1. Introduction
Rapid changes in the lifestyles of people around the world have occurred due to economic globalization, urbanization, and fast-paced development in recent years [
1]. These changes have significantly impacted people’s dietary habits, leading to poor health among many people due to the foods they consume. Gluten, a complex protein found in many grains [
2], contains alcohol-soluble proteins that are known to be pathogenic factors for celiac disease (CD) [
3] and have been implicated in other diseases such as type 1 diabetes [
4], rheumatoid arthritis [
5], and multiple sclerosis [
6], among others [
7,
8,
9]. Increasing evidence indicates that a considerable number of patients exhibit neurological dysfunction. The first systematic evidence of gluten-related neurological disease dates back to 1966 when electron microscopy and hematoxylin and eosin staining were used to examine neurodegenerative changes in muscle biopsies of adult patients with CD. Subsequent studies reported the first fatal case caused by neurological disease, comparing autopsy results with nine other CD cases presenting progressive central nervous system disorders. The case showed a common histopathological feature of progressive neurodegeneration in the cerebellum, deep gray matter, brainstem, and spinal cord [
10]. Another study demonstrated that following a gluten-free (GF) diet stabilized not only gastrointestinal symptoms but also neurological symptoms in patients with CD [
11]. This demonstrated that the long-term consumption of gluten led to neurological dysfunction. Moreover, GF food is the only way to achieve complete symptom relief among patients with CD [
12].
Diabetes mellitus (DM) is a chronic, systemic metabolic disease characterized by elevated blood glucose levels [
13]. Type 1 DM (T1DM) has a high comorbidity with CD [
7,
14]. Long-term gluten consumption also increases the risk of developing T1DM [
15]. Early studies in mice suggest a role of gluten in the pathogenesis of T1DM, as it alters the composition of the gut microbiota, which may further promote the development of T1DM [
16]. A lifelong GF diet reduced the incidence of autoimmune diabetes from 64% to 15% [
17]. Therefore, dietary changes are crucial for achieving and maintaining metabolic control and help reduce the burden of diabetes-related complications [
18].
GF foods refer to those that are completely free of gluten or do not contain gluten-containing grains [
19]. Although significant progress has been made in understanding and improving GF foods over the past two decades through assessing different ingredients, additives, and technologies, finely processed GF foods may contain higher amounts of sugar and oil, increasing the risk of diabetes and obesity. The development of GF products still faces challenges. Currently, the focus is more on plant-based functional foods because their health benefits primarily come from plant proteins. Some plant proteins are typically added to GF foods to improve their structure, texture, and glycemic control [
19]. Developing plant-protein-based GF foods is an important trend, especially in managing CD and diabetes. Rice is the second largest consumed cereal after wheat. It is a highly nutritious food source, rich in protein and an excellent source of carbohydrates, besides containing various vitamins and minerals such as niacin and thiamine [
20]. Also, it is GF, making it a good choice for individuals with gluten sensitivity. Rice flour also plays a vital role as an ingredient in traditional and new food products [
21,
22,
23], and frequently is the main ingredient applied in GF products both from food industry and food scientists. However, rice has a glycemic index (GI) ranging from 54 to 121 [
24]. The long-term consumption of foods with a GI between 79 and 82 significantly increases the risk of developing diabetes [
25]. Consuming certain different types of plant-based proteins can reduce the incremental area under the blood glucose curve (iAUC) after a meal [
26], which can help control blood sugar levels. Therefore, rice is not the most appropriate food choice for patients with diabetes. Legumes such as soy and peas are an important source of plant proteins and are rich in carbohydrates, fibers, proteins, and various micronutrients [
27]. Consuming legume protein is associated with a reduced incidence of diabetes [
28]. Therefore, it is recommended that individuals choose low-GI GF foods rich in legume protein, which helps control blood sugar levels and alleviates related diseases.
In the development of GF foods, GI is a way to evaluate the speed and extent of the rise in blood sugar caused by the carbohydrates present in food within 2 h of consumption [
29]. Compared with regular foods, most GF foods have a lower GI and can help control blood sugar [
30]. The incremental area under the glucose curve (IAUC), calculated by measuring the increase in the blood sugar level after a meal relative to the fasting level, serves as an evaluation index for the blood glucose response to each test meal. GI refers to the percentage increase in the area under the blood glucose curve after consuming a target amount (usually 50 g) of available carbohydrates in a test food compared with the corresponding increase after consuming the same amount of available carbohydrates in a reference food (such as glucose). Foods are classified into high GI (>70), medium GI (55–70), and low GI (<55) based on their GI values. High-GI foods can lead to a rapid release of carbohydrates, resulting in an increase in blood glucose concentration [
31]. In contrast, low-GI foods have a slower digestion and absorption rate, leading to a gradual rise in blood glucose levels [
32]. This positively affects the prevention and management of diabetes, obesity, cardiovascular disease, hyperlipidemia, and hypercholesterolemia [
33,
34]. For patients with both CD and T1DM, maintaining good glycemic control and adhering to a strict low-GI GF diet is essential to avoid complications associated with these two diseases.
Nonhuman primate (NHP) models have been successfully established for studying CD-related issues [
35]. By feeding NHPs with gluten-containing food, gluten-sensitive macaques exhibited obvious CD symptoms, including chronic diarrhea, fat malabsorption, and intestinal lesions. These clinical, histological, and serological features were reversed through intervention with a GF diet [
36]. Therefore, these NHP models are of great significance for studying both fundamental and practical CD-related issues [
37]. Moreover, NHP models have been used to investigate the development and pathophysiological changes of obesity and diabetes [
38]. The blood glucose regulation and pathological characteristics exhibited by the cynomolgus monkeys are quite similar to the clinical features of certain human diseases. Additionally, captive macaques have a longer lifespan, and their living environment and dietary habits are relatively fixed and uniform, making it more feasible to study diseases through dietary interventions in NHPs [
39]. Thus, NHP models can effectively help with learning about many diseases and facilitate long-term research on the effects of dietary interventions on animals.
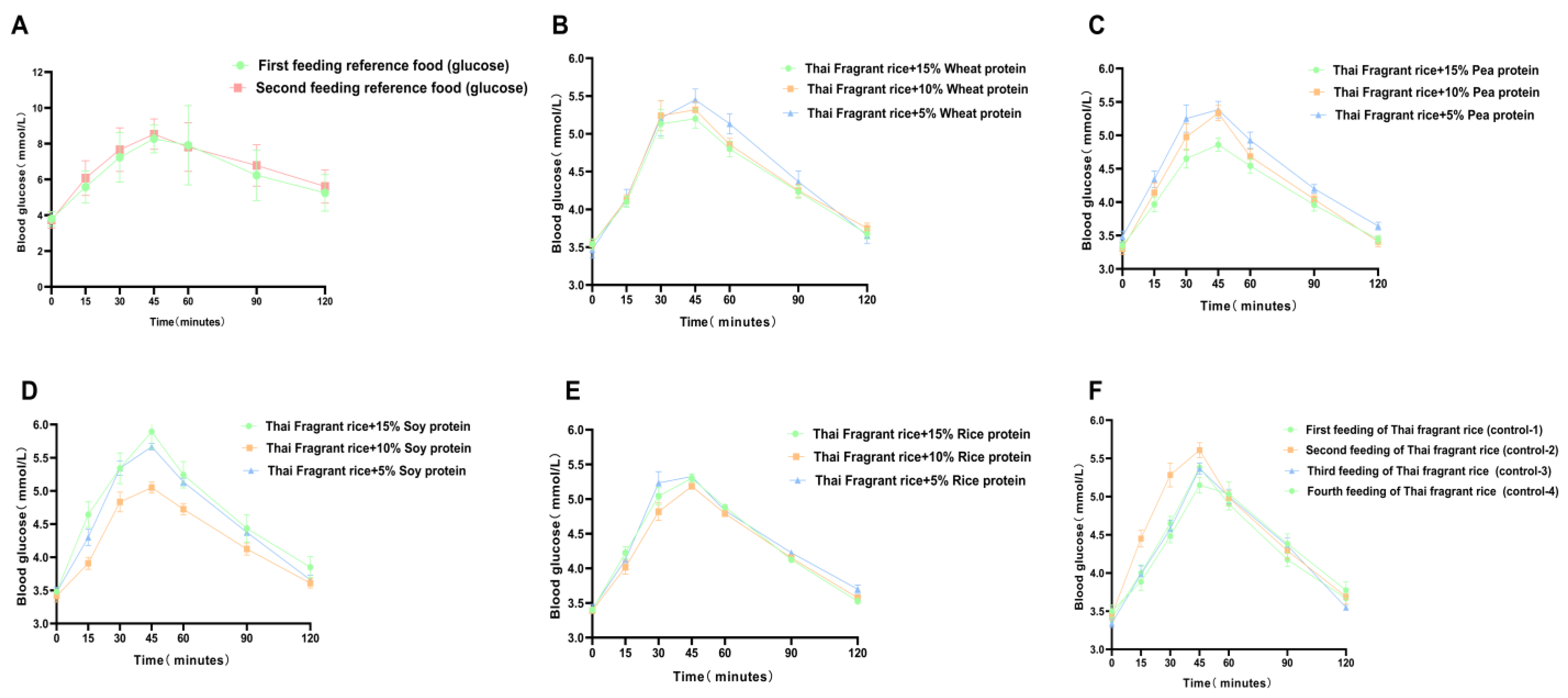
This study aimed to investigate the effect of GF rice cakes made with different ratios of plant-based protein and rice flour, and whole-grain rice cakes, on the postprandial blood glucose response in nonhuman primates. Thirteen test foods were prepared, including GF rice cakes mixed with 5%, 10%, or 15% of a single plant-based protein (rice protein, soy protein, and pea protein) and whole-grain rice cakes mixed with 5%, 10%, or 15% wheat protein as well as plain rice flour. Twelve healthy cynomolgus macaques were tested for fasting and postprandial fingertip blood glucose levels 15, 30, 45, 60, 90, and 120 min after consuming each test food (50 g). The GI and IAUC were analyzed for each test food, and the nutritional components, including protein, fat, starch, ash, and amino acids, were determined. It provides valuable insights into the selection and long-term consumption of GF foods with added plant-based protein for blood glucose control. This is particularly important for those with gluten intolerance and diabetes, and those who regularly consume gluten-containing foods and are at risk of developing neurodegenerative diseases.
4. Discussion
A high incidence of CD and T1DM [
40] is considered a risk factor for metabolic diseases. Therefore, a critical task for these patients is to maintain good blood sugar control while adhering to a strict GF diet. Postprandial hyperglycemia is a significant concern for those diagnosed with prediabetes or diabetes, as it can lead to elevated glycated hemoglobin; such patients are advised to consume low-GI foods. The main determinants of postprandial glycemic response are the amount of carbohydrates ingested and the common constituents of the entire food (such as water, fat, protein, and fiber), processing techniques, and external factors. Some amino acids also affect postprandial glycemic response. Currently, only a few studies have examined the impact of GF diets on patients with T1DM and CD [
41]. Also, only a limited number of small prospective and retrospective studies have discussed the glycemic benefits of GF diets [
42]. Previous research has demonstrated a significant difference in glucose response to GF pasta between healthy participants and patients with CD, with healthy individuals exhibiting a significantly higher glucose response than patients with CD [
43]. A study found that the fasting blood glucose levels increased significantly after 12 months on a GF diet [
44]. In healthy individuals, the postprandial blood glucose response was higher with GF bread than with bread containing gluten [
43]. Therefore, some GF foods may not be suitable for patients with abnormal glucose metabolism, and hence, further research on GF foods is needed. Although GF products are essential dietary needs for patients with CD, consumers without celiac disease need to consider that GF products are not necessarily a “healthier” food choice. Consumers should choose GF products that meet their individual needs while also paying attention to a balanced diet to avoid the negative effects of certain foods. The introduction of the GI concept provides a basis for patients with diabetes to make rational choices about carbohydrate-containing foods. A low-GI diet improves insulin sensitivity, lowers plasma triglyceride levels, reduces the risk of diabetes and heart disease, and helps treat obesity [
45]. Low-GI foods stay in the digestive tract longer, have a lower absorption rate, and release glucose slowly, leading to lower postprandial blood glucose responses. The changes in postprandial blood glucose responses represent the balance between glucose entering and exiting the bloodstream. Lowering the GI of foods is a promising method, especially by adding some plant-based protein substitutes for traditional refined flour and starch materials, such as non-gluten grains and legumes, instead of using refined basic flours and starches, such as rice and corn flour, corn, potatoes, and cassava starch, because increasing the protein content can alter the digestion rate of starch, dilute the amount of available carbohydrates, and lower the GI.
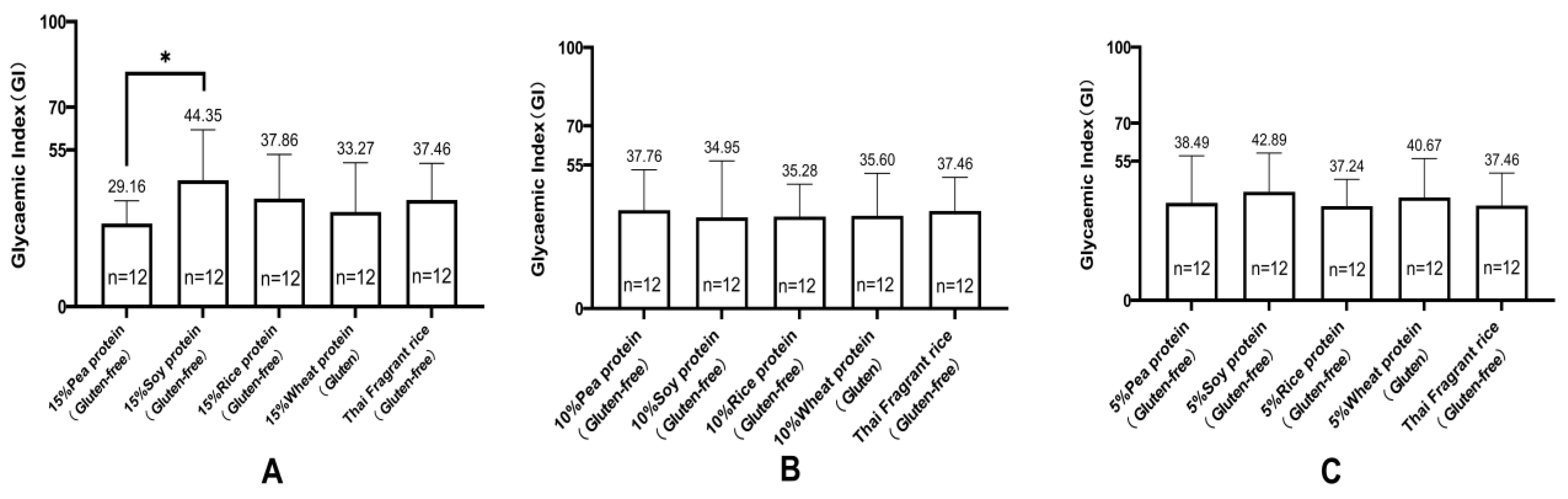
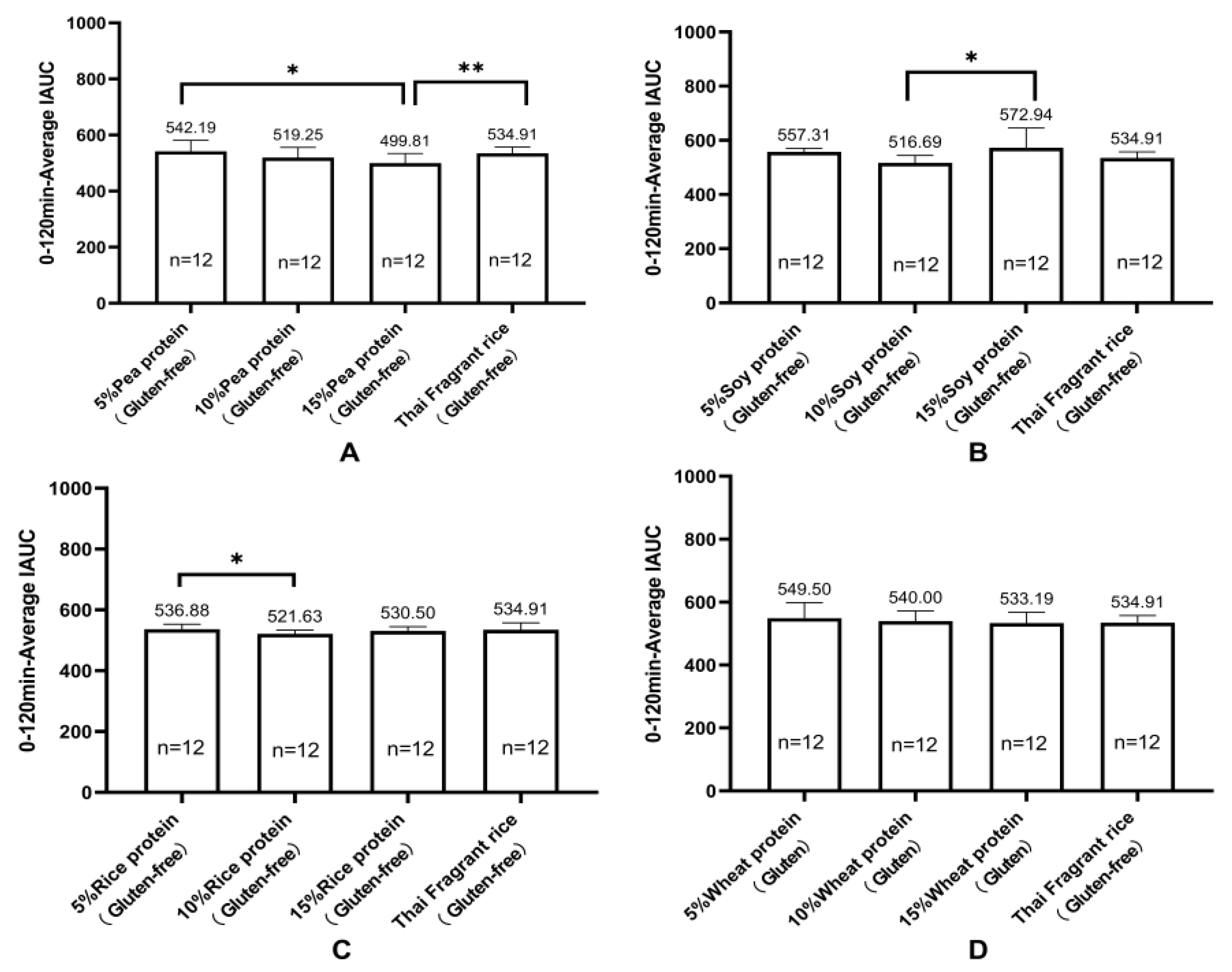
Research has shown a significant correlation between the consumption of legume protein and a reduced incidence of diabetes. Legume protein can mitigate postprandial blood glucose response. Green pea legumes produce lower blood glucose peak responses in mixed diets with different types of legumes. Chickpeas, lentils, and green peas generate significantly lower postprandial blood glucose responses than pasta (~35%). Isolated green pea protein produces a lower glucose area under the curve (AUC), making it a valuable food component for improving blood sugar control [
46], which is consistent with our research results. Some plant-based proteins are widely used to produce GF products. In this study, pea protein, rice protein, soy protein, and wheat protein were selected and processed into gluten-containing and GF rice cakes to examine their impact on postprandial blood glucose response in nonhuman primates. The GI values of the 10 GF rice cakes and 3 gluten-containing rice cakes ranged from 29 to 47, all with low GI (GI < 50). Rice cakes made from 15% pea protein and rice flour had a lower GI, while those made from 15% soy protein and rice flour had a higher GI. Meanwhile, the IAUC of postprandial blood glucose was the lowest for GF rice cakes made from 15% pea protein and rice flour. The IAUC of postprandial blood glucose was also highest for GF rice cakes made from 15% soy protein and rice flour. Additionally, the IAUC of GF rice cakes made with 15% pea protein was significantly lower than that of GF rice cakes made with 5% pea protein, 15% soy protein, 15% rice protein, and 15% wheat protein to produce gluten-containing rice cakes. Adding pea protein to make GF rice cakes had a minimal impact on postprandial blood glucose, which has important implications for a deeper understanding of the impact of nutrient content on blood sugar and the development of healthier food products. The impact of amino acids on blood glucose levels is mainly related to their metabolic pathways. Specifically, when branched-chain amino acids, including leucine, isoleucine, and valine, and aromatic amino acids, including phenylalanine, tyrosine, and tryptophan, are metabolized in the body, they generate ketones or biogenic amines that can stimulate insulin secretion and lower blood glucose levels [
47]. Additionally, specific amino acids such as arginine, serine, and lysine, which are commonly found in proteins, can improve insulin sensitivity and promote glucose uptake through different metabolic pathways after ingestion, thereby affecting blood sugar levels [
48]. Glycine can also promote insulin secretion, thereby reducing blood glucose levels and decreasing the peak value and AUC of postprandial blood glucose [
49,
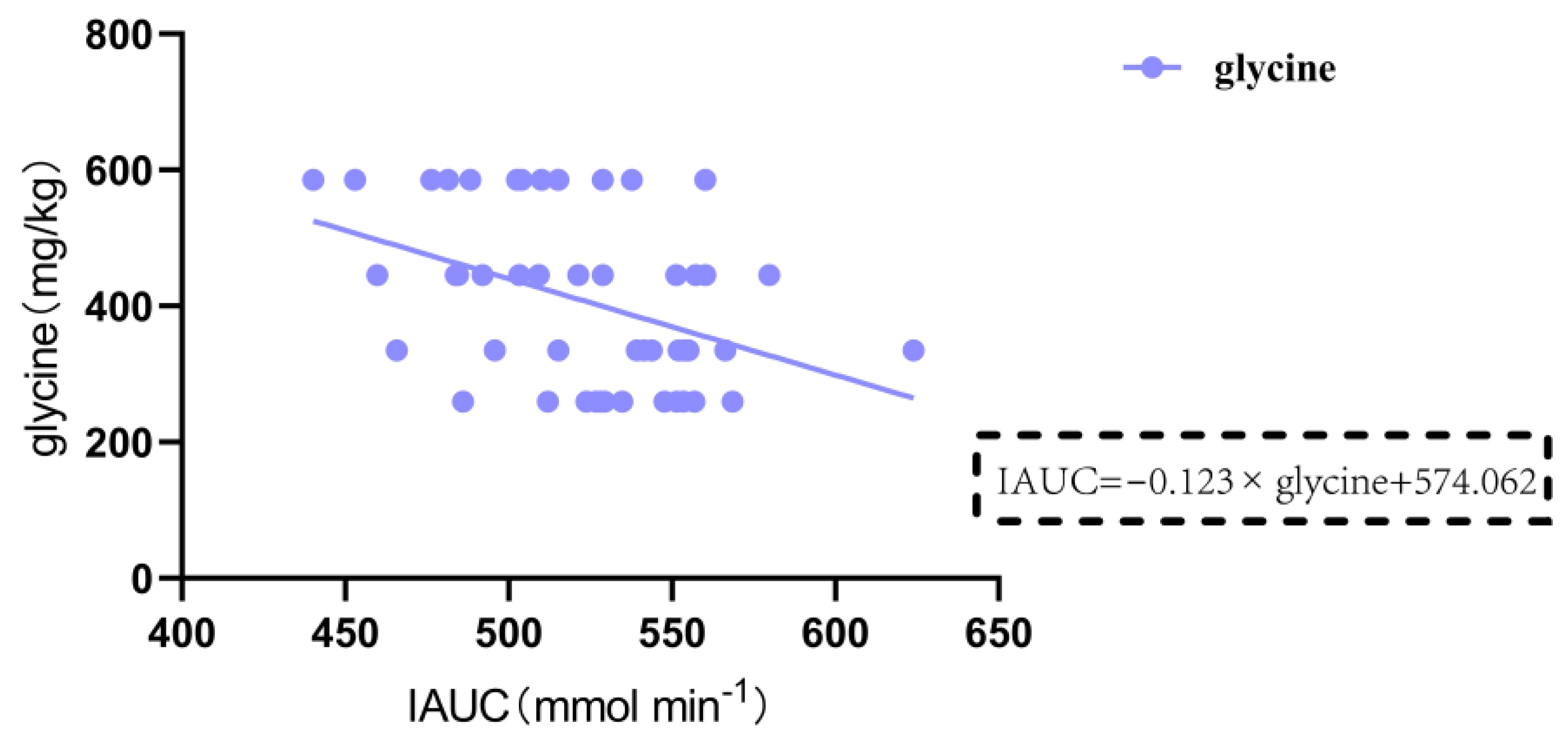
50]. We employed a multiple regression analysis to analyze the relationship between the IAUC of blood glucose from 0 to 120 min after eating and various nutritional components. The results demonstrated that the content of lysine was negatively correlated with IAUC in gluten-containing and GF rice cakes with added 15% plant protein (p = 0.013 < 0.05). Glycine was significantly negatively correlated with IAUC in GF rice cakes with added 5%, 10%, and 15% pea protein and pure rice flour GF rice cakes (p = 0.003 < 0.01). In the future, we will further study the different effects of the intake of lysine and glycine on blood sugar levels. Apart from the types and amounts of amino acids, other nutritional components in food, such as fat, and an individual’s metabolic status can also affect postprandial blood glucose levels. An increase in fat content in food leads to a slower rise in blood glucose levels, and the peak value of postprandial blood glucose is also lowered. This suggests that the AUC of postprandial blood glucose is accordingly reduced. We found that the fat content in GF rice cakes with added 5%, 10%, and 15% soy protein and pure rice flour GF rice cakes was significantly negatively correlated with IAUC (p = 0.002 < 0.01), and an increase in fat content in food led to a significant decrease in IAUC at 2 h after the meal.
In this study, we used captive nonhuman primates, specifically the cynomolgus monkeys, as experimental animals to investigate the effects of gluten-containing and GF foods on postprandial blood glucose response. These nonhuman primates share many biological similarities with humans and exhibit similar morphology and functionality, making them a crucial animal model for such studies. Furthermore, their diet is relatively homogeneous, consisting mainly of various grains as well as supplemented nutrients such as milk, powdered milk, eggs, fish meal, bone meal, and salt. Disinfected vegetables and fruits are also added to ensure that the animals receive sufficient amounts of nutrients, such as vitamin C and minerals. We can ensure the relative stability of nutrient content in the feed through diversified feeding regimes. We also provide appropriate amounts of animal-sourced food to animals to ensure that they consume more than 50 types of nutrients, including carbohydrates, proteins, fats, vitamins, and minerals. We control the amount of food intake, especially for long-term captive monkeys, where overeating may lead to digestive problems or obesity. Nonhuman primates have a long history of application in translational medicine research, particularly in exploring the mechanisms underlying metabolic diseases and evaluating novel therapeutic approaches. Additionally, their longer lifespan facilitates the investigation and monitoring of the effects of long-term dietary patterns on animal health indices, such as gluten-containing and GF diets, high-sugar diets, high-fat diets, and so forth.