An Overview of Different Vitamin D Compounds in the Setting of Adiposity
Abstract
1. Introduction
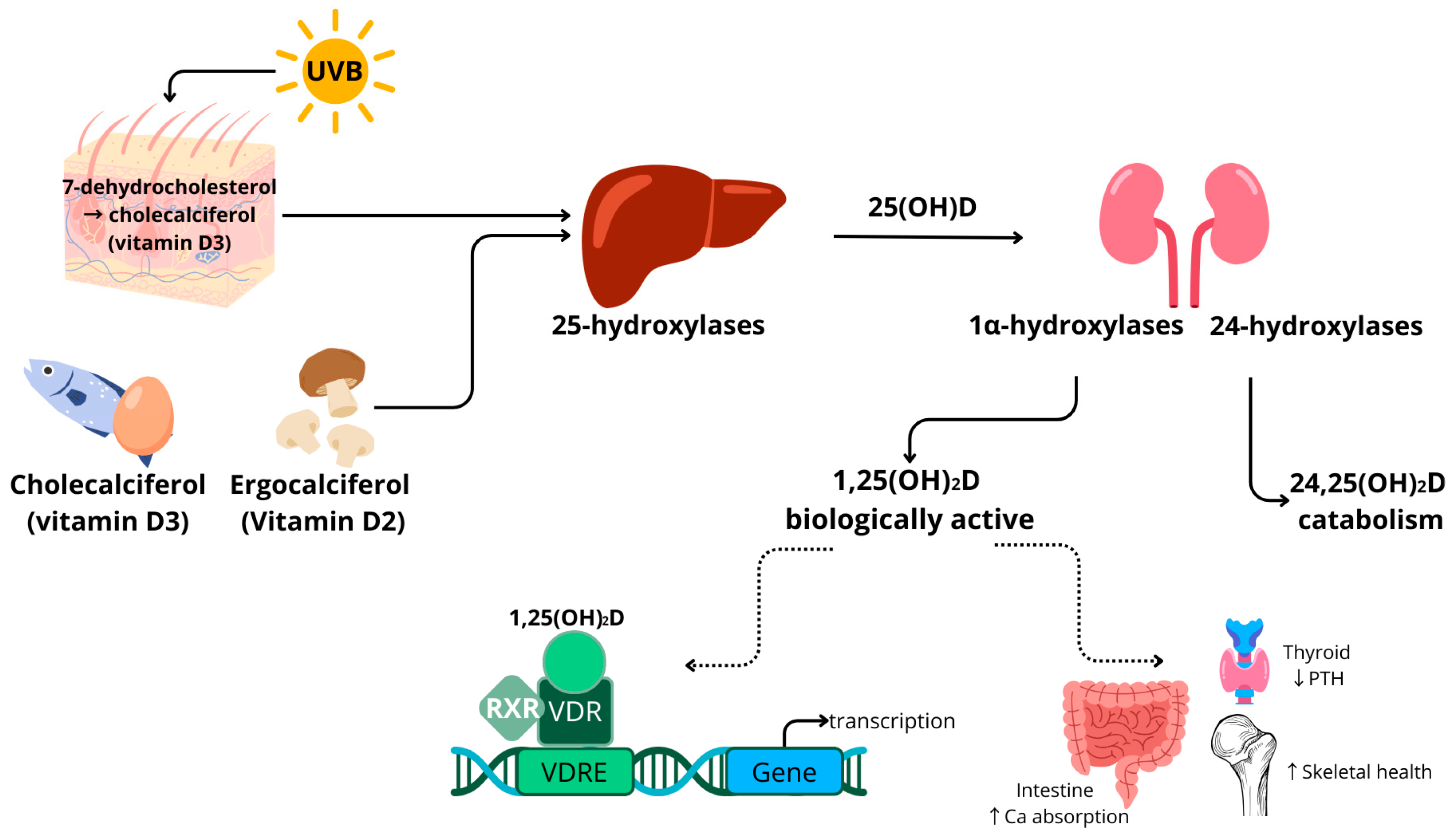
1.1. Vitamin D Metabolism
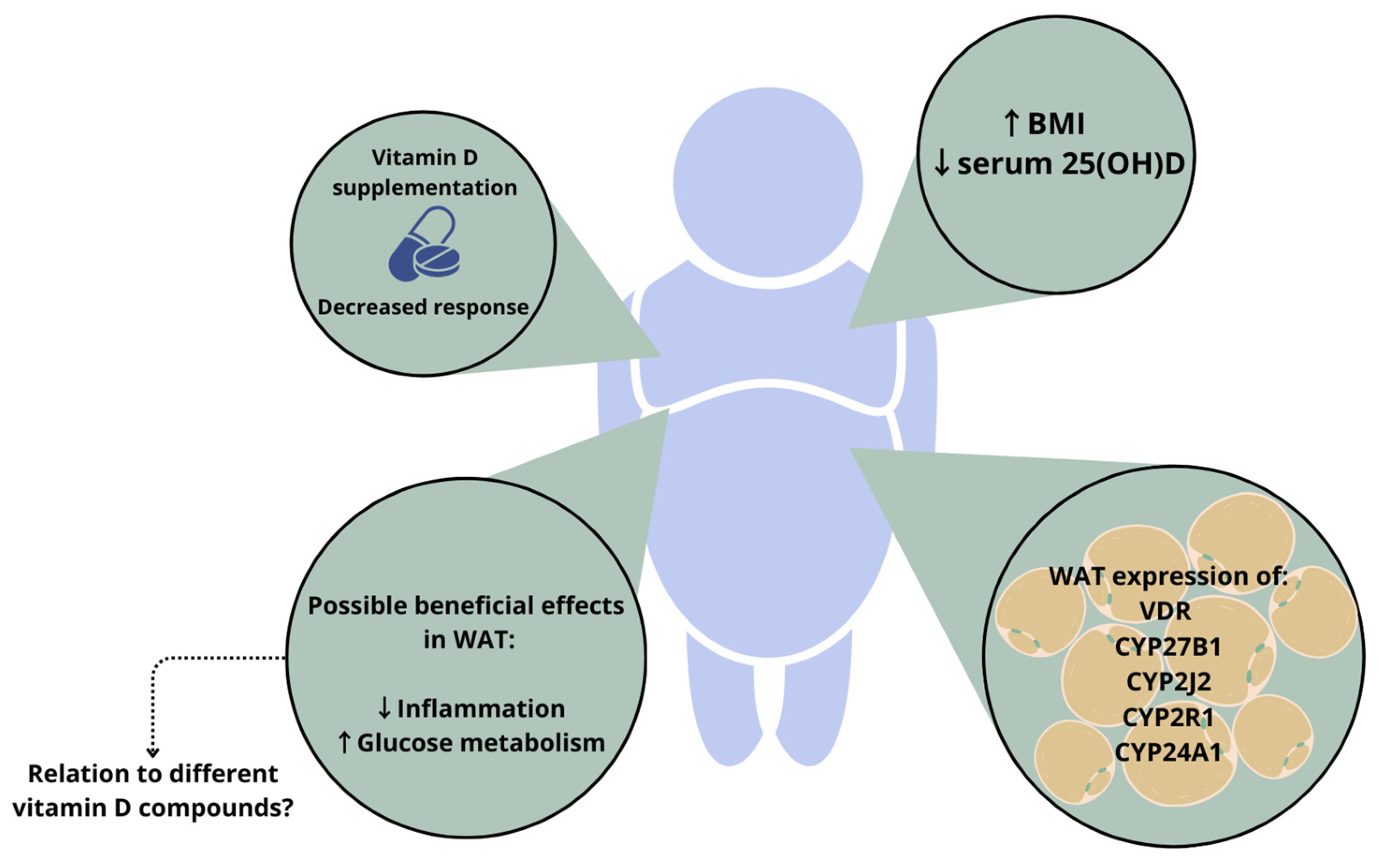
1.2. Vitamin D in Adiposity
2. Relevance of Different Vitamin D Compounds
2.1. Free Vitamin D Compound
2.2. 1,25-Dihydroxyvitamin D (1,25(OH)2D)
2.3. 24,25-Dihydroxyvitamin D (24,25(OH)2D)
2.4. C-3 Epimers of Vitamin D Compounds
3. Measuring Vitamin D Compounds in Human Adipose Tissue with LC-MS/MS
| Author, Year | MS, Ionisation | Sample Weight (g) | Sample Preparation | Measured Compounds | LOD LOQ | Recovery (R%) |
|---|---|---|---|---|---|---|
| Blum et al., 2008 [43] | LC-MS APCI | 0.2–0.25 |
| D3 | - | 72% |
| Höller et al., 2010 [127] | LC-MS APCI | 0.5 |
| 25(OH)D3 | LOD 5 ng/g | - |
| Beckman et al., 2013 [63] | LC DAD | - |
| D2 D3 | - | 98.5 ± 5% |
| Piccolo et al., 2013 [128] | LC-MS/MS APCI | 0.1–0.5 |
| 25(OH)D3 | ||
| Lipkie et al., 2013 [129] | LC-MS/MS ESI | - |
| 25(OH)D2 25(OH)D3 D2 D3 | ||
| Malmberg et al., 2014 [130] | SIMS-TOF | D3 25(OH)D3 1,25(OH)2D3 | - | - | ||
| Burild et al., 2014 [131] Didriksen et al., 2015 [132] Martinaityte et al., 2017 [133] | LC-MS/MS ESI | 0.2–1.0 |
| D3 25(OH)D3 | LOQ < 0.1 ng/g | - |
| Bonnet et al., 2019 [134] Bonnet et al., 2021 [135] | LC-MS/MS ESI | 0.05 |
| D3 25(OH)D3 1,25(OH)2D3 | - | - |
| Best et al., 2021 [136] | LC-MS/MS | 0.01 |
| D3 25(OH)D3 | - | - |
4. Conclusions
Funding
Conflicts of Interest
References
- Holick, M.F. Vitamin D and bone health. J. Nutr. 1996, 126, 1159S–1164S. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The role of vitamin D for bone health and fracture prevention. Curr. Osteoporos. Rep. 2006, 4, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Deficiency of sunlight and vitamin D. BMJ 2008, 336, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Vitamin D deficiency: Defining, prevalence, causes, and strategies of addressing. Calcif. Tissue Int. 2020, 106, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.A.; de Silva, N.L.; Sumanatilleke, M.; de Silva, S.D.N.; Gamage, K.K.K.; Dematapitiya, C.; Kuruppu, D.C.; Ranasinghe, P.; Pathmanathan, S.; Katulanda, P. Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 1484–1502. [Google Scholar] [CrossRef]
- Shah, K.; Saxena, D.; Mavalankar, D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM Int. J. Med. 2021, 114, 175–181. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.-S.; Magkos, F.; Dalamaga, M. Vitamin D and obesity: Current evidence and controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of vitamin D levels on inflammatory status: A systematic review of immune cell studies. PLoS ONE 2015, 10, e0141770. [Google Scholar] [CrossRef]
- Park, C.Y.; Kim, T.Y.; Yoo, J.S.; Seo, Y.; Pae, M.; Han, S.N. Effects of 1, 25-dihydroxyvitamin D3 on the inflammatory responses of stromal vascular cells and adipocytes from lean and obese mice. Nutrients 2020, 12, 364. [Google Scholar] [CrossRef]
- Nimitphong, H.; Guo, W.; Holick, M.F.; Fried, S.K.; Lee, M.J. Vitamin D inhibits adipokine production and inflammatory signaling through the vitamin D receptor in human adipocytes. Obesity 2021, 29, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.-F. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.-J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhou, Q.; Wang, Y.; Fu, S.; Li, Z.; Chen, Q. Serum and supplemental vitamin D levels and insulin resistance in T2DM populations: A meta-analysis and systematic review. Sci. Rep. 2023, 13, 12343. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.A.; Daneshzad, E.; Fung, T.T.; Zahidi, F.; Muhammadi, M.; Bellissimo, N.; Azadbakht, L. What is the impact of vitamin D supplementation on glycemic control in people with type-2 diabetes: A systematic review and meta-analysis of randomized controlled trails. BMC Endocr. Disord. 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Clement, K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Magno, C.P.; Lane, K.T.; Hinojosa, M.W.; Lane, J.S. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 2008, 207, 928–934. [Google Scholar] [CrossRef]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25 (OH) D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 2017, 173, 105–116. [Google Scholar] [CrossRef]
- Binkley, N.; Borchardt, G.; Siglinsky, E.; Krueger, D. Does vitamin D metabolite measurement help predict 25 (OH) D change following vitamin D supplementation? Endocr. Pract. 2017, 23, 432–441. [Google Scholar] [CrossRef]
- Makris, K.; Sempos, C.; Cavalier, E. The measurement of vitamin D metabolites part II—The measurement of the various vitamin D metabolites. Hormones 2020, 19, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Wildman, R.P.; Muntner, P.; Reynolds, K.; McGinn, A.P.; Rajpathak, S.; Wylie-Rosett, J.; Sowers, M.R. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch. Intern. Med. 2008, 168, 1617–1624. [Google Scholar] [CrossRef]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Soll, D.; Gawron, J.; Pletsch-Borba, L.; Spranger, J.; Mai, K. Long-term impact of the metabolic status on weight loss-induced health benefits. Nutr. Metab. 2022, 19, 25. [Google Scholar] [CrossRef]
- Meadows, A.; Daníelsdóttir, S. What’s in a word? On weight stigma and terminology. Front. Psychol. 2016, 7, 1527. [Google Scholar] [CrossRef]
- Puhl, R.M. What words should we use to talk about weight? A systematic review of quantitative and qualitative studies examining preferences for weight-related terminology. Obes. Rev. 2020, 21, e13008. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Stokes, C.S.; Lammert, F. Vitamin D supplementation: Less controversy, more guidance needed. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef]
- Saponaro, F.; Saba, A.; Zucchi, R. An update on vitamin D metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Rao, D.S.; Raghuramulu, N. Food chain as origin of vitamin D in fish. Comp. Biochem. Physiol. Part A Physiol. 1996, 114, 15–19. [Google Scholar]
- Atsuko, T.; Toshio, O.; Makoto, T.; Tadashi, K. Possible origin of extremely high contents of vitamin D3 in some kinds of fish liver. Comp. Biochem. Physiol. Part A Physiol. 1991, 100, 483–487. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Osteopath. Med. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Risco, F.; Traba, M. Influence of magnesium on the in vitro synthesis of 24, 25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3. Magnes. Res. 1992, 5, 5–14. [Google Scholar] [PubMed]
- Plum, L.A.; DeLuca, H.F. Vitamin D, disease and therapeutic opportunities. Nat. Rev. Drug Discov. 2010, 9, 941–955. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef]
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the calcium-parathyroid hormone-vitamin D axis. Vitam. D Clin. Med. 2018, 50, 1–13. [Google Scholar]
- Al-Zohily, B.; Al-Menhali, A.; Gariballa, S.; Haq, A.; Shah, I. Epimers of vitamin D: A review. Int. J. Mol. Sci. 2020, 21, 470. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.d.F.; Assis, A.d.; Santos, C.d.S.; Santos, D.d. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A. Vitamin D insufficiency in overweight and obese children and adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Dolnikowski, G.; Seyoum, E.; Harris, S.S.; Booth, S.L.; Peterson, J.; Saltzman, E.; Dawson-Hughes, B. Vitamin D3 in fat tissue. Endocrine 2008, 33, 90–94. [Google Scholar] [CrossRef]
- Himbert, C.; Ose, J.; Delphan, M.; Ulrich, C.M. A systematic review of the interrelation between diet-and surgery-induced weight loss and vitamin D status. Nutr. Res. 2017, 38, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Lespessailles, E.; Toumi, H. Vitamin D alteration associated with obesity and bariatric surgery. Exp. Biol. Med. 2017, 242, 1086–1094. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Rahme, M.; Fuleihan, G.E.-H. Vitamin D metabolism in bariatric surgery. Endocrinol. Metab. Clin. 2017, 46, 947–982. [Google Scholar] [CrossRef]
- Li, J.; Byrne, M.E.; Chang, E.; Jiang, Y.; Donkin, S.S.; Buhman, K.K.; Burgess, J.R.; Teegarden, D. 1α, 25-Dihydroxyvitamin D hydroxylase in adipocytes. J. Steroid Biochem. Mol. Biol. 2008, 112, 122–126. [Google Scholar] [CrossRef]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.-J. 25-hydroxyvitamin D3 and 1, 25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef]
- Wamberg, L.; Christiansen, T.; Paulsen, S.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.I.; Kuryłowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Kozniewski, K.; Puzianowska-Kuznicka, M. Vitamin D receptor gene expression in adipose tissue of obese individuals is regulated by miRNA and correlates with the pro-inflammatory cytokine level. Int. J. Mol. Sci. 2019, 20, 5272. [Google Scholar] [CrossRef]
- Elkhwanky, M.S.; Kummu, O.; Piltonen, T.T.; Laru, J.; Morin-Papunen, L.; Mutikainen, M.; Tavi, P.; Hakkola, J. Obesity represses CYP2R1, the vitamin D 25-hydroxylase, in the liver and extrahepatic tissues. JBMR Plus 2020, 4, e10397. [Google Scholar] [CrossRef]
- Ochs-Balcom, H.M.; Chennamaneni, R.; Millen, A.E.; Shields, P.G.; Marian, C.; Trevisan, M.; Freudenheim, J.L. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am. J. Clin. Nutr. 2011, 93, 5–10. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Anguita-Ruiz, A.; Leis, R.; Aguilera, C.M. Genetic factors and molecular mechanisms of vitamin D and obesity relationship. Ann. Nutr. Metab. 2018, 73, 89–99. [Google Scholar] [CrossRef]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.-M.; Buring, J.E. Association of Body Weight With Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, E.G.; Lips, P.; Schoonmade, L.J.; Lanham-New, S.A.; van Schoor, N.M. Comparison of the effect of daily vitamin D2 and vitamin D3 supplementation on serum 25-hydroxyvitamin D concentration (total 25 (OH) D, 25 (OH) D2 and 25 (OHD3) and importance of body mass index: A systematic review and meta-analysis. Adv. Nutr. 2023. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Rocca, M.S.; De Filippis, V.; Opocher, G.; Azzena, B.; Vettor, R.; Plebani, M.; Foresta, C. Impaired release of vitamin D in dysfunctional adipose tissue: New cues on vitamin D supplementation in obesity. J. Clin. Endocrinol. Metab. 2017, 102, 2564–2574. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D deficiency: Consequence or cause of obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Di Nisio, A.; Mele, C.; Scappaticcio, L.; Savastano, S.; Colao, A.; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Obesity and hypovitaminosis D: Causality or casualty? Int. J. Obes. Suppl. 2019, 9, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Earthman, C.; Beckman, L.; Masodkar, K.; Sibley, S. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.-Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Cordeiro, A.; Santos, A.; Bernardes, M.; Ramalho, A.; Martins, M.J. Vitamin D metabolism in human adipose tissue: Could it explain low vitamin D status in obesity? Horm. Mol. Biol. Clin. Investig. 2018, 33, 20170003. [Google Scholar] [CrossRef] [PubMed]
- Beckman, L.M.; Earthman, C.P.; Thomas, W.; Compher, C.W.; Muniz, J.; Horst, R.L.; Ikramuddin, S.; Kellogg, T.A.; Sibley, S.D. Serum 25 (OH) vitamin D concentration changes after Roux-en-Y gastric bypass surgery. Obesity 2013, 21, E599–E606. [Google Scholar] [CrossRef]
- Cominacini, M.; Fumaneri, A.; Ballerini, L.; Braggio, M.; Valenti, M.T.; Dalle Carbonare, L. Unraveling the Connection: Visceral Adipose Tissue and Vitamin D Levels in Obesity. Nutrients 2023, 15, 4259. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Alberti, K.G.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef] [PubMed]
- van Etten, E.; Mathieu, C. Immunoregulation by 1, 25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Melsen, F.; Christensen, E.I.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. The free hormone hypothesis: When, why, and how to measure the free hormone levels to assess vitamin D, thyroid, sex hormone, and cortisol status. JBMR Plus 2021, 5, e10418. [Google Scholar] [CrossRef]
- Mendel, C.M. The free hormone hypothesis: A physiologically based mathematical model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Bikle, D.D.; Malmstroem, S.; Schwartz, J. Current controversies: Are free vitamin metabolite levels a more accurate assessment of vitamin D status than total levels? Endocrinol. Metab. Clin. 2017, 46, 901–918. [Google Scholar] [CrossRef]
- Schwartz, J.; Lai, J.; Lizaola, B.; Kane, L.; Weyland, P.; Terrault, N.; Stotland, N.; Bikle, D. Variability in free 25 (OH) vitamin D levels in clinical populations. J. Steroid Biochem. Mol. Biol. 2014, 144, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Shieh, A.; Ma, C.; Chun, R.F.; Wittwer-Schegg, J.; Swinkels, L.; Huijs, T.; Wang, J.; Donangelo, I.; Hewison, M.; Adams, J.S. Associations between change in total and free 25-hydroxyvitamin D with 24, 25-dihydroxyvitamin D and parathyroid hormone. J. Clin. Endocrinol. Metab. 2018, 103, 3368–3375. [Google Scholar] [CrossRef] [PubMed]
- Marques-Pamies, M.; López-Molina, M.; Pellitero, S.; Santillan, C.S.; Martínez, E.; Moreno, P.; Tarascó, J.; Granada, M.L.; Puig-Domingo, M. Differential behavior of 25 (OH) D and f25 (OH) D3 in patients with morbid obesity after bariatric surgery. Obes. Surg. 2021, 31, 3990–3995. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Osmancevic, A.; Jansson, N.; Hulthén, L.; Holmäng, A.; Larsson, I. Increased vitamin D-binding protein and decreased free 25 (OH) D in obese women of reproductive age. Eur. J. Nutr. 2014, 53, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Paredez, B.; Hidalgo-Bravo, A.; León-Reyes, G.; León-Maldonado, L.S.; Aquino-Gálvez, A.; Castillejos-López, M.; Denova-Gutiérrez, E.; Flores, Y.N.; Salmerón, J.; Velázquez-Cruz, R. Total, bioavailable, and free 25-hydroxyvitamin D equally associate with adiposity markers and metabolic traits in mexican adults. Nutrients 2021, 13, 3320. [Google Scholar] [CrossRef]
- Brommage, R.; Deluca, H.F. Evidence that 1, 25-dihydroxyvitamin D3 is the physiologically active metabolite of vitamin D3. Endocr. Rev. 1985, 6, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Marcotorchino, J.; Tourniaire, F.; Landrier, J.-F. Vitamin D, adipose tissue, and obesity. Horm. Mol. Biol. Clin. Investig. 2013, 15, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Karhu, T.; Lehtonen, S.; Lehenkari, P.; Carlberg, C.; Saarnio, J.; Sebert, S.; Hyppönen, E.; Järvelin, M.R.; Herzig, K.H. Inhibition of cytokine secretion from adipocytes by 1, 25-dihydroxyvitamin D3 via the NF-κB pathway. FASEB J. 2012, 26, 4400–4407. [Google Scholar] [CrossRef]
- Wamberg, L.; Cullberg, K.; Rejnmark, L.; Richelsen, B.; Pedersen, S. Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: Results from an in vitro study and a randomized controlled trial. Horm. Metab. Res. 2013, 45, 456–462. [Google Scholar] [CrossRef]
- Bellia, A.; Garcovich, C.; D’Adamo, M.; Lombardo, M.; Tesauro, M.; Donadel, G.; Gentileschi, P.; Lauro, D.; Federici, M.; Lauro, R. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2013, 8, 33–40. [Google Scholar] [CrossRef]
- Sha, S.; Gwenzi, T.; Chen, L.-J.; Brenner, H.; Schöttker, B. About the associations of vitamin D deficiency and biomarkers of systemic inflammatory response with all-cause and cause-specific mortality in a general population sample of almost 400,000 UK Biobank participants. Eur. J. Epidemiol. 2023, 38, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Mawer, E.B.; Backhouse, J.; Holman, C.A.; Lumb, G.; Stanbury, S. The distribution and storage of vitamin D and its metabolites in human tissues. Clin. Sci. 1972, 43, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Bing, C.; Wood, I.S. Adipose tissue and adipokines—Energy regulation from the human perspective. J. Nutr. 2006, 136, 1935S–1939S. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2023, 204, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, obesity, and leptin resistance: Where are we 25 years later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Prolo, P.; Wong, M.-L.; Licinio, J. Leptin. Int. J. Biochem. Cell Biol. 1998, 30, 1285–1290. [Google Scholar] [CrossRef]
- Paul, R.F.; Hassan, M.; Nazar, H.S.; Gillani, S.; Afzal, N.; Qayyum, I. Effect of body mass index on serum leptin levels. J. Ayub Med. Coll. Abbottabad 2011, 23, 40–43. [Google Scholar] [PubMed]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of leptin with obesity and insulin resistance. Cureus 2020, 12, e12178. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2011, 8, 1031–1063. [Google Scholar]
- Menendez, C.; Lage, M.; Peino, R.; Baldelli, R.; Concheiro, P.; Dieguez, C.; Casanueva, F. Retinoic acid and vitamin D3 powerfully inhibit in vitro leptin secretion by human adipose tissue. J. Endocrinol. 2001, 170, 425–432. [Google Scholar] [CrossRef][Green Version]
- Hajimohammadi, M.; Shab-Bidar, S.; Neyestani, T. Vitamin D and serum leptin: A systematic review and meta-analysis of observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Vilarrasa, N.; Vendrell, J.; Maravall, J.; Elío, I.; Solano, E.; San José, P.; García, I.; Virgili, N.; Soler, J.; Gómez, J.M. Is plasma 25 (OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine 2010, 38, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lwow, F.; Bohdanowicz-Pawlak, A. Vitamin D and selected cytokine concentrations in postmenopausal women in relation to metabolic disorders and physical activity. Exp. Gerontol. 2020, 141, 111107. [Google Scholar] [CrossRef]
- de Souza, W.N.; Norde, M.M.; Oki, É.; Rogero, M.M.; Marchioni, D.M.; Fisberg, R.M.; Martini, L.A. Association between 25-hydroxyvitamin D and inflammatory biomarker levels in a cross-sectional population-based study, São Paulo, Brazil. Nutr. Res. 2016, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maetani, M.; Maskarinec, G.; Franke, A.A.; Cooney, R.V. Association of leptin, 25-hydroxyvitamin D, and parathyroid hormone in women. Nutr. Cancer 2009, 61, 225–231. [Google Scholar] [CrossRef][Green Version]
- Lorente-Cebrián, S.; Eriksson, A.; Dunlop, T.; Mejhert, N.; Dahlman, I.; Åström, G.; Sjölin, E.; Wåhlén, K.; Carlberg, C.; Laurencikiene, J. Differential effects of 1α, 25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur. J. Nutr. 2012, 51, 335–342. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Tian, L.; Zheng, L.; Wang, X.; Liu, W.; Zhang, Y.; Huang, G. Association of serum 25-hydroxyvitamin D3 with adipokines and inflammatory marker in persons with prediabetes mellitus. Clin. Chim. Acta 2017, 468, 152–158. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Gibney, M.J.; Connor, A.O.; Mion, B.; Kaluskar, S.; Cashman, K.D.; Flynn, A.; Shanahan, F.; Brennan, L. Biochemical and metabolomic phenotyping in the identification of a vitamin D responsive metabotype for markers of the metabolic syndrome. Mol. Nutr. Food Res. 2011, 55, 679–690. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Jammah, A.; Racz, M.; Khawaja, R.A.; Hasanato, R.; El-Fawal, H.A.; Mousa, S.A.; Mason, D.L. Effect of vitamin D supplementation on glucose control and inflammatory response in type II diabetes: A double blind, randomized clinical trial. Int. J. Endocrinol. Metab. 2015, 13, e22604. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Hanwell, H.E.; Schnabl, K.; Yazdanpanah, M.; Kimball, S.; Fu, L.; Sidhom, G.; Rousseau, D.; Cole, D.E.; Vieth, R. The ratio of serum 24, 25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J. Steroid Biochem. Mol. Biol. 2011, 126, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Dugar, A.; Hoofnagle, A.N.; Sanchez, A.P.; Ward, D.M.; Corey-Bloom, J.; Cheng, J.H.; Ix, J.H.; Ginsberg, C. The Vitamin D Metabolite Ratio (VMR) is a Biomarker of Vitamin D Status That is Not Affected by Acute Changes in Vitamin D Binding Protein. Clin. Chem. 2023, 69, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, C.; Hoofnagle, A.N.; Katz, R.; Becker, J.O.; Kritchevsky, S.B.; Shlipak, M.G.; Sarnak, M.J.; Ix, J.H. The vitamin D metabolite ratio is independent of vitamin D binding protein concentration. Clin. Chem. 2021, 67, 385–393. [Google Scholar] [CrossRef]
- Herrmann, M.; Farrell, C.-J.L.; Pusceddu, I.; Fabregat-Cabello, N.; Cavalier, E. Assessment of vitamin D status–a changing landscape. Clin. Chem. Lab. Med. (CCLM) 2017, 55, 3–26. [Google Scholar] [CrossRef]
- Martineau, C.; Naja, R.P.; Husseini, A.; Hamade, B.; Kaufmann, M.; Akhouayri, O.; Arabian, A.; Jones, G.; St-Arnaud, R. Optimal bone fracture repair requires 24R, 25-dihydroxyvitamin D 3 and its effector molecule FAM57B2. J. Clin. Investig. 2018, 128, 3546–3557. [Google Scholar] [CrossRef]
- Verma, A.; Schwartz, Z.; Boyan, B.D. 24R, 25-dihydroxyvitamin D3 modulates tumorigenicity in breast cancer in an estrogen receptor-dependent manner. Steroids 2019, 150, 108447. [Google Scholar] [CrossRef] [PubMed]
- Schorr, P.; Kovacevic, B.; Volmer, D.A. Overestimation of 3α-over 3β-25-Hydroxyvitamin D3 Levels in Serum: A Mechanistic Rationale for the Different Mass Spectral Properties of the Vitamin D Epimers. J. Am. Soc. Mass Spectrom. 2021, 32, 1116–1125. [Google Scholar] [CrossRef]
- Chen, Y.-C.; He, Y.-Y.; Li, Y.-M.; Wu, B.-T.; Yang, Y.-W.; Feng, J.-F. The importance of analyzing the serum C3-epimer level for evaluating vitamin D storage in some special populations. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5334–5343. [Google Scholar]
- Bailey, D.; Veljkovic, K.; Yazdanpanah, M.; Adeli, K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin. Biochem. 2013, 46, 190–196. [Google Scholar] [CrossRef]
- Arroyo, E.; Leber, C.A.; Burney, H.N.; Li, Y.; Li, X.; Lu, T.-s.; Jones, G.; Kaufmann, M.; Ting, S.M.; Hiemstra, T.F. Epimeric vitamin D and cardiovascular structure and function in advanced CKD and after kidney transplantation. Nephrol. Dial. Transplant. 2023, gfad168. [Google Scholar] [CrossRef]
- Hazell, T.J.; Gallo, S.; Berzina, l.; Vanstone, C.A.; Rodd, C.; Weiler, H.A. Plasma 25-hydroxyvitamin D, more so than its epimer, has a linear relationship to leaner body composition across infancy in healthy term infants. Appl. Physiol. Nutr. Metab. 2014, 39, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Chailurkit, L.; Aekplakorn, W.; Ongphiphadhanakul, B. Serum C3 epimer of 25-hydroxyvitamin D and its determinants in adults: A national health examination survey in Thais. Osteoporos. Int. 2015, 26, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Inflammatory markers in non-obese women with polycystic ovary syndrome are not elevated and show no correlation with vitamin D metabolites. Nutrients 2022, 14, 3540. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Shah, I.; Yasin, J.; Alessa, A. Vitamin D [25 (OH) D] metabolites and epimers in obese subject: Interaction and correlations with adverse metabolic health risk factors. J. Steroid Biochem. Mol. Biol. 2022, 215, 106023. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Perez-Lopez, F.R.; Lopez-Baena, M.T.; Perez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef]
- van den Ouweland, J.M. Analysis of vitamin D metabolites by liquid chromatography-tandem mass spectrometry. TrAC Trends Anal. Chem. 2016, 84, 117–130. [Google Scholar] [CrossRef]
- Binkley, N.; Dawson-Hughes, B.; Durazo-Arvizu, R.; Thamm, M.; Tian, L.; Merkel, J.; Jones, J.; Carter, G.; Sempos, C. Vitamin D measurement standardization: The way out of the chaos. J. Steroid Biochem. Mol. Biol. 2017, 173, 117–121. [Google Scholar] [CrossRef]
- Müller, M.J.; Volmer, D.A. Mass spectrometric profiling of vitamin D metabolites beyond 25-hydroxyvitamin D. Clin. Chem. 2015, 61, 1033–1048. [Google Scholar] [CrossRef]
- Alexandridou, A.; Volmer, D.A. Sample preparation techniques for extraction of vitamin D metabolites from non-conventional biological sample matrices prior to LC–MS/MS analysis. Anal. Bioanal. Chem. 2022, 414, 4613–4632. [Google Scholar] [CrossRef]
- Alexandridou, A.; Schorr, P.; Stokes, C.S.; Volmer, D.A. Analysis of vitamin D metabolic markers by mass spectrometry: Recent progress regarding the “gold standard” method and integration into clinical practice. Mass Spectrom. Rev. 2021, 42, 1647–1687. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.S.; Lammert, F.; Volmer, D.A. Analytical methods for quantification of vitamin D and implications for research and clinical practice. Anticancer Res. 2018, 38, 1137–1144. [Google Scholar] [PubMed]
- Qi, Y.; Müller, M.; Stokes, C.S.; Volmer, D.A. Rapid quantification of 25-hydroxyvitamin D3 in human serum by matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2018, 29, 1456–1462. [Google Scholar] [CrossRef]
- Abu Kassim, N.S.; Gomes, F.P.; Shaw, P.N.; Hewavitharana, A.K. Simultaneous quantitative analysis of nine vitamin D compounds in human blood using LC–MS/MS. Bioanalysis 2016, 8, 397–411. [Google Scholar] [CrossRef]
- Alexandridou, A.; Schorr, P.; Volmer, D.A. Comparing derivatization reagents for quantitative LC–MS/MS analysis of a variety of vitamin D metabolites. Anal. Bioanal. Chem. 2023, 415, 4689–4701. [Google Scholar] [CrossRef]
- Höller, U.; Quintana, A.P.; Gössl, R.; Olszewski, K.; Riss, G.; Schattner, A.; Nunes, C.S. Rapid determination of 25-hydroxy vitamin D3 in swine tissue using an isotope dilution HPLC-MS assay. J. Chromatogr. B 2010, 878, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, B.D.; Dolnikowski, G.; Seyoum, E.; Thomas, A.P.; Gertz, E.R.; Souza, E.C.; Woodhouse, L.R.; Newman, J.W.; Keim, N.L.; Adams, S.H. Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients 2013, 5, 3352–3366. [Google Scholar] [CrossRef]
- Lipkie, T.E.; Janasch, A.; Cooper, B.R.; Hohman, E.E.; Weaver, C.M.; Ferruzzi, M.G. Quantification of vitamin D and 25-hydroxyvitamin D in soft tissues by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2013, 932, 6–11. [Google Scholar] [CrossRef]
- Malmberg, P.; Karlsson, T.; Svensson, H.; Lönn, M.; Carlsson, N.-G.; Sandberg, A.-S.; Jennische, E.; Osmancevic, A.; Holmäng, A. A new approach to measuring vitamin D in human adipose tissue using time-of-flight secondary ion mass spectrometry: A pilot study. J. Photochem. Photobiol. B Biol. 2014, 138, 295–301. [Google Scholar] [CrossRef]
- Burild, A.; Frandsen, H.L.; Poulsen, M.; Jakobsen, J. Quantification of physiological levels of vitamin D3 and 25-hydroxyvitamin D3 in porcine fat and liver in subgram sample sizes. J. Sep. Sci. 2014, 37, 2659–2663. [Google Scholar] [CrossRef]
- Didriksen, A.; Burild, A.; Jakobsen, J.; Fuskevåg, O.M.; Jorde, R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur. J. Endocrinol. 2015, 172, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Martinaityte, I.; Kamycheva, E.; Didriksen, A.; Jakobsen, J.; Jorde, R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J. Clin. Endocrinol. Metab. 2017, 102, 3731–3738. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Margier, M.; Svilar, L.; Couturier, C.; Reboul, E.; Martin, J.-C.; Landrier, J.-F.; Defoort, C. Simple fast quantification of cholecalciferol, 25-hydroxyvitamin D and 1, 25-dihydroxyvitamin D in adipose tissue using LC-HRMS/MS. Nutrients 2019, 11, 1977. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Tourniaire, F.; Mounien, L.; Landrier, J.-F. Four days high fat diet modulates vitamin D metabolite levels and enzymes in mice. J. Endocrinol. 2021, 248, 87–93. [Google Scholar] [CrossRef]
- Best, C.M.; Riley, D.V.; Laha, T.J.; Pflaum, H.; Zelnick, L.R.; Hsu, S.; Thummel, K.E.; Foster-Schubert, K.E.; Kuzma, J.N.; Cromer, G. Vitamin D in human serum and adipose tissue after supplementation. Am. J. Clin. Nutr. 2021, 113, 83–91. [Google Scholar] [CrossRef]
- Fda, U. Bioanalytical Method Validation Guidance for Industry; US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research and Center for Veterinary Medicine: 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 15 November 2023).
- Hymøller, L.; Jensen, S.K. Vitamin D analysis in plasma by high performance liquid chromatography (HPLC) with C30 reversed phase column and UV detection–easy and acetonitrile-free. J. Chromatogr. A 2011, 1218, 1835–1841. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyksma, E.E.; Alexandridou, A.; Mai, K.; Volmer, D.A.; Stokes, C.S. An Overview of Different Vitamin D Compounds in the Setting of Adiposity. Nutrients 2024, 16, 231. https://doi.org/10.3390/nu16020231
Spyksma EE, Alexandridou A, Mai K, Volmer DA, Stokes CS. An Overview of Different Vitamin D Compounds in the Setting of Adiposity. Nutrients. 2024; 16(2):231. https://doi.org/10.3390/nu16020231
Chicago/Turabian StyleSpyksma, Eva E., Anastasia Alexandridou, Knut Mai, Dietrich A. Volmer, and Caroline S. Stokes. 2024. "An Overview of Different Vitamin D Compounds in the Setting of Adiposity" Nutrients 16, no. 2: 231. https://doi.org/10.3390/nu16020231
APA StyleSpyksma, E. E., Alexandridou, A., Mai, K., Volmer, D. A., & Stokes, C. S. (2024). An Overview of Different Vitamin D Compounds in the Setting of Adiposity. Nutrients, 16(2), 231. https://doi.org/10.3390/nu16020231






