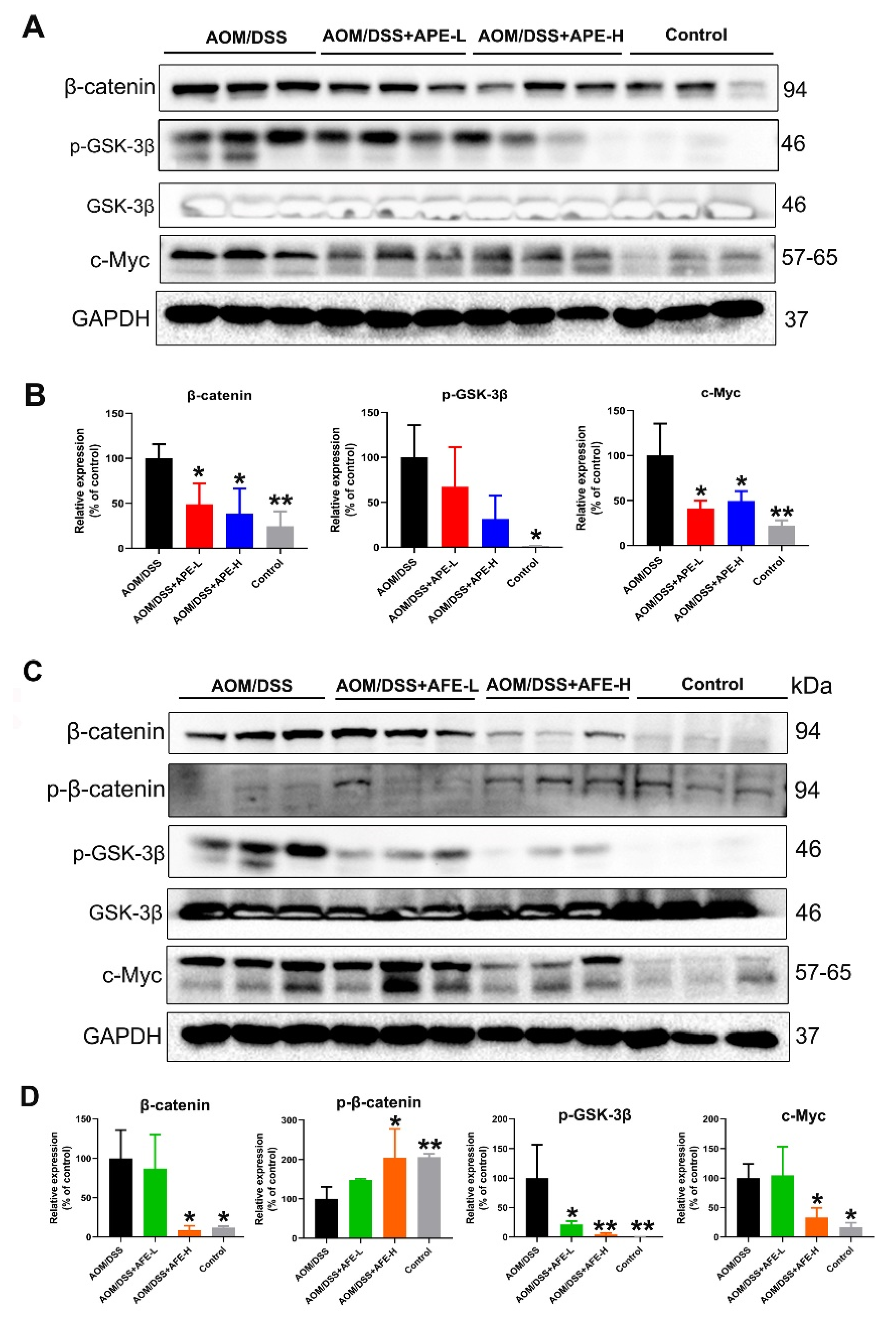
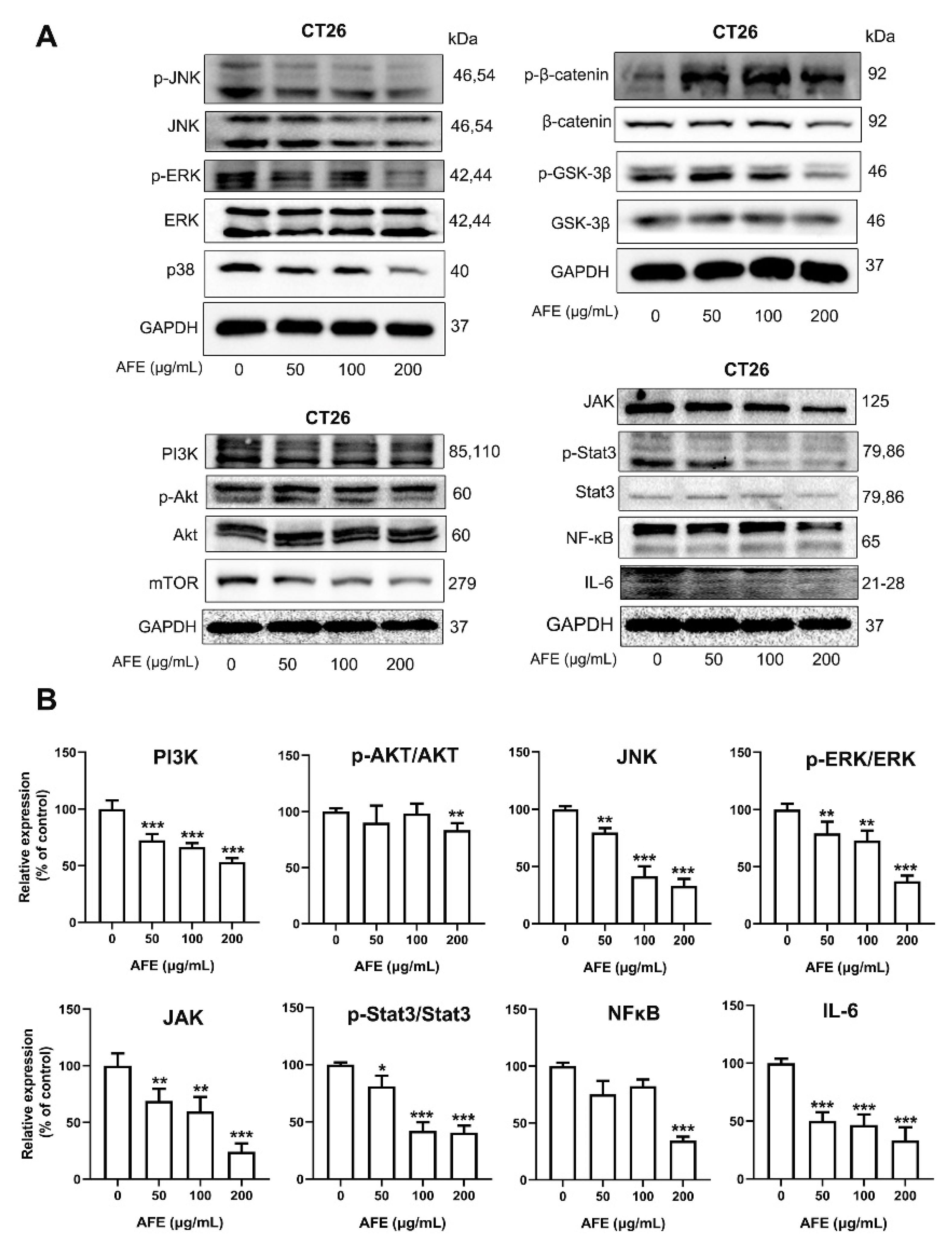

| Score | Weight Loss (%) | Stool Consistency | Visible Fecal Blood |
|---|---|---|---|
| 0 | <1 | Normal pellets | Normal |
| 1 | 1–5 | Loose feces | Slightly bloody |
| 2 | 6–10 | ||
| 3 | 11–15 | ||
| 4 | >15 | Watery diarrhea | Blood in the entire colon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Huang, X.; Chen, X.; Wang, M.; Tian, L.; Zhou, H.; Yang, W.; He, F.; Yin, W. Chemopreventive Effects of Polysaccharides and Flavonoids from Okra Flowers in Azomethane/Dextran Sulfate Sodium-Induced Murine Colitis-Associated Cancer. Nutrients 2023, 15, 4820. https://doi.org/10.3390/nu15224820
Deng Y, Huang X, Chen X, Wang M, Tian L, Zhou H, Yang W, He F, Yin W. Chemopreventive Effects of Polysaccharides and Flavonoids from Okra Flowers in Azomethane/Dextran Sulfate Sodium-Induced Murine Colitis-Associated Cancer. Nutrients. 2023; 15(22):4820. https://doi.org/10.3390/nu15224820
Chicago/Turabian StyleDeng, Yuanle, Xiaoyi Huang, Xiaotong Chen, Meng Wang, Li Tian, Heting Zhou, Wenyu Yang, Fang He, and Wenya Yin. 2023. "Chemopreventive Effects of Polysaccharides and Flavonoids from Okra Flowers in Azomethane/Dextran Sulfate Sodium-Induced Murine Colitis-Associated Cancer" Nutrients 15, no. 22: 4820. https://doi.org/10.3390/nu15224820
APA StyleDeng, Y., Huang, X., Chen, X., Wang, M., Tian, L., Zhou, H., Yang, W., He, F., & Yin, W. (2023). Chemopreventive Effects of Polysaccharides and Flavonoids from Okra Flowers in Azomethane/Dextran Sulfate Sodium-Induced Murine Colitis-Associated Cancer. Nutrients, 15(22), 4820. https://doi.org/10.3390/nu15224820








