Feeding Practices, Parent Perceptions, and Diet Diversity in a Sample of Children Aged 0–5 Years from Western Sydney, Australia: A Mixed Methods Study
Abstract
1. Introduction
- (a)
- Describe the feeding history (including breastfeeding and squeeze pouch use), diet diversity, and nutrient intake in a sample of children aged 0–5 years from Western Sydney.
- (b)
- Explore the factors underlying decisions around feeding practices made by parents for the children in this sample.
2. Materials and Methods
2.1. Participants
2.2. Study Design and Data Collection
2.2.1. Design
2.2.2. Qualitative Data Collection
2.2.3. Quantitative Data Collection
2.3. Analysis
2.3.1. Statistical Analysis
2.3.2. Thematic Analysis
3. Results
3.1. Quantitative
3.1.1. Participants
3.1.2. Feeding History
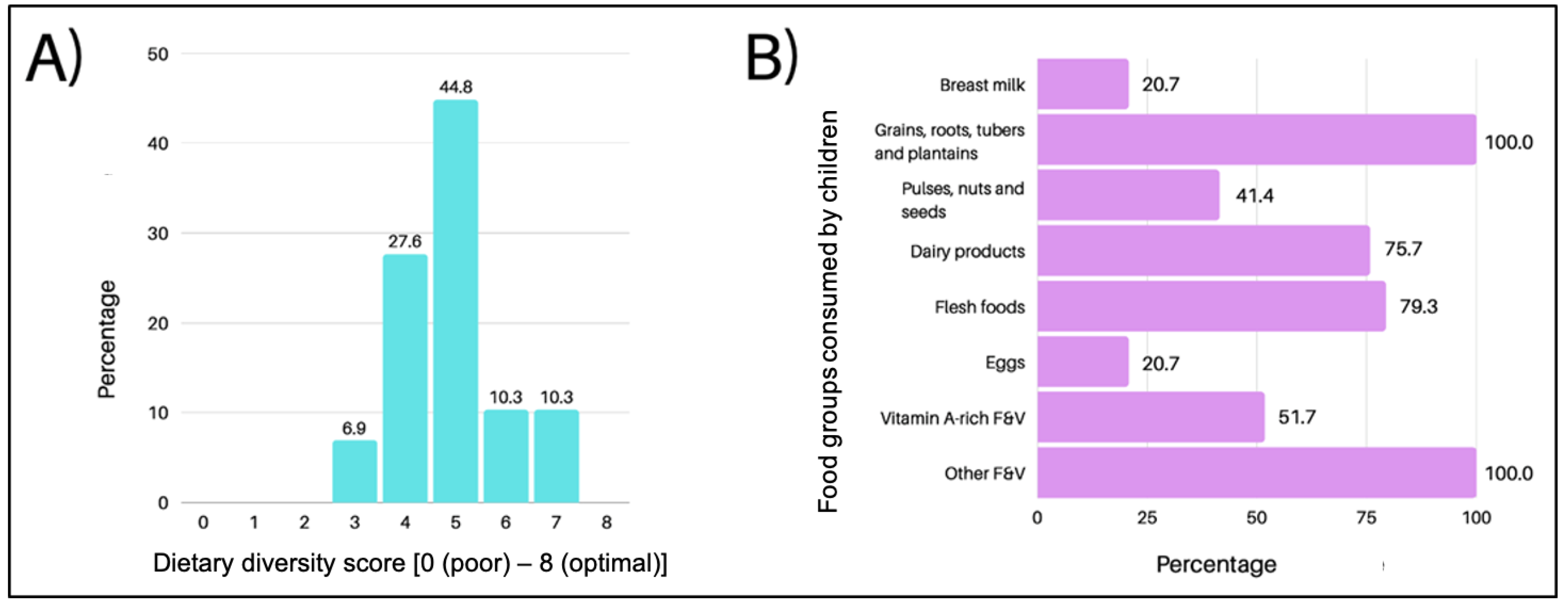
3.1.3. Diet Diversity Score
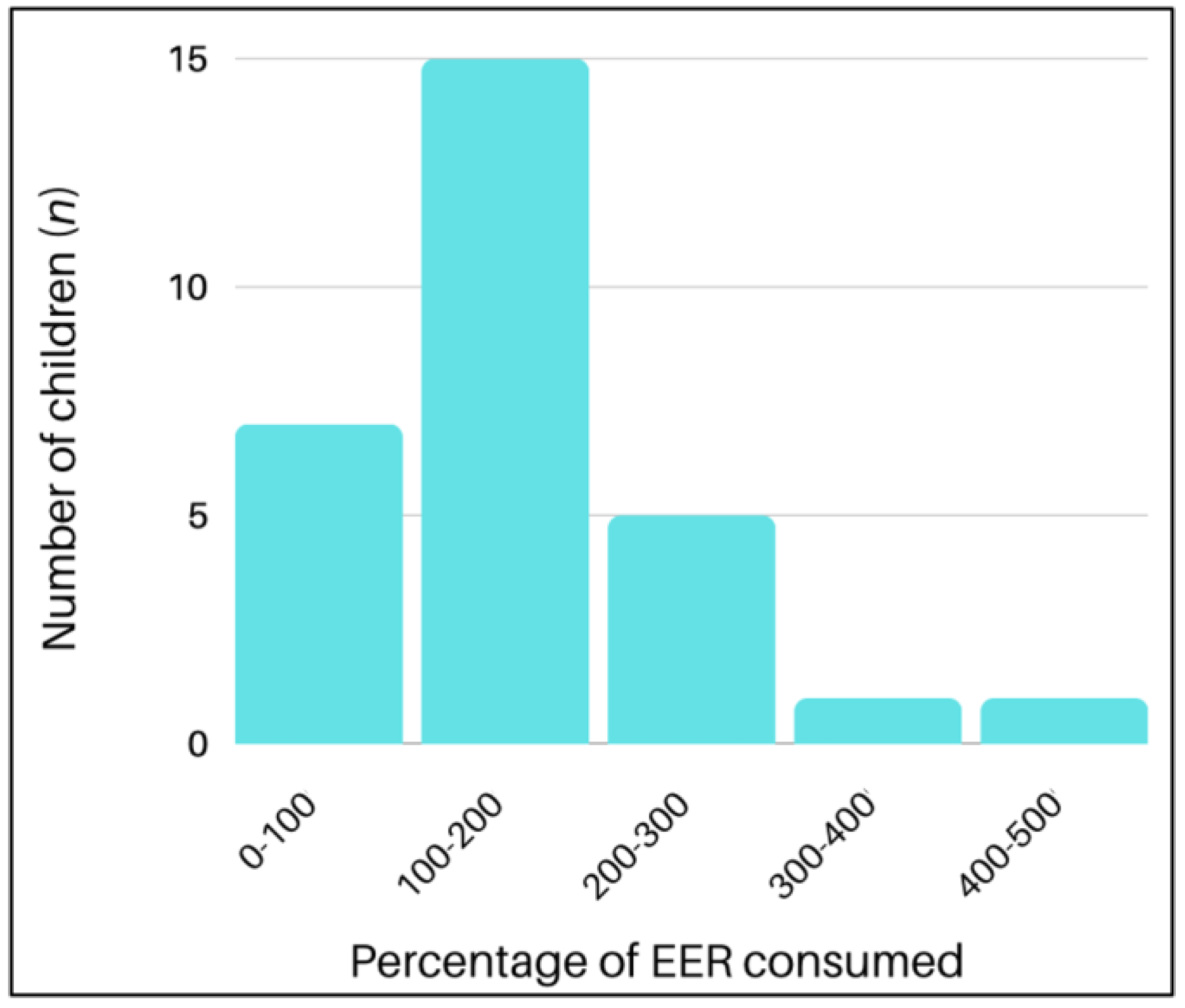
3.1.4. Nutrient Intakes
3.2. Qualitative Analysis
3.2.1. Mothers’ Perceptions of Their Children’s Diet
3.2.2. External Influences
3.2.3. Personal Experiences
3.2.4. Feeding Choices and Behaviours
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhal, A. The role of infant nutrition in the global epidemic of non-communicable disease. Proc. Nutr. Soc. 2016, 75, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef]
- Mikkilä, V.; Räsänen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent dietary patterns identified from childhood to adulthood: The cardiovascular risk in Young Finns Study. Br. J. Nutr. 2005, 93, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Australia’s Health 2020: In Brief; Australia’s Health Series No. 17 Cat. No. AUS 232; AIHW: Canberra, Australia, 2020.
- Hardy, L.L.; Jin, K.; Mihrshahi, S.; Ding, D. Trends in overweight, obesity, and waist-to-height ratio among Australian children from linguistically diverse backgrounds, 1997 to 2015. Int. J. Obes. 2019, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Infant Feeding Guidelines; National Health and Medical Research Council: Canberra, Australia, 2012.
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Australian Institute of Health and Welfare. Nutrition across the Life Stages; Cat. No. PHE 227; AIHW: Canberra, Australia, 2018.
- Johnson, B.J.; Bell, L.K.; Zarnowiecki, D.; Rangan, A.M.; Golley, R.K. Contribution of Discretionary Foods and Drinks to Australian Children’s Intake of Energy, Saturated Fat, Added Sugars and Salt. Children 2017, 4, 104. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.R.; Russell, C.G.; Campbell, K.J.; Woods, J.L. Nutrition and packaging characteristics of toddler foods and milks in Australia. Public Health Nutr. 2021, 24, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Moumin, N.A.; Netting, M.J.; Golley, R.K.; Mauch, C.E.; Makrides, M.; Green, T.J. Does Food Intake of Australian Toddlers 12–24 Months Align with Recommendations: Findings from the Australian Feeding Infants and Toddlers Study (OzFITS) 2021. Nutrients 2022, 14, 2890. [Google Scholar] [CrossRef]
- New South Wales Government. Western Sydney District Data Profile. Available online: https://www.facs.nsw.gov.au/download?file=725857 (accessed on 17 September 2022).
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info™, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Australian Bureau of Statistics: SEIFA 2016 by Postal Area Code. Available online: https://explore.data.abs.gov.au (accessed on 17 September 2022).
- Australian Institute of Health and Welfare. 2010 Australian National Infant Feeding Survey: Indicator Results; AIHW: Canberra, Australia, 2011.
- Subar, A.F.; Kirkpatrick, S.I.; Mittl, B.; Zimmerman, T.P.; Thompson, F.E.; Bingley, C.; Willis, G.; Islam, N.G.; Baranowski, T.; McNutt, S.; et al. The Automated Self-Administered 24-h dietary recall (ASA24): A resource for researchers, clinicians, and educators from the National Cancer Institute. J. Acad. Nutr. Diet. 2012, 112, 1134–1137. [Google Scholar] [CrossRef]
- World Health Organization; United Nations Children’s Fund (UNICEF). Indicators for Assessing Infant and Young Child Feeding Practices: Definitions and Measurement Methods; World Health Organization: Geneva, Switzerland; United Nations Children’s Fund (UNICEF): Geneva, Switzerland, 2021. [Google Scholar]
- National Health and Medical Research Council, Australian Government Department of Health and Ageing, New Zealand Ministry of Health. Nutrient Reference Values for Australia and New Zealand; National Health and Medical Research Council: Canberra, Australia, 2006.
- Braun, V.; Clarke, V. Thematic analysis. In APA Handbook of Research Methods in Psychology, Vol 2: Research Designs: Quantitative, Qualitative, Neuropsychological, and Biological; American Psychological Association: Washington, DC, USA, 2012; pp. 57–71. [Google Scholar]
- Burr, V. Social Constructionism, 3rd ed.; Routledge/Taylor & Francis Group: New York, NY, USA, 2015. [Google Scholar]
- Chimoriya, R.; Scott, J.A.; John, J.R.; Bhole, D.S.; Hayen, A.; Kolt, G.S.; Arora, A. Determinants of Full Breastfeeding at 6 Months and Any Breastfeeding at 12 and 24 Months among Women in Sydney: Findings from the HSHK Birth Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 5384. [Google Scholar] [CrossRef]
- Arora, A.; Manohar, N.; Hector, D.; Bhole, S.; Hayen, A.; Eastwood, J.; Scott, J.A. Determinants for early introduction of complementary foods in Australian infants: Findings from the HSHK birth cohort study. Nutr. J. 2020, 19, 16. [Google Scholar] [CrossRef]
- Brunacci, K.A.; Salmon, L.; McCann, J.; Gribble, K.; Fleming, C.A.K. The big squeeze: A product content and labelling analysis of ready-to-use complementary infant food pouches in Australia. BMC Public Health 2023, 23, 656. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.; Aquino, D.; Hadgraft, N.; Thompson, F.; Marley, J.V. Poor nutrition from first foods: A cross-sectional study of complementary feeding of infants and young children in six remote Aboriginal communities across northern Australia. Nutr. Diet. 2017, 74, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Rutishauser, I.; Knezevic, N. Foods, nutrients and portions consumed by a sample of Australian children aged 16–24 months. Nutr. Diet. 2008, 65, 56–65. [Google Scholar] [CrossRef]
- Polak, R.; Phillips, E.M.; Campbell, A. Legumes: Health Benefits and Culinary Approaches to Increase Intake. Clin. Diabetes 2015, 33, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Grieger, J.A.; Wycherley, T.P.; Golley, R.K. Theoretical Reductions in Discretionary Choices Intake via Moderation, Substitution, and Reformulation Dietary Strategies Show Improvements in Nutritional Profile: A Simulation Study in Australian 2- to 18-Year-Olds. J. Acad. Nutr. Diet. 2019, 119, 782–798.e786. [Google Scholar] [CrossRef]
- Webb, K.L.; Lahti-Koski, M.; Rutishauser, I.; Hector, D.J.; Knezevic, N.; Gill, T.; Peat, J.K.; Leeder, S.R. Consumption of ‘extra’ foods (energy-dense, nutrient-poor) among children aged 16-24 months from western Sydney, Australia. Public Health Nutr. 2006, 9, 1035–1044. [Google Scholar] [CrossRef]
- Holmes, K.L.; Rollo, M.E.; Collins, C.E. Do the contemporary dietary patterns of children align with national food and nutrient recommendations? J. Hum. Nutr. Diet. 2018, 31, 670–682. [Google Scholar] [CrossRef]
- McKeown, N.M.; Fahey, G.C., Jr.; Slavin, J.; van der Kamp, J.W. Fibre intake for optimal health: How can healthcare professionals support people to reach dietary recommendations? BMJ 2022, 378, e054370. [Google Scholar] [CrossRef]
- Bailey, R.L.; Catellier, D.J.; Jun, S.; Dwyer, J.T.; Jacquier, E.F.; Anater, A.S.; Eldridge, A.L. Total Usual Nutrient Intakes of US Children (Under 48 Months): Findings from the Feeding Infants and Toddlers Study (FITS) 2016. J. Nutr. 2018, 148, 1557s–1566s. [Google Scholar] [CrossRef]
- Chakona, G. Social circumstances and cultural beliefs influence maternal nutrition, breastfeeding and child feeding practices in South Africa. Nutr. J. 2020, 19, 47. [Google Scholar] [CrossRef]
- Arden, M.A. Conflicting influences on UK mothers’ decisions to introduce solid foods to their infants. Matern. Child. Nutr. 2010, 6, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.C.; Hesketh, K.D.; Crawford, D.A.; Campbell, K.J. Mothers’ perceptions of the influences on their child feeding practices—A qualitative study. Appetite 2016, 105, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Kourlaba, G.; Kondaki, K.; Grammatikaki, E.; Roma-Giannikou, E.; Manios, Y. Diet quality of preschool children and maternal perceptions/misperceptions: The GENESIS study. Public Health 2009, 123, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, K.; Burrows, T.; Collins, C. Peer education is a feasible method of disseminating information related to child nutrition and feeding between new mothers. BMC Public Health 2014, 14, 1262. [Google Scholar] [CrossRef]
- Young, K.G.; Duncanson, K.; Burrows, T. Influence of grandparents on the dietary intake of their 2-12-year-old grandchildren: A systematic review. Nutr. Diet. 2018, 75, 291–306. [Google Scholar] [CrossRef]
- Frey, E.; Bonfiglioli, C.; Brunner, M.; Frawley, J. Parents’ Use of Social Media as a Health Information Source for Their Children: A Scoping Review. Acad. Pediatr. 2022, 22, 526–539. [Google Scholar] [CrossRef]
- Faith, M.S.; Scanlon, K.S.; Birch, L.L.; Francis, L.A.; Sherry, B. Parent-child feeding strategies and their relationships to child eating and weight status. Obes. Res. 2004, 12, 1711–1722. [Google Scholar] [CrossRef]
| Mothers (n = 29) | Children (n = 29) | |
|---|---|---|
| Age (years) | 35 ± 3.6 1 | 2 (1–3) |
| Sex (n (%)) | ||
| Female | 29 (100.0) | 15 (51.7) |
| Male | 0 (0.0) | 14 (48.3) |
| Ethnicity (n (%)) | ||
| European | 15 (51.7) | - |
| Aboriginal and/or Torres Strait Islander | 1 (3.4) | 2 (6.9) |
| Vietnamese | 6 (20.7) | - |
| Chinese | 4 (13.8) | - |
| Indonesian | 1 (3.4) | - |
| Mixed Asian | 2 (6.9) | - |
| Education (n (%)) | ||
| Certificate or lower | 4 (13.8) | - |
| Diploma, degree, or higher | 25 (86.2) | - |
| In paid employment (n (%)) | 17 (58.6) | - |
| Socioeconomic status 2 (n (%)) | ||
| IRSAD deciles 1–4 | 11 (37.9) | 11 (37.9) |
| IRSAD deciles 5–7 | 6 (20.7) | 6 (20.7) |
| IRSAD deciles 8–10 | 12 (41.4) | 12 (41.4) |
| Anthropometry | ||
| Weight (kg) | 63 (46–105) | 12.5 (11–14.8) |
| Length/height (m) | 1.64 ± 0.1 | 0.9 ± 0.1 3 |
| BMI (kg/m2) | 23.4 (19.3–40) | 16.2 ± 3.0 3 |
| BMI z-score | - | 0.7 (−1.4–1.5) 3 |
| n (%) | |
|---|---|
| First feed | |
| Breastmilk | 27 (93.1) |
| Infant formula | 2 (6.9) |
| Breastfeeding | |
| Continuing | 6 (20.7) |
| Ceased | 23 (79.3) |
| Ceased < 6 months | 4 (17.4) |
| Ceased 6–12 months | 8 (34.8) |
| Ceased > 12 months | 11 (47.8) |
| Age at introduction to solid foods | |
| 4 months | 8 (27.6) |
| 5 months | 10 (34.5) |
| 6 months | 11 (37.9) |
| Previously used squeeze pouches 1 | 24 (82.8) |
| Introduced at <6 months | 4 (16.7) |
| Introduced at 6 months | 9 (37.5) |
| Introduced at >6 months | 10 (41.7) |
| Intake Mean ± SD or Median (IQR) | Meeting Requirements 1 n (%) | |
|---|---|---|
| Energy (kJ) | 6000.7 ± 3093.3 | 22 (75.9) |
| Macronutrients | ||
| Protein (g) | 56.6 ± 28.0 | 28 (96.6) |
| Total fat (g) | 44.4 (32.0–72.5) | 7 (24.1) 2 |
| Monounsaturated fat (g) | 16.5 (10.1–24.8) | - |
| Polyunsaturated fat (g) | 5.2 (3.3–8.5) | - |
| Saturated fat (g) | 19.4 (13.5–30.9) | - |
| Carbohydrates (g) | 158.8 (111.9–207.9) | 9 (31.0) 3 |
| Fibre (g) | 17.0 ± 8.0 | 14 (53.8) 4 |
| Micronutrients | ||
| Calcium (mg) | 701.0 ± 318.8 | 23 (79.3) |
| Iron (mg) | 6.3 (4.0–11.6) | 23 (79.3) |
| Sodium (mg) | 1516.8 (1077.4–2095.0) | 28 (96.6) |
| Zinc (mg) | 6.9 ± 3.4 | 28 (96.6) |
| Thiamine (mg) | 1.1 (0.6–1.7) | 26 (89.7) |
| Riboflavin (mg) | 1.6 ± 0.9 | 26 (89.7) |
| Niacin (mg) | 12.3 (7.1–17.5) | 25 (86.2) |
| Folate (µg) | 284.7 (220.2–408.7) | 27 (93.1) |
| Vitamin B6 (mg) | 0.8 (0.6–1.1) | 26 (89.7) |
| Vitamin B12 (µg) | 3.0 ± 1.8 | 27 (93.1) |
| Vitamin C (mg) | 38.6 (28.0–75.2) | 22 (75.9) |
| Vitamin E (mg) | 5.2 (3.7–8.2) | 16 (55.2) |
| Other | ||
| Total sugars (g) | 68.1 (41.9–89.8) | - |
| Caffeine (mg) | 0.0 (0.0–0.6) | - |
| Cholesterol (mg) | 128.3 (67.5–362.1) | - |
| Sub-Theme | Quote |
|---|---|
| Perceptions of a child’s readiness to eat solids | “She just hit a point where she just started picking up foods off my plate and giving them to herself” (Mother, child aged 9 months) |
| Perceptions about a child’s diet | “Now, she knows exactly what’s in the fridge and she’s always snacking. And it’s quite healthy as well. So, she eats a lot of apples and a lot of fruit.” (Mother, child aged 5 years) |
| Sub-Theme | Quote |
|---|---|
| Advice from family and friends | “Oh, mum and friends. Friends are because—a couple of mummy friends would because they were just like,” Oh, “they post pictures of their child to say they eat this or broccoli or this at this age…” “So it’s also set further examples and what works for them and tested by them. My mum is because she feeds me, so I trust her with that one as well.” (Mother, child aged 3 years). |
| Advice from mother’s groups | “A lot of the support and the advice I got was from other women in my mother’s group who had done all the research into introducing solids…” (Mother, child aged 18 months). |
| Advice from healthcare providers | “It’s only when we experience something like we’re worried about if he’s not gaining weight well or he’s getting sick, then we would chat to the doctor and nurse about that, but so far, we haven’t really talked much about solid foods.” (Mother, child aged 13 months). |
| Information from the internet and social media | “I did read a lot of books. So I read a lot on weaning books, especially Gina Ford’s book, which talks about when to start baby on solids, what to feed them and everything.” (Mother, child aged 13 months). “So I use Facebook forums and groups, and I just look at the information and resources that they have and then just go from it to Google on a theme.” (Mother, child aged 20 months). |
| Uncertainty about advice | “I did a lot of research online and through books and just speaking with other people and came to what was the most common feature. So, a lot of them said berries—they could have a reaction. So I steer clear of them for a little while. Same with honey, it was quite—although there was all different data, there was a majority. So I went with the majority.” (Mother, child aged 2 years). |
| Sub-Theme | Quote |
|---|---|
| Mother’s upbringing and own experiences | “I guess growing up in an Asian household, rice is number one. So I tend to feed him a lot of rice and noodles.” (Mother, child aged 2 years). “Two out of three of my oldest kids are lactose-intolerant. And just the fact that she was quite small … made me think that maybe she had an issue with lactose.” (Mother, child aged 22 months). |
| Sub-Theme | Quote |
|---|---|
| Mother’s choice of foods to provide | “He’ll eat whatever the family is eating which is a wide range of meats, pasta, rice, potato, salads, veggies, cereal, bread, sandwiches.” (Mother, child aged 2 years). |
| Pressure to eat | “…I always had a thing that he has to finish what I gave him ‘cause, otherwise, he would be behind someone exactly the same age as him.” (Mother, child aged 3 years). “So he can eat as much as he likes, but he tells me he’s full, that’s fine, he doesn’t have to finish it but he then can’t have something else…” (Mother, child aged 4 years). |
| Giving their child a choice of foods | “I think maybe you can provide him with options but you still need to watch what you give him.” (Mother, child aged 10 months). “…if my child doesn’t wanna finish their meal, I don’t make them. Learning to listen to their bodies and they can come back and finish it later.” (Mother, child aged 5 years). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyer, A.; Kent, K.; Brunacci, K.; Agho, K.E.; Fleming, C.A.K. Feeding Practices, Parent Perceptions, and Diet Diversity in a Sample of Children Aged 0–5 Years from Western Sydney, Australia: A Mixed Methods Study. Nutrients 2024, 16, 198. https://doi.org/10.3390/nu16020198
Iyer A, Kent K, Brunacci K, Agho KE, Fleming CAK. Feeding Practices, Parent Perceptions, and Diet Diversity in a Sample of Children Aged 0–5 Years from Western Sydney, Australia: A Mixed Methods Study. Nutrients. 2024; 16(2):198. https://doi.org/10.3390/nu16020198
Chicago/Turabian StyleIyer, Anjana, Katherine Kent, Kaitlyn Brunacci, Kingsley Emwinyore Agho, and Catharine A. K. Fleming. 2024. "Feeding Practices, Parent Perceptions, and Diet Diversity in a Sample of Children Aged 0–5 Years from Western Sydney, Australia: A Mixed Methods Study" Nutrients 16, no. 2: 198. https://doi.org/10.3390/nu16020198
APA StyleIyer, A., Kent, K., Brunacci, K., Agho, K. E., & Fleming, C. A. K. (2024). Feeding Practices, Parent Perceptions, and Diet Diversity in a Sample of Children Aged 0–5 Years from Western Sydney, Australia: A Mixed Methods Study. Nutrients, 16(2), 198. https://doi.org/10.3390/nu16020198







