Adherence to Mediterranean Diet and Biomarkers of Redox Balance and Inflammation in Old Patients Hospitalized in Internal Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Laboratory Measurements
2.3. RNA Isolation and Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
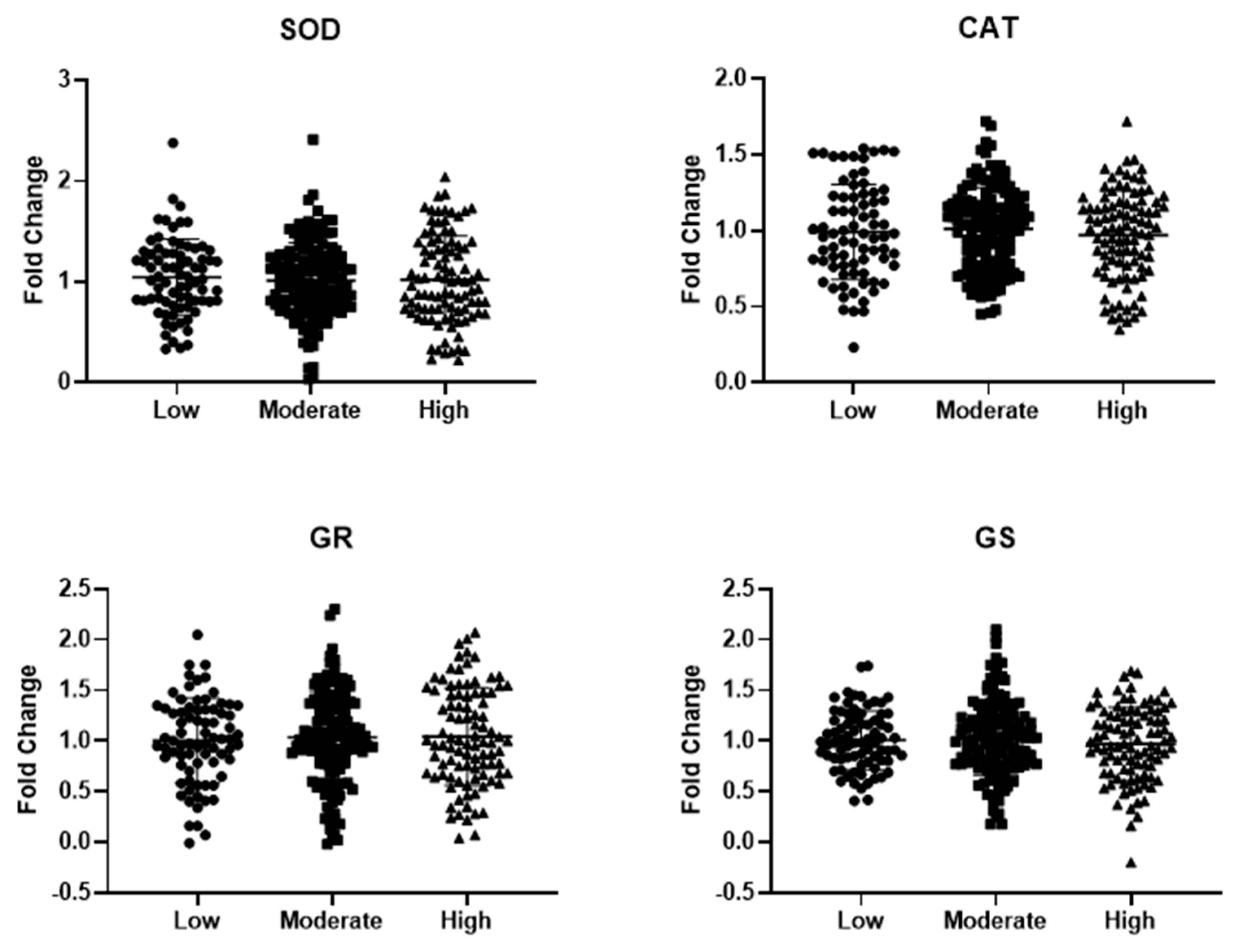
3.2. MD Adherence and Circulating Markers of Redox Balance
3.3. MD Adherence and Circulating Markers of Inflammation/Immune Response
3.4. Association between Circulating Markers of Redox Balance and Inflammation/Immune Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean Diet and Nutritional Adequacy: A Review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Roman, B.; Estruch, R. Scientific Evidence of Interventions Using the Mediterranean Diet: A Systematic Review. Nutr. Rev. 2006, 64, S27–S47. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and Control of Type 2 Diabetes by Mediterranean Diet: A Systematic Review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.; Hartley, L.; Flowers, N.; Clarke, A.; Hooper, L.; Thorogood, M.; Stranges, S. “Mediterranean” Dietary Pattern for the Primary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2013, 2012, CD009825. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; De La Torre, R.; Martínez-González, M.Á.; Martínez-Lapiscina, E.H.; Fitó, M.; Pérez-Heras, A.; Salas-Salvadó, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Würtele, G.; Spiegelhalder, B.; Bartsch, H. Olive-Oil Consumption and Health: The Possible Role of Antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; La Sala, L.; Pujadas, G.; De Nigris, V.; Testa, R.; Bucciarelli, L.; Rondinelli, M.; Genovese, S. The Protective Effect of the Mediterranean Diet on Endothelial Resistance to GLP-1 in Type 2 Diabetes: A Preliminary Report. Cardiovasc. Diabetol. 2014, 13, 140. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The Immune Protective Effect of the Mediterranean Diet against Chronic Low-Grade Inflammatory Diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Vilarnau, C.; Stracker, D.M.; Funtikov, A.; da Silva, R.; Estruch, R.; Bach-Faig, A. Worldwide Adherence to Mediterranean Diet between 1960 and 2011. Eur. J. Clin. Nutr. 2019, 72, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Roman, B.; Carta, L.; Martínez-González, Á.M.; Serra-Majem, L. Effectiveness of the Mediterranean Diet in the Elderly. Clin. Interv. Aging 2008, 3, 97–109. [Google Scholar] [PubMed]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The Origins of Age-Related Proinflammatory State. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Lo Buglio, A.; Bellanti, F.; Talia, M.; Romano, A.D.; Serviddio, G.; Vendemiale, G. Reliability of Serum Procalcitonin Concentration for the Diagnosis of Sepsis in Elderly Patient with Chronic Kidney Disease. J. Gerontol. Geriatr. 2016, 64, 49–54. [Google Scholar]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative Stress Is Increased in Sarcopenia and Associated with Cardiovascular Disease Risk in Sarcopenic Obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic Criteria for Malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Morley, J.E. Anorexia of Ageing: A Key Component in the Pathogenesis of Both Sarcopenia and Cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 523–526. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, Age-Related Diseases and Oxidative Stress: What to Do Next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Lo Buglio, A.; Bellanti, F.; Capurso, C.; Paglia, A.; Vendemiale, G. Adherence to Mediterranean Diet, Malnutrition, Length of Stay and Mortality in Elderly Patients Hospitalized in Internal Medicine Wards. Nutrients 2019, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Agnoli, C.; Krogh, V.; Grioni, S.; Sieri, S.; Palli, D.; Masala, G.; Sacerdote, C.; Vineis, P.; Tumino, R.; Frasca, G.; et al. A Priori-Defined Dietary Patterns Are Associated with Reduced Risk of Stroke in a Large Italian Cohort. J. Nutr. 2011, 141, 1552–1558. [Google Scholar] [CrossRef]
- Serviddio, G.; Romano, A.D.; Greco, A.; Rollo, T.; Bellanti, F.; Altomare, E.; Vendemiale, G. Frailty Syndrome Is Associated with Altered Circulating Redox Balance and Increased Markers of Oxidative Stress. Int. J. Immunopathol. Pharmacol. 2009, 22, 819–827. [Google Scholar] [CrossRef]
- Masako, T.; Toshiyuki, M.; Kaoru, M.; Kageaki, A. Fluorescent Substances in Mouse and Human Sera as a Parameter of in Vivo Lipid Peroxidation. Biochim. Biophys. Acta Lipids Lipid Metab. 1985, 834, 196–204. [Google Scholar] [CrossRef]
- Molloy, R.M.; Mc Connell, R.I.; Lamont, J.V.; FitzGerald, S.P. Automation of Biochip Array Technology for Quality Results. Clin. Chem. Lab. Med. 2005, 43, 1303–1313. [Google Scholar] [CrossRef]
- Galbete, C.; Schwingshackl, L.; Schwedhelm, C.; Boeing, H.; Schulze, M.B. Evaluating Mediterranean Diet and Risk of Chronic Disease in Cohort Studies: An Umbrella Review of Meta-Analyses. Eur. J. Epidemiol. 2018, 33, 909–931. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses of Observational Studies and Randomised Trials. Eur. J. Clin. Nutr. 2018, 72, e21. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A.; Shab-Bidar, S.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to the Mediterranean Diet in Relation to All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. 2019, 10, 1029–1039. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. The Role of Antioxidants in the Mediterranean Diet. Lipids 2001, 36, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Estruch, R. Mediterranean Diet, Antioxidants and Cancer: The Need for Randomized Trials. Eur. J. Cancer Prev. 2004, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean Diet and 10-Year Incidence (2002–2012) of Diabetes: Correlations with Inflammatory and Oxidative Stress Biomarkers in the ATTICA Cohort Study. Diabetes Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, J.; Stefanadis, C. Status and Management of Blood Lipids in Greek Adults and Their Relation to Socio-Demographic, Lifestyle and Dietary Factors: The ATTICA Study: Blood Lipids Distribution in Greece. Atherosclerosis 2004, 173, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between Adherence to the Mediterranean Diet and Oxidative Stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [PubMed]
- Berndt, C.; Lillig, C.H.; Flohé, L. Redox Regulation by Glutathione Needs Enzymes. Front. Pharmacol. 2014, 5, 168. [Google Scholar] [CrossRef]
- Meister, A.; Tate, S.S. Glutathione and Related Gamma-Glutamyl Compounds: Biosynthesis and Utilization. Annu. Rev. Biochem. 1976, 45, 559–604. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Sureda, A.; del Mar Bibiloni, M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef]
- Dai, J.; Miller, A.H.; Bremner, J.D.; Goldberg, J.; Jones, L.; Shallenberger, L.; Buckham, R.; Murrah, N.V.; Veledar, E.; Wilson, P.W.; et al. Adherence to the Mediterranean Diet Is Inversely Associated with Circulating Interleukin-6 among Middle-Aged Men: A Twin Study. Circulation 2008, 117, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a Healthy Dietary Pattern Results in Significant Reductions in C-Reactive Protein Levels in Adults: A Meta-Analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Bucci, L.; Capri, M.; Salvioli, S.; Scurti, M.; Pini, E.; Monti, D.; Franceschi, C. Immunosenescence and Immunogenetics of Human Longevity. Neuroimmunomodulation 2008, 15, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Spazzafumo, L.; Olivieri, F.; Abbatecola, A.M.; Castellani, G.; Monti, D.; Lisa, R.; Galeazzi, R.; Sirolla, C.; Testa, R.; Ostan, R.; et al. Remodelling of Biological Parameters during Human Ageing: Evidence for Complex Regulation in Longevity and in Type 2 Diabetes. Age 2013, 35, 419–429. [Google Scholar] [CrossRef]
- Martucci, M.; Ostan, R.; Biondi, F.; Bellavista, E.; Fabbri, C.; Bertarelli, C.; Salvioli, S.; Capri, M.; Franceschi, C.; Santoro, A. Mediterranean Diet and Inflammaging within the Hormesis Paradigm. Nutr. Rev. 2017, 75, 442–455. [Google Scholar] [CrossRef]
- Moskalev, A.; Stambler, I.; Caruso, C. Innate and Adaptive Immunity in Aging and Longevity: The Foundation of Resilience. Aging Dis. 2020, 11, 1363–1373. [Google Scholar] [CrossRef]
- Jia, H.; Huang, W.; Liu, C.; Tang, S.; Zhang, J.; Chen, C.; Tian, Y.; Zhong, W. Immunosenescence Is a Therapeutic Target for Frailty in Older Adults: A Narrative Review. Ann. Transl. Med. 2022, 10, 1142. [Google Scholar] [CrossRef]
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The Interplay between Immunosenescence and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-Lymphocyte Ratio and Mortality in the United States General Population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef]
- Lacy, P.; Stow, J.L. Cytokine Release from Innate Immune Cells: Association with Diverse Membrane Trafficking Pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox Regulation of the Immune Response. Cell Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mikkilä, V.; Räsänen, L.; Raitakari, O.T.; Pietinen, P.; Viikari, J. Consistent Dietary Patterns Identified from Childhood to Adulthood: The Cardiovascular Risk in Young Finns Study. Br. J. Nutr. 2005, 93, 923–931. [Google Scholar] [CrossRef]

| Adherence to Mediterranean Diet | ||||
|---|---|---|---|---|
| Low (n = 76) | Moderate (n = 135) | High (n = 95) | p | |
| Age (years) | 75.9 ± 9.82 | 75.4 ± 7.44 | 74.6 ± 6.66 | 0.600 |
| Sex (M/F) | 36/40 | 54/81 | 39/56 | 0.564 |
| Comorbidities (n, %) | 28 (36.8) | 57 (42.2) | 35 (36.8) | 0.633 |
| Polypharmacotherapy (n, %) | 12 (15.8) | 29 (21.5) | 25 (26.3) | 0.251 |
| Hemoglobin (g/dL) | 11.2 ± 2.03 | 11.7 ± 2.02 | 14.2 ± 3.34 ***,††† | <0.001 |
| Glucose (mg/dL) | 123 ± 47.8 | 119 ± 54.1 | 120 ± 47.8 | 0.819 |
| Albumin (mg/dL) | 2.99 ± 0.51 | 3.06 ± 0.52 | 3.14 ± 0.49 | 0.159 |
| Total cholesterol (mg/dL) | 152 ± 48.0 | 151 ± 49.0 | 146 ± 39.1 | 0.593 |
| Creatinine (mg/dL) | 1.19 ± 0.81 | 1.20 ± 0.70 | 1.19 ± 0.46 | 0.986 |
| Blood Urea Nitrogen (mg/dL) | 67.2 ± 40.7 | 70.2 ± 55.2 | 67.7 ± 40.3 | 0.884 |
| Triglycerides (mg/dL) | 120 ± 62.0 | 115 ± 49.1 | 128 ± 70.0 | 0.314 |
| Adherence to Mediterranean Diet | ||||
|---|---|---|---|---|
| Low (n = 76) | Moderate (n = 135) | High (n = 95) | p | |
| GSH (µM) | 39.6 ± 22.1 | 49.0 ± 22.6 * | 64.6 ± 24.7 ***,††† | <0.001 |
| GSSG (µM) | 6.39 ± 3.03 | 5.59 ± 2.72 | 5.31 ± 3.15 | 0.051 |
| GSSG/GSH (%) | 28.4 ± 37.6 | 18.2 ± 24.9 * | 10.3 ± 9.23 *** | <0.001 |
| HNE–protein adducts (AUF) | 48.4 ± 23.1 | 36.6 ± 21.0 *** | 27.6 ± 17.3 ***,†† | <0.001 |
| MDA–protein adducts (AUF) | 93.3 ± 51.8 | 75.0 ± 41.6 ** | 59.5 ± 38.0 ***,† | <0.001 |
| Adherence to Mediterranean Diet | ||||
|---|---|---|---|---|
| Low (n = 76) | Moderate (n = 135) | High (n = 95) | p | |
| ESR (mm/h) | 59.1 ± 36.7 | 42.7 ± 30.6 *** | 18.0 ± 15.9 ***,††† | <0.001 |
| CRP (mg/L) | 49.7 ± 58.7 | 55.3 ± 79.2 | 57.8 ± 35.7 | 0.698 |
| Fibrinogen (mg/dL) | 408 ± 189 | 410 ± 167 | 326 ± 73.3 **,††† | <0.001 |
| Ferritin (ng/mL) | 358 ± 733 | 273 ± 443 | 103 ± 111 **,† | 0.002 |
| α2-globulins (g/dL) | 12.5 ± 3.72 | 12.8 ± 3.67 | 9.42 ± 2.13 ***,††† | <0.001 |
| White blood cells (n/mm2) | 8945 ± 4616 | 8284 ± 4449 | 7735 ± 3553 | 0.180 |
| Neutrophils (n/mm2) | 7983 ± 1047 | 6125 ± 4306 | 3872 ± 1185 ***,† | <0.001 |
| Lymphocytes (n/mm2) | 1278 ± 843 | 1516 ± 938 | 1970 ± 692 ***,††† | <0.001 |
| NLR | 7.53 ± 5.88 | 5.89 ± 7.69 | 2.15 ± 0.92 ***,††† | <0.001 |
| Adherence to Mediterranean Diet | ||||
|---|---|---|---|---|
| Low (n = 76) | Moderate (n = 135) | High (n = 95) | p | |
| IL-1α (pg/mL) | 0.53 ± 0.06 | 0.53 ± 0.05 | 0.53 ± 0.09 | 0.839 |
| IL-1β (pg/mL) | 0.96 ± 0.23 | 0.91 ± 0.29 | 0.92 ± 0.24 | 0.446 |
| IL-2 (pg/mL) | 0.99 ± 0.53 | 0.93 ± 0.43 | 0.94 ± 0.60 | 0.725 |
| IL-4 (pg/mL) | 0.81 ± 0.22 | 0.84 ± 0.16 | 0.84 ± 0.30 | 0.595 |
| IL-6 (pg/mL) | 21.8 ± 4.98 | 11.9 ± 5.55 *** | 3.37 ± 1.56 ***,††† | <0.001 |
| IL-8 (pg/mL) | 100 ± 29.9 | 103 ± 35.7 | 99.0 ± 43.9 | 0.608 |
| IL-10 (pg/mL) | 1.98 ± 0.97 | 1.95 ± 1.17 | 1.69 ± 0.71 | 0.090 |
| TNF (pg/mL) | 13.2 ± 2.10 | 6.52 ± 3.36 *** | 4.60 ± 1.86 ***,††† | <0.001 |
| IFN-γ (pg/mL) | 2.26 ± 0.86 | 2.27 ± 0.93 | 2.41 ± 1.12 | 0.485 |
| VEGF (pg/mL) | 253 ± 43.9 | 262 ± 35.7 | 261 ± 54.2 | 0.357 |
| EGF (pg/mL) | 13.9 ± 1.33 | 13.4 ± 2.04 | 13.6 ± 3.46 | 0.295 |
| ESR | Fibrinogen | Ferritin | α2-glob | Neutro | Lympho | NLR | IL-6 | TNF | GSSG/GSH | HNE | MDA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ESR | 1 | 0.400 *** | 0.469 *** | 0.491 *** | 0.533 *** | −0.354 *** | 0.499 *** | 0.556 *** | 0.142 * | 0.317 *** | 0.310 *** | 0.266 *** |
| Fibrinogen | 0.400 *** | 1 | 0.297 *** | 0.407 *** | 0.185 ** | −0.18 | 0.120 * | 0.175 ** | 0.213 *** | 0.134 * | −0.002 | 0.075 |
| Ferritin | 0.469 *** | 0.297 *** | 1 | 0.166 ** | 0.358 *** | −0.247 *** | 0.465 *** | 0.250 *** | 0.002 | 0.263 *** | 0.186 ** | 0.069 |
| α2-glob | 0.491 *** | 0.407 *** | 0.166 ** | 1 | 0.287 *** | −0.176 ** | 0.193 ** | 0.318 *** | 0.303 *** | 0.205 *** | 0.131 * | 0.159 ** |
| Neutro | 0.533 *** | 0.185 ** | 0.358 *** | 0.287 *** | 1 | −0.242 *** | 0.832 *** | 0.413 *** | 0.060 | 0.341 *** | 0.260 *** | 0.247 *** |
| Lympho | −0.354 *** | −0.18 | −0.247 *** | −0.176 ** | −0.242 *** | 1 | 0.478 *** | 0.370 *** | 0.055 | −0.208 *** | −0.282 *** | −0.185 *** |
| NLR | 0.499 *** | 0.120 * | 0.465 *** | 0.193 ** | 0.832 *** | 0.478 *** | 1 | 0.444 *** | 0.019 | 0.481 *** | 0.265 *** | 0.301 *** |
| IL-6 | 0.556 *** | 0.175 ** | 0.250 *** | 0.318 *** | 0.413 *** | 0.370 *** | 0.444 *** | 1 | 0.381 *** | 0.365 *** | 0.360 *** | 0.344 *** |
| TNF | −0.142 * | 0.213 *** | 0.002 | −0.303 *** | −0.060 | 0.055 | −0.019 | 0.381 *** | 1 | 0.058 | 0.094 | 0.013 |
| GSSG/GSH | 0.317 *** | 0.134 * | 0.263 *** | 0.205 *** | 0.341 *** | −0.208 *** | 0.481 *** | 0.365 *** | 0.058 | 1 | 0.205 *** | 0.375 *** |
| HNE | 0.310 *** | −0.002 | 0.186 ** | 0.131 * | 0.260 *** | −0.282 *** | 0.265 *** | 0.360 *** | 0.094 | 0.205 *** | 1 | 0.233 *** |
| MDA | 0.266 *** | 0.075 | 0.069 | 0.159 ** | 0.247 *** | −0.185 *** | 0.301 *** | 0.344 *** | 0.013 | 0.375 *** | 0.233 *** | 1 |
| Unstandardized Coefficients | Standardized Coefficients | t-Value | p-Value | ||
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| Dependent: GSSG/GSH | |||||
| Constant | 5.020 | 6.923 | 0.725 | 0.469 | |
| ESR | 0.075 | 0.055 | 0.119 | 1.373 | 0.171 |
| Fibrinogen | −0.008 | 0.008 | −0.063 | 0.910 | 0.364 |
| Ferritin | 0.007 | 0.003 | 0.176 | 2.438 | 0.051 |
| α2-glob | 0.478 | 0.375 | 0.095 | 1.274 | 0.204 |
| Neutro | −0.001 | 0.001 | −0.193 | −1.724 | 0.086 |
| Lympho | 0.001 | 0.002 | 0.041 | 0.551 | 0.582 |
| NLR | 0.496 | 0.369 | 0.164 | 1.344 | 0.180 |
| IL-6 | 0.311 | 0.187 | 0.129 | 1.660 | 0.098 |
| TNF | 0.198 | 0.298 | 0.048 | 0.667 | 0.506 |
| Model verification: ANOVA, F = 3.503; p < 0.001 | |||||
| Dependent: HNE | |||||
| Constant | 44.563 | 7.781 | 5.527 | <0.001 | |
| ESR | 0.095 | 0.061 | 0.130 | 1.553 | 0.122 |
| Fibrinogen | −0.020 | 0.009 | −0.143 | −2.134 | 0.034 |
| Ferritin | 0.005 | 0.003 | 0.106 | 1.511 | 0.132 |
| α2-glob | −0.206 | 0.421 | −0.035 | −0.490 | 0.625 |
| Neutro | 0.001 | 0.001 | 0.173 | 1.600 | 0.111 |
| Lympho | −0.005 | 0.002 | −0.201 | −2.793 | 0.006 |
| NLR | −0.662 | 0.415 | −0.189 | −1.595 | 0.112 |
| IL-6 | 0.461 | 0.211 | 0.164 | 2.188 | 0.030 |
| TNF | −0.374 | 0.334 | −0.078 | −1.117 | 0.265 |
| Model verification: ANOVA, F = 5.711; p < 0.001 | |||||
| Dependent: MDA | |||||
| Constant | 49.287 | 16.046 | 3.053 | 0.003 | |
| ESR | 0.118 | 0.128 | 0.082 | 0.925 | 0.356 |
| Fibrinogen | −0.003 | 0.019 | −0.009 | −0.130 | 0.896 |
| Ferritin | −0.004 | 0.007 | −0.041 | −0.577 | 0.578 |
| α2-glob | 0.441 | 0.875 | 0.038 | 0.504 | 0.615 |
| Neutro | 0.000 | 0.001 | −0.009 | −0.077 | 0.939 |
| Lympho | −0.002 | 0.004 | −0.049 | −0.647 | 0.519 |
| NLR | −0.114 | 0.861 | −0.016 | −0.132 | 0.895 |
| IL-6 | 1.281 | 0.437 | 0.232 | 2.931 | 0.004 |
| TNF | 0.808 | 0.694 | 0.086 | 1.165 | 0.295 |
| Model verification: ANOVA, F = 2.165; p = 0.025 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellanti, F.; Lo Buglio, A.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Serviddio, G.; Vendemiale, G. Adherence to Mediterranean Diet and Biomarkers of Redox Balance and Inflammation in Old Patients Hospitalized in Internal Medicine. Nutrients 2024, 16, 3359. https://doi.org/10.3390/nu16193359
Bellanti F, Lo Buglio A, Dobrakowski M, Kasperczyk A, Kasperczyk S, Serviddio G, Vendemiale G. Adherence to Mediterranean Diet and Biomarkers of Redox Balance and Inflammation in Old Patients Hospitalized in Internal Medicine. Nutrients. 2024; 16(19):3359. https://doi.org/10.3390/nu16193359
Chicago/Turabian StyleBellanti, Francesco, Aurelio Lo Buglio, Michał Dobrakowski, Aleksandra Kasperczyk, Sławomir Kasperczyk, Gaetano Serviddio, and Gianluigi Vendemiale. 2024. "Adherence to Mediterranean Diet and Biomarkers of Redox Balance and Inflammation in Old Patients Hospitalized in Internal Medicine" Nutrients 16, no. 19: 3359. https://doi.org/10.3390/nu16193359
APA StyleBellanti, F., Lo Buglio, A., Dobrakowski, M., Kasperczyk, A., Kasperczyk, S., Serviddio, G., & Vendemiale, G. (2024). Adherence to Mediterranean Diet and Biomarkers of Redox Balance and Inflammation in Old Patients Hospitalized in Internal Medicine. Nutrients, 16(19), 3359. https://doi.org/10.3390/nu16193359








