Age-Related Effects of Olive Oil Polyphenol Ingestion on Oxidation of Low-Density Lipoprotein in Healthy Japanese Men: A Randomized Controlled Double-Blind Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Participants
2.2.1. Selection Criteria
2.2.2. Exclusion Criteria
2.3. Sample Size
2.4. Test Food and Control Food
2.5. Dietary Survey
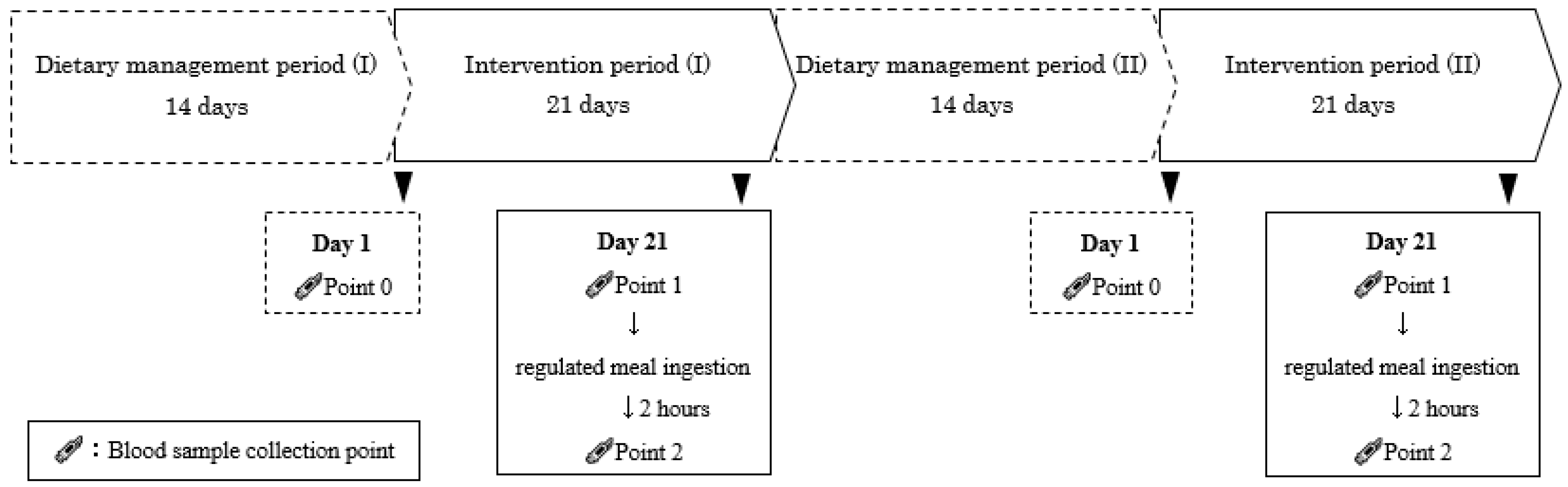
2.6. Study Design
2.6.1. Dietary Management
2.6.2. Lifestyle Management
2.6.3. Specific Compliance before Test Day
2.7. Outcomes
2.8. Blood Biochemical Analysis
2.9. Adverse Events
2.10. Statistical Analysis
3. Results
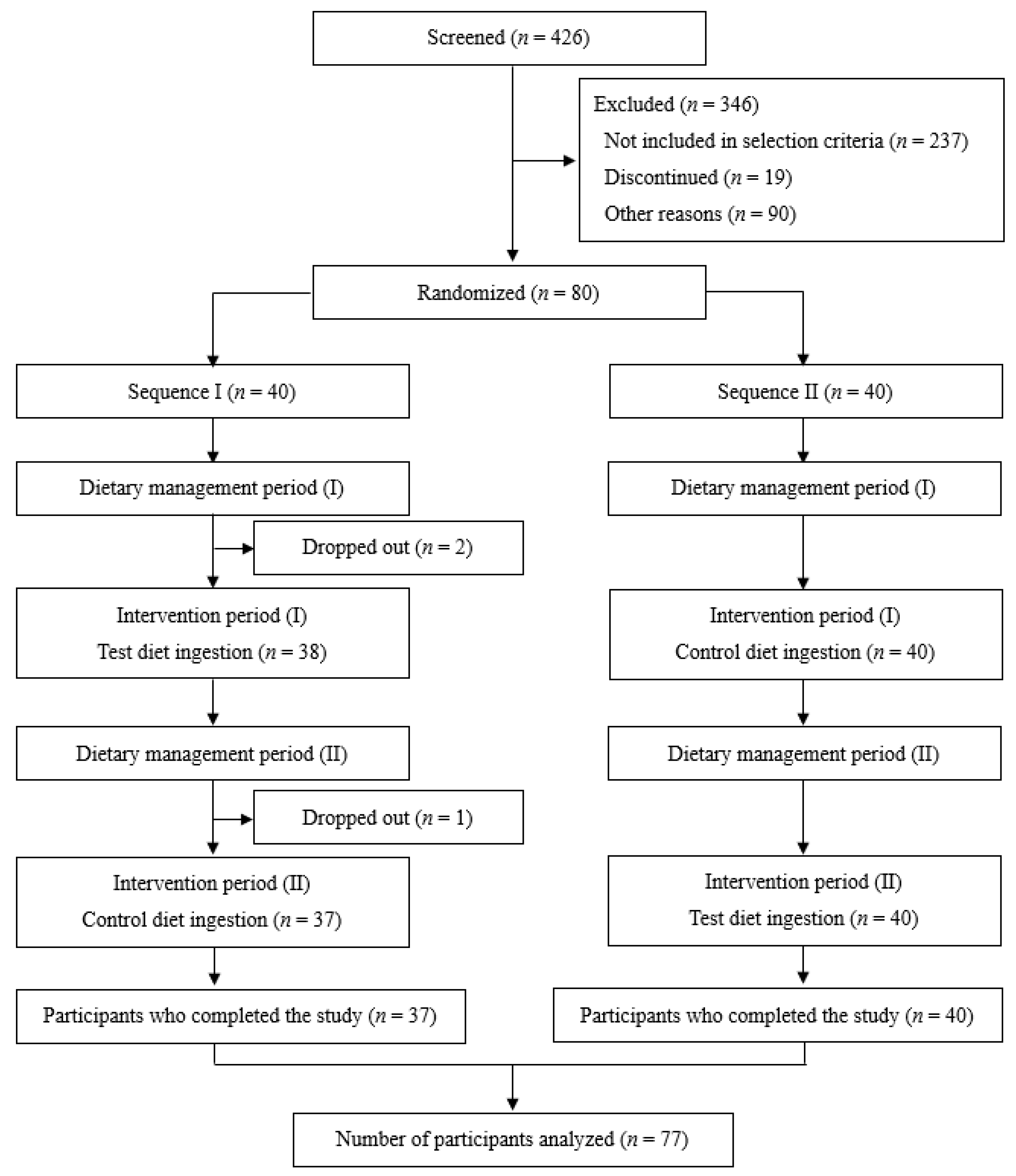
3.1. Participants
3.2. Adverse Events
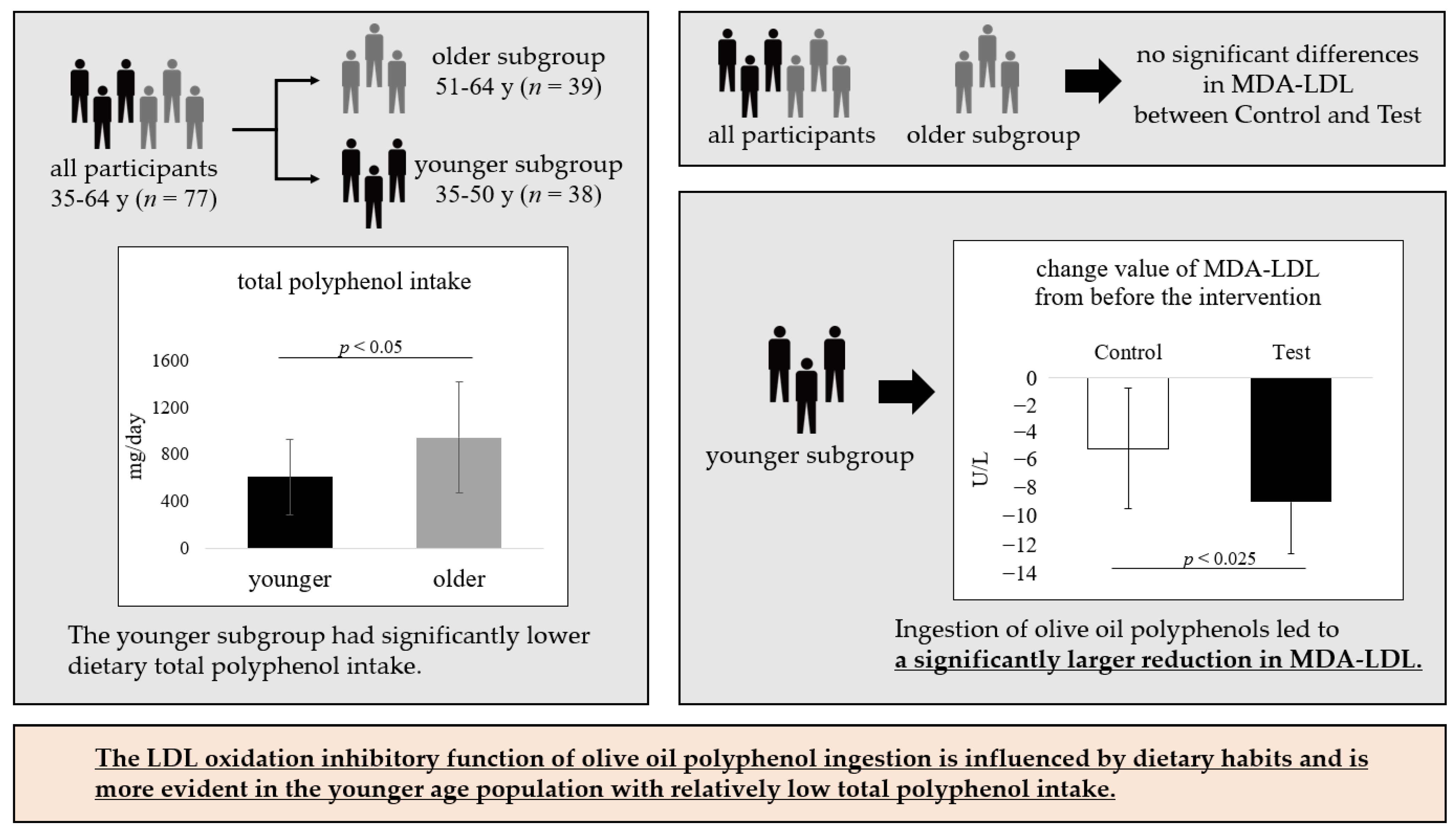
3.3. Nutrient Intake
3.4. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, The Top 10 Causes of Death. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 June 2024).
- Ministry of Health, Labour and Welfare, Japan. Overview Report of Vital Statistics in FY 2020. Available online: https://www.mhlw.go.jp/english/database/db-hw/orvf/SummaryReport2020.html (accessed on 1 June 2024).
- Doundoulakis, I.; Farmakis, I.T.; Theodoridis, X.; Konstantelos, A.; Christoglou, M.; Kotzakioulafi, E.; Chrysoula, L.; Siargkas, A.; Karligkiotis, A.; Kyprianou, G.; et al. Effects of Dietary Interventions on Cardiovascular Outcomes: A Network Meta-Analysis. Nutr. Rev. 2024, 82, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.J.; Kromhout, D. Atherosclerosis-Epidemiological Studies on the Health Effects of a Mediterranean Diet. Eur. J. Nutr. 2004, 43, i2–i5. [Google Scholar] [CrossRef]
- Isaakidis, A.; Maghariki, J.E.l.; Carvalho-Barros, S.; Gomes, A.M.; Correia, M. Is There More to Olive Oil than Healthy Lipids? Nutrients 2023, 15, 3625. [Google Scholar] [CrossRef] [PubMed]
- Flynn, M.M.; Tierney, A.; Itsiopoulos, C. Is Extra Virgin Olive Oil the Critical Ingredient Driving the Health Benefits of a Mediterranean Diet? A Narrative Review. Nutrients 2023, 15, 2916. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive Oil Intake and Risk of Cardiovascular Disease and Mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sayón-Orea, C.; Bullón-Vela, V.; Bes-Rastrollo, M.; Rodríguez-Artalejo, F.; Yusta-Boyo, M.J.; García-Solano, M. Effect of Olive Oil Consumption on Cardiovascular Disease, Cancer, Type 2 Diabetes, and All-Cause Mortality: A Systematic Review and Meta-Analysis. Clin. Nutr. 2022, 41, 2659–2682. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma Oxidized Low-Density Lipoprotein, a Strong Predictor for Acute Coronary Heart Disease Events in Apparently Healthy, Middle-Aged Men from the General Population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.-C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The Effect of High-Polyphenol Extra Virgin Olive Oil on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef]
- Fitó, M.; Covas, M.I.; Lamuela-Raventós, R.M.; Vila, J.; Torrents, L.; de la Torre, C.; Marrugat, J. Protective Effect of Olive Oil and Its Phenolic Compounds against Low Density Lipoprotein Oxidation. Lipids 2000, 35, 633–638. [Google Scholar] [CrossRef]
- Derakhshandeh-Rishehri, S.-M.; Kazemi, A.; Shim, S.R.; Lotfi, M.; Mohabati, S.; Nouri, M.; Faghih, S. Effect of Olive Oil Phenols on Oxidative Stress Biomarkers: A Systematic Review and Dose-Response Meta-Analysis of Randomized Clinical Trials. Food Sci. Nutr. 2023, 11, 2393–2402. [Google Scholar] [CrossRef]
- Corona, G.; Tzounis, X.; Assunta Dessì, M.; Deiana, M.; Debnam, E.S.; Visioli, F.; Spencer, J.P.E. The Fate of Olive Oil Polyphenols in the Gastrointestinal Tract: Implications of Gastric and Colonic Microflora-Dependent Biotransformation. Free Radic. Res. 2006, 40, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and Sensory Properties of Virgin Olive Oil Hydrophilic Phenols: Agronomic and Technological Aspects of Production That Affect Their Occurrence in the Oil. J. Chromatogr. A. 2004, 1054, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic Compounds and Squalene in Olive Oils: The Concentration and Antioxidant Potential of Total Phenols, Simple Phenols, Secoiridoids, Lignansand Squalene. Food Chem. Toxicol. 2000, 38, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.-I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.-J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The Effect of Polyphenols in Olive Oil on Heart Disease Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- Marrugat, J.; Covas, M.-I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; López-Sabater, M.C.; de la Torre, R.; Farré, M. Effects of Differing Phenolic Content in Dietary Olive Oils on Lipids LDLOxidation—A Randomized Controlled Trial. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Renzo, L.; Romeo, F. Effects of Postprandial Hydroxytyrosol Derivates on Oxidation of, L.D.L.; Cardiometabolic State Gene Expression: ANutrigenomic Approach for Cardiovascular Prevention. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef]
- Capogna, D.; Gómez, M.I. Olive Oil: An Overview of the Japanese Market. OCL—Oilseeds fats, Crop. Lipids 2016, 23, D608. [Google Scholar] [CrossRef]
- International Olive Council, Olive Oil Imports in Japon. Available online: https://www.internationaloliveoil.org/olive-oil-imports-in-japon/ (accessed on 1 June 2024).
- Mori, N.; Sawada, N.; Ishihara, J.; Kotemori, A.; Takachi, R.; Murai, U.; Kobori, M.; Tsugane, S. Validity of a Food Frequency Questionnaire for the Estimation of Total Polyphenol Intake Estimates Its Major Food Sources in the Japanese Population: The JPHCFFQValidation Study. J. Nutr. Sci. 2021, 10, e35. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary Polyphenol Intake in Europe: The European Prospective Investigation into Cancer Nutrition (EPIC) Study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Suzuki-Sugihara, N.; Saita, E.; Takahashi, Y.; Kondo, K. Estimated Dietary Polyphenol Intake and Major Food and Beverage Sources among Elderly Japanese. Nutrients 2015, 7, 10269–10281. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Tashiro, T.; Kumagai, A.; Ohyanagi, H.; Horiuchi, T.; Takizawa, K.; Sugihara, N.; Kishimoto, Y.; Taguchi, C.; Tani, M.; et al. Coffee Beverages Are the Major Contributors to Polyphenol Consumption from Food Beverages in Japanese Middle-Aged Women. J. Nutr. Sci. 2014, 3, e48. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, C.; Kishimoto, Y.; Takeuchi, I.; Tanaka, M.; Iwashima, T.; Fukushima, Y.; Kondo, K. Estimated Dietary Polyphenol Intake Its Seasonal Variations among Japanese University Students. J. Nutr. Sci. Vitaminol. 2019, 65, 192–195. [Google Scholar] [CrossRef]
- Taguchi, C.; Kishimoto, Y.; Kondo, K.; Tohyama, K.; Goda, T. Serum Gamma-Glutamyltransferase Is Inversely Associated with Dietary Total and Coffee-Derived Polyphenol Intakes in Apparently Healthy Japanese Men. Eur. J. Nutr. 2018, 57, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total Specific Polyphenol Intakes in Midlife Are Associated with Cognitive Function Measured 13 Years Later. J. Nutr. 2012, 142, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remón, A.; Pérez-Jiménez, J.; Martínez-González, M.A.; Covas, M.I.; Corella, D.; Salas-Salvadó, J.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Dietary Intake and Major Food Sources of Polyphenols in a Spanish Population at High Cardiovascular Risk: The PREDIMED Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef]
- Linseisen, J.; Welch, A.A.; Ocké, M.; Amiano, P.; Agnoli, C.; Ferrari, P.; Sonestedt, E.; Chajès, V.; Bueno-de-Mesquita, H.B.; Kaaks, R.; et al. Dietary Fat Intake in the European Prospective Investigation into Cancer and Nutrition: Results from the 24-h Dietary Recalls. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S61–S80. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare, Japan. The National Health and Nutrition Survey in Japan, 2019. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/download_files/eiyouchousa/2019.pdf (accessed on 1 June 2024).
- Tyrrell, D.J.; Goldstein, D.R. Ageing and Atherosclerosis: Vascular Intrinsic and Extrinsic Factors and Potential Role of IL-6. Nat. Rev. Cardiol. 2021, 18, 58–68. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare, Japan. Ethical Guidelines for Medical and Biological Research Involving Human Subjects (Only Japanese Text Available). 2013. Available online: https://www.mhlw.go.jp/content/001077424.pdf (accessed on 1 June 2024).
- Ministry of Justice, Japan. Act on the Protection of Personal Information (Only Japanese Text Available). 2003. Available online: https://laws.e-gov.go.jp/law/415AC0000000057 (accessed on 1 June 2024).
- UMIN-CTR URL. Available online: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000056328 (accessed on 1 June 2024).
- Badeau, M.; Adlercreutz, H.; Kaihovaara, P.; Tikkanen, M.J. Estrogen A-Ring Structure and Antioxidative Effect on Lipoproteins. J. Steroid Biochem. Mol. Biol. 2005, 96, 271–278. [Google Scholar] [CrossRef]
- International Olive Council. Method of Analysis, Determination of Biophenols in Olive Oils by HPLC. 2017, 1–8. COI/T.20/Doc. No 29/Rev.1. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/10/COI-T20-Doc.-29-REV-1-2017-Eng.pdf (accessed on 1 March 2024).
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. Jpn. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Fukushima, Y.; Ohie, T.; Yonekawa, Y.; Yonemoto, K.; Aizawa, H.; Mori, Y.; Watanabe, M.; Takeuchi, M.; Hasegawa, M.; Taguchi, C.; et al. Coffee Green Tea as a Large Source of Antioxidant Polyphenols in the Japanese Population. J. Agric. Food Chem. 2009, 57, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Zech, L.A.; Gregg, R.E.; Schaefer, E.J.; Hoeg, J.M.; Sprecher, D.L.; Brewer, H.B.J. Estimation of VLDL Cholesterol in Hyperlipidemia. Clin. Chim. Acta. 1985, 151, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Squillaro, T.; Galderisi, U.; Peluso, G. Polyphenols, the Healthy Brand of Olive Oil: Insights and Perspectives. Nutrients 2021, 13, 3831. [Google Scholar] [CrossRef] [PubMed]
- Pedret, A.; Catalán, Ú.; Fernández-Castillejo, S.; Farràs, M.; Valls, R.-M.; Rubió, L.; Canela, N.; Aragonés, G.; Romeu, M.; Castañer, O.; et al. Impact of Virgin Olive Oil and Phenol-Enriched Virgin Olive Oils on the HDL Proteome in Hypercholesterolemic Subjects: A Double Blind, Randomized, Controlled, Cross-Over Clinical Trial (VOHF Study). PLoS ONE 2015, 10, e0129160. [Google Scholar] [CrossRef] [PubMed]
- Biel, S.; Mesa, M.-D.; de la Torre, R.; Espejo, J.-A.; Fernández-Navarro, J.-R.; Fitó, M.; Sánchez-Rodriguez, E.; Rosa, C.; Marchal, R.; Alche, J.D.D.; et al. The NUTRAOLEOUM Study, a Randomized Controlled Trial, for Achieving Nutritional Added Value for Olive Oils. BMC Complement Altern. Med. 2016, 16, 404. [Google Scholar] [CrossRef] [PubMed]
- EFSA. EFSA Panel on Dietetic Products, Nutrition and allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage. EFSA J. 2011, 10, 2848. [Google Scholar]
- UE. European Commission Regulation EC No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health; Official Journal of the European Union: Brussels, Belgium, 2012. [Google Scholar]
- Ministry of Health, Labour and Welfare, Japan. Dietary Reference Intakes for Japanese (2020) (Only Japanese Text Available). Available online: https://www.mhlw.go.jp/content/10904750/000586553.pdf (accessed on 1 June 2024).
- Japan Atherosclerosis Society. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022. (Only Japanese Text Available). Available online: https://www.j-athero.org/jp/wp-content/uploads/publications/pdf/GL2022_s/jas_gl2022_3_230210.pdf (accessed on 1 June 2024).
- Taguchi, C.; Fukushima, Y.; Kishimoto, Y.; Saita, E.; Suzuki-Sugihara, N.; Yoshida, D.; Kondo, K. Polyphenol Intake from Beverages in Japan over an 18-Year Period (1996–2013): Trends by Year, Age, Gender and Season. J. Nutr. Sci. Vitaminol. 2015, 61, 338–344. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive Oil Phenolics Are Dose-Dependently Absorbed in Humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Gimeno, E.; de la Torre-Carbot, K.; Lamuela-Raventós, R.M.; Castellote, A.I.; Fitó, M.; de la Torre, R.; Covas, M.-I.; López-Sabater, M.C. Changes in the Phenolic Content of Low Density Lipoprotein after Olive Oil Consumption in Men ARandomized Crossover Controlled Trial. Br. J. Nutr. 2007, 98, 1243–1250. [Google Scholar] [CrossRef]
- Covas, M.-I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL Phenolic Content and LDL Oxidation Are Modulated by Olive Oil Phenolic Compounds in Humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in Vivo Fate of Hydroxytyrosol and Tyrosol, Antioxidant Phenolic Constituents of Olive Oil, after Intravenous and Oral Dosing of Labeled Compounds to Rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics Bioavailability of Hydroxytyrosol Are Dependent on the Food Matrix in Humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, R.; Varì, R.; Scazzocchio, B.; Filesi, C.; Santangelo, C.; Giovannini, C.; Matarrese, P.; D’Archivio, M.; Masella, R. Tyrosol, the Major Extra Virgin Olive Oil Compound, Restored Intracellular Antioxidant Defences in Spite of Its Weak Antioxidative Effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, S.J.; Bast, A.; Haenen, G.R.M.M. New Insights into Controversies on the Antioxidant Potential of the Olive Oil Antioxidant Hydroxytyrosol. J. Agric. Food Chem. 2007, 55, 7609–7614. [Google Scholar] [CrossRef]
- Chen, X.-L.; Kunsch, C. Induction of Cytoprotective Genes through Nrf2/Antioxidant Response Element Pathway: A New Therapeutic Approach for the Treatment of Inflammatory Diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by Olive Oil and Wine Polyphenols and Neuroprotection. Antioxidants 2017, 6, 73. [Google Scholar] [CrossRef]
- de Graaf, J.; Hak-Lemmers, H.L.; Hectors, M.P.; Demacker, P.N.; Hendriks, J.C.; Stalenhoef, A.F. Enhanced Susceptibility to in Vitro Oxidation of the Dense Low Density Lipoprotein Subfraction in Healthy Subjects. Arterioscler. Thromb. J. Vasc. Biol. 1991, 11, 298–306. [Google Scholar] [CrossRef]
- Chancharme, L.; Thérond, P.; Nigon, F.; Lepage, S.; Couturier, M.; Chapman, M.J. Cholesteryl Ester Hydroperoxide Lability Is a Key Feature of the Oxidative Susceptibility of Small, Dense LDL. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 810–820. [Google Scholar] [CrossRef][Green Version]
- Hirayama, S.; Miida, T. Small Dense LDL: An Emerging Risk Factor for Cardiovascular Disease. Clin. Chim. Acta. 2012, 414, 215–224. [Google Scholar] [CrossRef]
- Chary, A.; Tohidi, M.; Hedayati, M. Association of LDL-Cholesterol Subfractions with Cardiovascular Disorders: A Systematic Review. BMC Cardiovasc. Disord. 2023, 23, 533. [Google Scholar] [CrossRef] [PubMed]
- Griffin, B.A.; Freeman, D.J.; Tait, G.W.; Thomson, J.; Caslake, M.J.; Packard, C.J.; Shepherd, J. Role of Plasma Triglyceride in the Regulation of Plasma Low Density Lipoprotein (LDL) Subfractions: Relative Contribution of Small, Dense LDL to Coronary Heart Disease Risk. Atherosclerosis 1994, 106, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Couillard, C.; Pascot, A.; Bergeron, N.; Prud’homme, D.; Bergeron, J.; Tremblay, A.; Bouchard, C.; Mauriège, P.; Després, J.P. The Small, Dense LDL Phenotype as a Correlate of Postprandial Lipemia in Men. Atherosclerosis 2000, 153, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Bronze, M.R.; Figueira, M.E.; Siwy, J.; Mischak, H.; Combet, E.; Mullen, W. Impact of a 6-Wk Olive Oil Supplementation in Healthy Adults on Urinary Proteomic Biomarkers of Coronary Artery Disease, Chronic Kidney Disease, and Diabetes (Types 1 and 2): A Randomized, Parallel, Controlled, Double-Blind Study. Am. J. Clin. Nutr. 2015, 101, 44–54. [Google Scholar] [CrossRef]
- Takahashi, T.; Kato, S.; Ito, J.; Shimizu, N.; Parida, I.S.; Itaya-Takahashi, M.; Sakaino, M.; Imagi, J.; Yoshinaga, K.; Yoshinaga-Kiriake, A.; et al. Dietary Triacylglycerol Hydroperoxide Is Not Absorbed, yet It Induces the Formation of Other Triacylglycerol Hydroperoxides in the Gastrointestinal Tract. Redox Biol. 2022, 57, 102471. [Google Scholar] [CrossRef]
| Characteristics | ||
|---|---|---|
| Age | years | 50.4 ± 7.7 |
| BMI | kg/m2 | 24.1 ± 2.1 |
| TG | mg/dL | 92.7 ± 41.8 |
| LDL-C | mg/dL | 126.2 ± 9.4 |
| HDL-C | mg/dL | 60.0 ± 13.4 |
| MDA-LDL | U/L | 113.6 ± 31.9 |
| Control | Test | ||
|---|---|---|---|
| Energy | kcal | 1758 ± 496 | 1754 ± 498 |
| Carbohydrate | g | 218.8 ± 72.5 | 219.7 ± 72.1 |
| Protein | g | 61.5 ± 22.2 | 61.0 ± 22.3 |
| Fat | g | 64.7 ± 20.0 | 64.0 ± 20.1 |
| Saturated fatty acid | g | 14.9 ± 5.7 | 14.8 ± 5.7 |
| Monounsaturated fatty acid | g | 29.4 ± 7.6 | 29.2 ± 7.7 |
| Polyunsaturated fatty acid | g | 14.1 ± 4.8 | 13.8 ± 4.9 |
| Vitamin E | mg | 9.9 ± 2.6 | 9.8 ± 2.6 |
| β-Carotene | mg | 2.1 ± 1.5 | 2.0 ± 1.5 |
| Total polyphenol | mg | 777 ± 461 | 783 ± 420 |
| 35–50 Years (n = 38) | 51–64 Years (n = 39) | ||||
|---|---|---|---|---|---|
| Control | Test | Control | Test | ||
| Energy | kcal | 1647 ± 357 | 1637 ± 375 | 1866 ± 587 | 1869 ± 575 |
| Carbohydrate | g | 203.0 ± 51.4 | 204.9 ± 52.5 | 234.2 ± 86.3 | 234.2 ± 85.4 |
| Protein | g | 57.7 ± 20.8 | 57.3 ± 23.3 | 65.2 ± 23.1 | 64.5 ± 21.0 |
| Fat | g | 61.0 ± 17.3 | 59.5 ± 18.5 | 68.3 ± 21.9 | 68.5 ± 20.8 |
| Saturated fatty acid | g | 14.3 ± 5.0 | 14.2 ± 5.1 | 15.5 ± 6.4 | 15.4 ± 6.2 |
| Monounsaturated fatty acid | g | 28.0 ± 6.8 | 27.4 ± 7.4 | 30.7 ± 8.1 | 30.9 ± 7.6 |
| Polyunsaturated fatty acid | g | 12.9 ± 4.1 | 12.3 ± 4.2 | 15.2 ± 5.2 | 15.3 ± 5.0 |
| Vitamin E | mg | 9.1 ± 2.0 | 8.9 ± 2.0 | 10.6 ± 2.9 | 10.7 ± 2.9 |
| β-Carotene | mg | 1.8 ± 1.5 | 1.8 ± 1.6 | 2.3 ± 1.5 | 2.2 ± 1.5 |
| Total polyphenol | mg | 600 ± 323 | 619 ± 330 | 950 ± 511 * | 942 ± 441 * |
| Outcome | Control | Test | p Value | |
|---|---|---|---|---|
| MDA-LDL | U/L | 96.1 ± 3.2 | 99.7 ± 3.6 | 0.55 |
| sd-LDL | mg/dL | 33.3 ± 1.5 | 32.9 ± 1.6 | 0.55 |
| PAO | µmol/L | 1278.3 ± 20.1 | 1273.8 ± 21.7 | 0.66 |
| TG | mg/dL | 101.3 ± 7.1 | 97.8 ± 5.7 † | 0.07 |
| LDL-C | mg/dL | 128.0 ± 2.2 | 127.2 ± 2.2 | 0.45 |
| HDL-C | mg/dL | 59.6 ± 1.5 | 60.1 ± 1.5 | 0.29 |
| Outcome | Control | Test | p Value | ||
|---|---|---|---|---|---|
| MDA-LDL | U/L | Point 1 Point 2 | −2.6 ± 3.0 −2.4 ± 3.1 | −7.1 ± 3.2 −6.1 ± 2.9 | 0.88 0.053 |
| sd-LDL | mg/dL | Point 1 Point 2 | −2.4 ± 0.6 −1.8 ± 0.7 | −1.0 ± 0.8 −0.2 ± 0.8 | 0.10 0.10 |
| PAO | µmol/L | Point 1 Point 2 | 41.3 ± 11.3 26.6 ± 11.8 | 43.8 ± 13.6 31.9 ± 15.3 | 0.66 0.30 |
| TG | mg/dL | Point 1 Point 2 | −10.6 ± 5.0 15.4 ± 5.0 | −1.2 ± 4.5 22.0 ± 4.5 | 0.37 0.07 |
| LDL-C | mg/dL | Point 1 Point 2 | −2.1 ± 1.8 −3.4 ± 1.7 | −2.0 ± 1.5 −2.8 ± 1.6 | 0.46 0.66 |
| HDL-C | mg/dL | Point 1 Point 2 | 0.1 ± 0.7 −0.2 ± 0.7 | 0.4 ± 0.6 0.2 ± 0.6 | 0.37 0.76 |
| Outcome | Control | Test | p Value | ||
|---|---|---|---|---|---|
| MDA-LDL | U/L | Point 1 Point 2 | −3.7 ± 4.0 −5.0 ± 4.3 | −8.6 ± 3.8 −8.8 ± 3.7 * | 0.21 0.01 |
| sd-LDL | mg/dL | Point 1 Point 2 | −3.2 ± 0.9 −2.7 ± 1.0 | 1.0 ± 1.1 * 1.5 ± 1.1 * | 0.001 0.003 |
| PAO | µmol/L | Point 1 Point 2 | 35.3 ± 16.4 28.0 ± 17.3 | 68.5 ± 18.1 † 60.3 ± 20.3 † | 0.03 0.03 |
| TG | mg/dL | Point 1 Point 2 | −21.7 ± 8.8 3.5 ± 8.2 | 4.9 ± 4.8 † 29.7 ± 5.3 * | 0.03 0.01 |
| LDL-C | mg/dL | Point 1 Point 2 | −0.7 ± 2.9 −2.0 ± 2.8 | −1.0 ± 2.3 −2.1 ± 2.3 | 0.40 0.68 |
| HDL-C | mg/dL | Point 1 Point 2 | 0.8 ± 0.8 0.3 ± 0.8 | 0.7 ± 0.9 0.3 ± 0.9 | 1 0.83 |
| Outcome | Control | Test | p Value | ||
|---|---|---|---|---|---|
| MDA-LDL | U/L | Point 1 Point 2 | −1.6 ± 4.4 0.1 ± 4.5 | −5.5 ± 5.3 −3.5 ± 4.4 | 0.17 0.84 |
| sd-LDL | mg/dL | Point 1 Point 2 | −1.7 ± 0.8 −0.9 ± 0.9 | −2.9 ± 1.1 −1.9 ± 1.1 | 0.30 0.54 |
| PAO | µmol/L | Point 1 Point 2 | 46.8 ± 15.6 25.2 ± 16.4 | 19.8 ± 19.8 4.3 ± 22.1 | 0.15 0.54 |
| TG | mg/dL | Point 1 Point 2 | 0.2 ± 4.2 26.9 ± 5.3 | −7.2 ± 7.4 14.4 ± 7.2 | 0.41 0.84 |
| LDL-C | mg/dL | Point 1 Point 2 | −3.4 ± 2.0 −4.8 ± 2.1 | −3.0 ± 2.0 −3.4 ± 2.1 | 0.84 0.84 |
| HDL-C | mg/dL | Point 1 Point 2 | −0.6 ± 1.1 −0.6 ± 1.1 | 0.1 ± 1.0 0.2 ± 1.0 | 0.21 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujino, S.; Sadamitsu, S.; Nosaka, N.; Fushimi, T.; Kishimoto, Y.; Kondo, K. Age-Related Effects of Olive Oil Polyphenol Ingestion on Oxidation of Low-Density Lipoprotein in Healthy Japanese Men: A Randomized Controlled Double-Blind Crossover Trial. Nutrients 2024, 16, 3342. https://doi.org/10.3390/nu16193342
Tsujino S, Sadamitsu S, Nosaka N, Fushimi T, Kishimoto Y, Kondo K. Age-Related Effects of Olive Oil Polyphenol Ingestion on Oxidation of Low-Density Lipoprotein in Healthy Japanese Men: A Randomized Controlled Double-Blind Crossover Trial. Nutrients. 2024; 16(19):3342. https://doi.org/10.3390/nu16193342
Chicago/Turabian StyleTsujino, Shogo, Shohei Sadamitsu, Naohisa Nosaka, Tatsuya Fushimi, Yoshimi Kishimoto, and Kazuo Kondo. 2024. "Age-Related Effects of Olive Oil Polyphenol Ingestion on Oxidation of Low-Density Lipoprotein in Healthy Japanese Men: A Randomized Controlled Double-Blind Crossover Trial" Nutrients 16, no. 19: 3342. https://doi.org/10.3390/nu16193342
APA StyleTsujino, S., Sadamitsu, S., Nosaka, N., Fushimi, T., Kishimoto, Y., & Kondo, K. (2024). Age-Related Effects of Olive Oil Polyphenol Ingestion on Oxidation of Low-Density Lipoprotein in Healthy Japanese Men: A Randomized Controlled Double-Blind Crossover Trial. Nutrients, 16(19), 3342. https://doi.org/10.3390/nu16193342






