The Epidemiology and Clinical Management of Short Bowel Syndrome and Chronic Intestinal Failure in Crohn’s Disease in Italy: An IG-IBD Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Structure
2.2. Survey Distribution
2.3. Statistical Analysis
3. Results
3.1. Participants
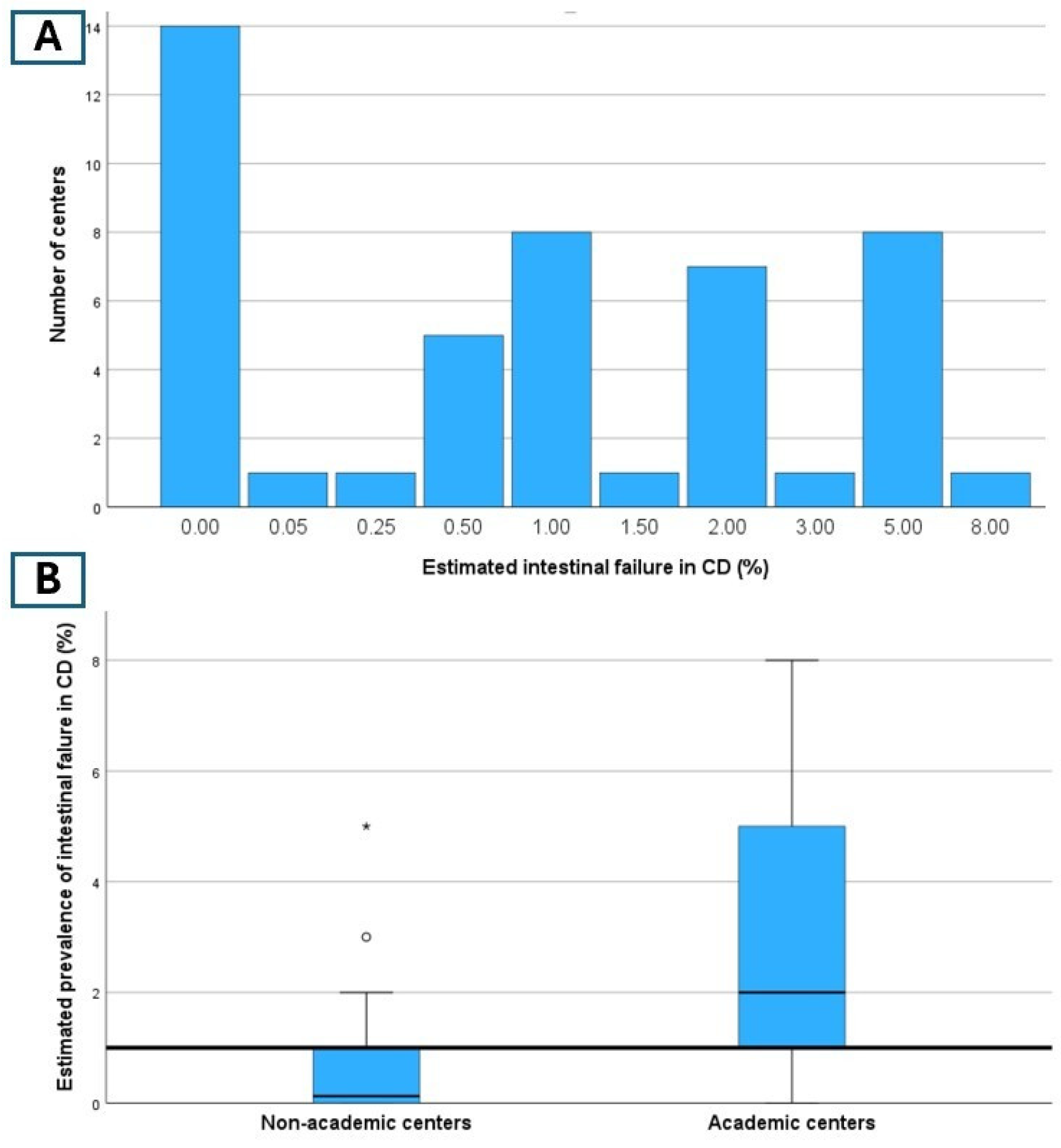
3.2. Epidemiology
3.3. Diagnostic Assessment
3.4. Clinical Management
3.5. Teduglutide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pironi, L.; Allard, J.P.; Joly, F.; Geransar, P.; Genestin, E.; Pape, U. Use of teduglutide in adults with short bowel syndrome-associated intestinal failure. Nutr. Clin. Pract. 2024, 39, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Cuerda, C.; Jeppesen, P.B.; Joly, F.; Jonkers, C.; Krznarić, Ž.; Lal, S.; Lamprecht, G.; Lichota, M.; Mundi, M.S.; et al. ESPEN guideline on chronic intestinal failure in adults–Update 2023. Clin. Nutr. 2023, 42, 1940–2021. [Google Scholar] [PubMed]
- Aksan, A.; Farrag, K.; Blumenstein, I.; Schröder, O.; Dignass, A.U.; Stein, J. Chronic intestinal failure and short bowel syndrome in Crohn’s disease. World J. Gastroenterol. 2021, 27, 3440–3465. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L.; Konrad, D.; Brandt, C.; Joly, F.; Wanten, G.; Agostini, F.; Chambrier, C.; Aimasso, U.; Zeraschi, S.; Kelly, D.; et al. Clinical classification of adult patients with chronic intestinal failure due to benign disease: An international multicenter cross-sectional survey. Clin. Nutr. 2018, 37, 728–738. [Google Scholar] [CrossRef]
- Watanabe, Y.; Miyoshi, N.; Fujino, S.; Takahashi, H.; Haraguchi, N.; Hata, T.; Matsuda, C.; Yamamoto, H.; Doki, Y.; Mori, M.; et al. Cumulative Inflammation Could Be a Risk Factor for Intestinal Failure in Crohn’s Disease. Dig. Dis. Sci. 2019, 64, 2280–2285. [Google Scholar] [CrossRef]
- Watanabe, K.; Sasaki, I.; Fukushima, K.; Futami, K.; Ikeuchi, H.; Sugita, A.; Nezu, R.; Mizushima, T.; Kameoka, S.; Kusunoki, M.; et al. Long-term incidence and characteristics of intestinal failure in Crohn’s disease: A multicenter study. J. Gastroenterol. 2014, 49, 231–238. [Google Scholar] [CrossRef]
- Pironi, L.; Boeykens, K.; Bozzetti, F.; Joly, F.; Klek, S.; Lal, S.; Lichota, M.; Mühlebach, S.; Van Gossum, A.; Wanten, G.; et al. ESPEN guideline on home parenteral nutrition. Clin. Nutr. 2020, 39, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.F.; Smith, C.E. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. J. Parenter. Enter. Nutr. 2014, 38 (Suppl. S1), 32S–37S. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’KEefe, S.J.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012, 143, 1473–1481.e3. [Google Scholar] [CrossRef]
- Seidner, D.L.; Fujioka, K.; Boullata, J.I.; Iyer, K.; Lee, H.; Ziegler, T.R. Reduction of Parenteral Nutrition and Hydration Support and Safety With Long-Term Teduglutide Treatment in Patients With Short Bowel Syndrome-Associated Intestinal Failure: STEPS-3 Study. Nutr. Clin. Pract. 2018, 33, 520–527. [Google Scholar] [CrossRef]
- Schwartz, L.K.; O’keefe, S.J.; Fujioka, K.; Gabe, S.M.; Lamprecht, G.; Pape, U.F.; Li, B.; Youssef, N.N.; Jeppesen, P.B. Long-Term Teduglutide for the Treatment of Patients With Intestinal Failure Associated With Short Bowel Syndrome. Clin. Transl. Gastroenterol. 2016, 7, e142. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Duc, N.T.M.; Thang, T.L.L.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. A Consensus-Based Checklist for Reporting of Survey Studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding short bowel syndrome: Current status and future perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Seguy, D.; Nuzzo, A.; Chambrier, C.; Beau, P.; Poullenot, F.; Thibault, R.; Debeir, L.A.; Layec, S.; Boehm, V.; et al. Six-month outcomes of teduglutide treatment in adult patients with short bowel syndrome with chronic intestinal failure: A real-world French observational cohort study. Clin. Nutr. 2020, 39, 2856–2862. [Google Scholar] [CrossRef]
- Borghini, R.; Villanacci, V.; Oberti, A.; Caronna, R.; Trecca, A. Long-term effects of Teduglutide on intestinal Mucosa in a patient with Crohn’s disease and short bowel syndrome: Clinical, endoscopic and histological data compared. Inflamm. Bowel Dis. 2021, 27, E152–E153. [Google Scholar] [CrossRef]
- Raghu, V.K.; Binion, D.G.; Smith, K.J. Cost-effectiveness of teduglutide in adult patients with short bowel syndrome: Markov modeling using traditional cost-effectiveness criteria. Am. J. Clin. Nutr. 2020, 111, 141–148. [Google Scholar] [CrossRef]
- Arhip, L.; Serrano-Moreno, C.; Romero, I.; Camblor, M.; Cuerda, C. The economic costs of home parenteral nutrition: Systematic review of partial and full economic evaluations. Clin. Nutr. 2021, 40, 339–349. [Google Scholar] [CrossRef]
- Pironi, L. Definition, classification, and causes of short bowel syndrome. Nutr. Clin. Pract. 2023, 38 (Suppl. S1), S9–S16. [Google Scholar] [CrossRef]
- Lam, K.; Schwartz, L.; Batisti, J.; Iyer, K.R. Single-center experience with the use of teduglutide in adult patients with short bowel syndrome. J. Parenter. Enter. Nutr. 2018, 42, 225–230. [Google Scholar] [CrossRef]
- Pevny, S.; Maasberg, S.; Rieger, A.; Karber, M.; Blüthner, E.; Knappe-Drzikova, B.; Thurmann, D.; Büttner, J.; Weylandt, K.-H.; Wiedenmann, B.; et al. Experience with teduglutide treatment for short bowel syndrome in clinical practice. Clin. Nutr. 2019, 38, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Puello, F.; Wall, E.; Herlitz, J.; Lozano, E.S.; Semrad, C.; Micic, D. Long-term outcomes with teduglutide from a single center. J. Parenter. Enter. Nutr. 2021, 45, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Ukleja, A.; To, C.; Alvarez, A.; Lara, L.F. Long-term therapy with teduglutide in parenteral support-dependent patients with short bowel syndrome: A case series. J. Parenter. Enter. Nutr. 2018, 42, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Long, M.D.; Shelton, E.; Young, L.M.; Farraye, F.A.; Yajnik, V.; Herfarth, H. Safety and efficacy of teduglutide (Gattex) in patients with Crohn’s disease and need for parenteral support due to short bowel syndrome-associated intestinal failure. J. Clin. Gastroenterol. 2017, 51, 508–511. [Google Scholar] [CrossRef]
- Compher, C.; Boullata, J.I.; Pickett-Blakely, O.; Schiavone, P.; Stoner, N.; Kinosian, B.P. Clinical management of patients with parenteral nutrition-dependent short bowel syndrome during teduglutide therapy. J. Parenter. Enter. Nutr. 2016, 40, 1183–1190. [Google Scholar] [CrossRef]
- Dibb, M.; Soop, M.; Teubner, A.; Shaffer, J.; Abraham, A.; Carlson, G.; Lal, S. Survival and nutritional dependence on home parenteral nutrition: Three decades of experience from a single referral centre. Clin. Nutr. 2017, 36, 570–576. [Google Scholar] [CrossRef]
- Walter, E.; Dawoud, C.; Hütterer, E.; Stift, A.; Harpain, F. Cost-effectiveness of teduglutide in adult patients with short bowel syndrome—A European socioeconomic perspective. Am. J. Clin. Nutr. 2024, 119, 1187–1199. [Google Scholar] [CrossRef]
- Fragkos, K.C.; Forbes, A. Citrulline as a marker of intestinal function and absorption in clinical settings: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 181–191. [Google Scholar] [CrossRef]
- Kannen, V.; Garcia, S.B.; Stopper, H.; Waaga-Gasser, A.M. Glucagon-like peptide 2 in colon carcinogenesis: Possible target for anti-cancer therapy? Pharmacol. Ther. 2013, 139, 87–94. [Google Scholar] [CrossRef]
- Greif, S.; Maasberg, S.; Wehkamp, J.; Fusco, S.; Zopf, Y.; Herrmann, H.J.; Lamprecht, G.; Jacob, T.; Schiefke, I.; von Websky, M.W.; et al. Long-term results of teduglutide treatment for chronic intestinal failure—Insights from a national, multi-centric patient home-care service program. Clin. Nutr. ESPEN 2022, 51, 222–230. [Google Scholar] [CrossRef]
- Fascì-Spurio, F.; Meucci, G.; Papi, C.; Saibeni, S. The use of oral corticosteroids in inflammatory bowel diseases in Italy: An IG-IBD survey. Dig. Liver Dis. 2017, 49, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
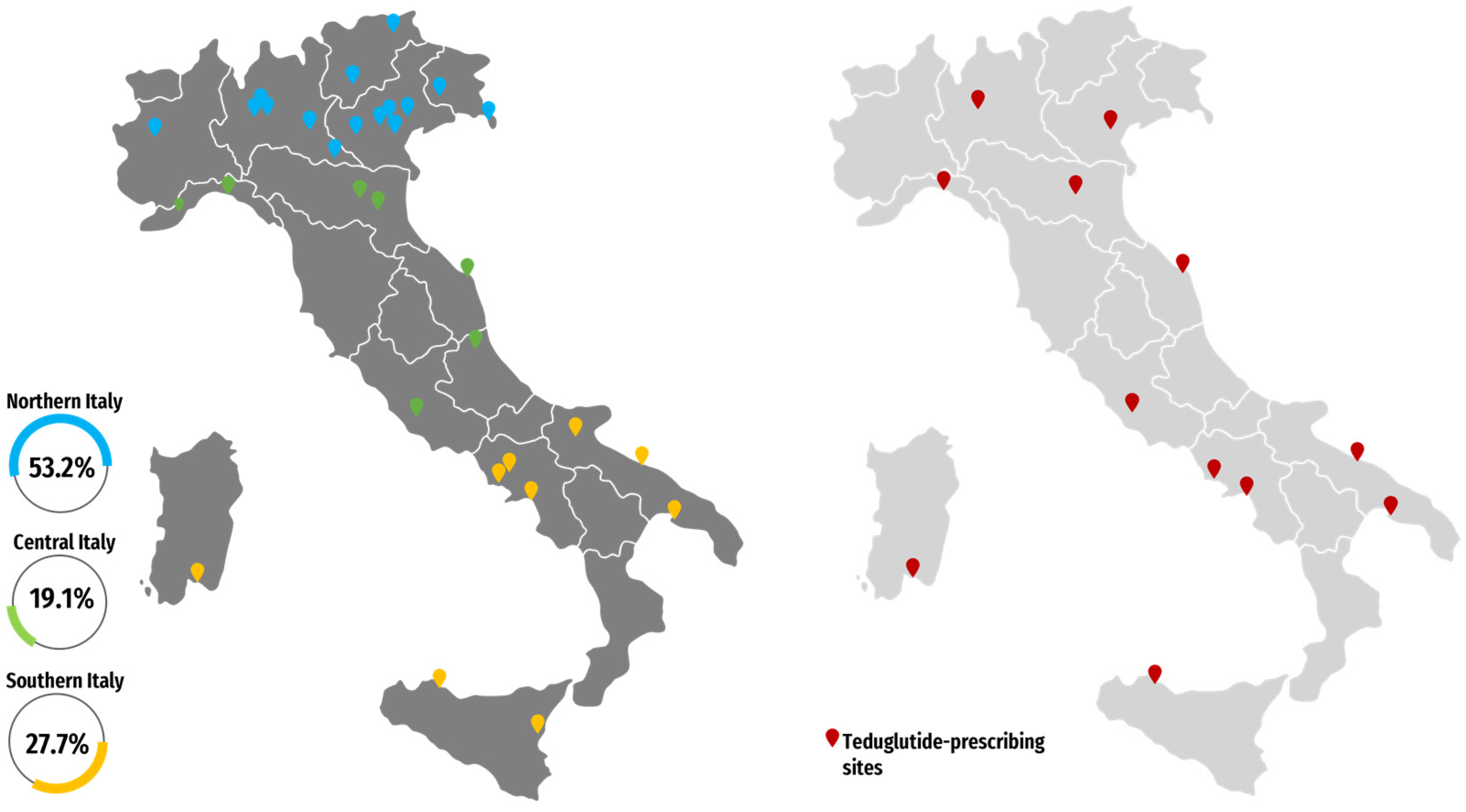


| Centers Included in the Analysis (n) | 47 |
|---|---|
| Academic centers, n (%) | 23 (48.9) |
| Geographic distribution, n (%) | |
| Northern Italy | 25 (53.2) |
| Central Italy | 9 (19.1) |
| Southern Italy | 13 (27.7) |
| Teduglutide prescribers, n (%) | 15 (31.9) |
| Northern Italy | 4 (26.7) |
| Central Italy | 5 (33.3) |
| Southern Italy | 6 (40.0) |
| Patient volume/center: Crohn’s disease with small bowel involvement, n (%) | |
| Less than 50 | 8 (17.0) |
| Between 50 and 200 | 16 (34.0) |
| Between 200 and 400 | 4 (8.5) |
| More than 400 | 19 (40.4) |
| Proportion of Crohn’s disease patients who underwent ≥1 intestinal resection/center, n (%) | |
| Between 0 and 5% | 6 (12.8) |
| Between 5 and 15% | 17 (36.2) |
| Between 15 and 30% | 13 (27.7) |
| More than 30% | 11 (23.4) |
| Question | Total n = 47 | Academic n = 23 | Non-Academic n = 24 | p | TED n = 15 | Non-TED n = 32 | p | >400 CD with SB Involvement n = 19 | <400 CD with SB Involvement n = 28 | p |
|---|---|---|---|---|---|---|---|---|---|---|
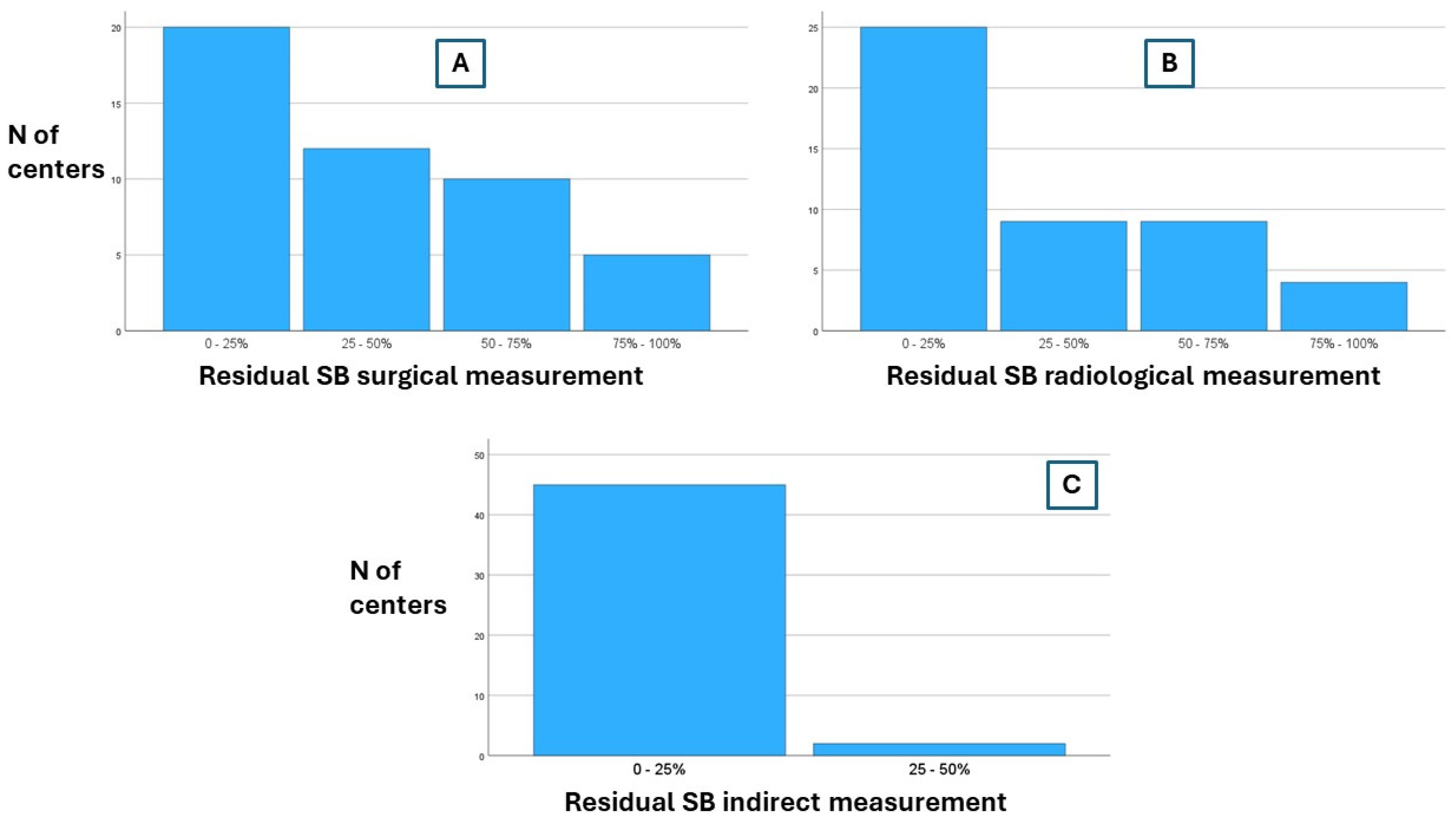
| Surgical measurement | 0.088 * | 1.000 * | 0.751 * | |||||||
| 0–25% | 20 (42.6) | 6 (26.1) | 14 (58.3) | 5 (33.3) | 15 (46.9) | 8 (42.1) | 12 (42.9) | |||
| 25–50% | 12 (25.5) | 7 (30.4) | 5 (20.8) | 5 (33.3) | 7 (21.9) | 4 (21.0) | 8 (28.6) | |||
| 50–75% | 10 (21.3) | 7 (30.4) | 3 (12.5) | 4 (26.7) | 6 (18.8) | 5 (26.3) | 5 (17.9) | |||
| 75–100% | 5 (10.6) | 3 (13.0) | 2 (8.3) | 1 (6.7) | 4 (12.5) | 2 (10.5) | 3 (10.7) | |||
| Radiological measurement | 0.290 * | 1.000 * | 1.000 * | |||||||
| 0–25% | 25 (53.2) | 10 (43.5) | 15 (65.2) | 10 (66.7) | 15 (46.9) | 10 (52.6) | 15 (53.6) | |||
| 25–50% | 9 (19.1) | 5 (21.7) | 4 (16.7) | 1 (6.7) | 8 (25.0) | 4 (21.0) | 5 (18.9) | |||
| 50–75% | 9 (19.1) | 6 (26.1) | 3 (12.5) | 4 (26.7) | 5 (15.6) | 3 (15.8) | 6 (21.4) | |||
| 75–100% | 4 (8.5) | 2 (8.7) | 2 (8.3) | 0 (0) | 4 (12.5) | 2 (10.5) | 2 (7.1) | |||
| Indirect measurement (citrullinemia, D-xylose absorption test) | ||||||||||
| 0–25% | 45 (95.7) | NA | NA | NA | ||||||
| 25–50% | 2 (4.3) | |||||||||
| 50–75% | 0 (0) | |||||||||
| 75–100% | 0 (0) |
| Question | n = Respondents to the Single Question (%) |
|---|---|
| Maximal duration of TED therapy in SBS-IF and CD patients? | n = 11 |
| 0 (0) |
| 4 (36.4) |
| 3 (27.3) |
| 4 (36.4) |
| Success rate (% of patients with at least a 30% reduction in the need for weekly parenteral nutrition)? | n = 11 |
| 2 (18.2) |
| 0 (0) |
| 6 (54.5) |
| 3 (27.3) |
| Reduction in the CDAI since the introduction of TED? | n = 11 |
| 4 (40.0) |
| 3 (30.0) |
| 2 (20.0) |
| 1 (10.0) |
| TED excluded due to cancer risk? | n = 26 |
| 20 (76.9) |
| 3 (11.5) |
| 2 (7.7) |
| 1 (3.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessarelli, T.; Topa, M.; Sorge, A.; Nandi, N.; Pugliese, D.; Macaluso, F.S.; Orlando, A.; Saibeni, S.; Costantino, A.; Stalla, F.; et al. The Epidemiology and Clinical Management of Short Bowel Syndrome and Chronic Intestinal Failure in Crohn’s Disease in Italy: An IG-IBD Survey. Nutrients 2024, 16, 3311. https://doi.org/10.3390/nu16193311
Pessarelli T, Topa M, Sorge A, Nandi N, Pugliese D, Macaluso FS, Orlando A, Saibeni S, Costantino A, Stalla F, et al. The Epidemiology and Clinical Management of Short Bowel Syndrome and Chronic Intestinal Failure in Crohn’s Disease in Italy: An IG-IBD Survey. Nutrients. 2024; 16(19):3311. https://doi.org/10.3390/nu16193311
Chicago/Turabian StylePessarelli, Tommaso, Matilde Topa, Andrea Sorge, Nicoletta Nandi, Daniela Pugliese, Fabio Salvatore Macaluso, Ambrogio Orlando, Simone Saibeni, Andrea Costantino, Francesco Stalla, and et al. 2024. "The Epidemiology and Clinical Management of Short Bowel Syndrome and Chronic Intestinal Failure in Crohn’s Disease in Italy: An IG-IBD Survey" Nutrients 16, no. 19: 3311. https://doi.org/10.3390/nu16193311
APA StylePessarelli, T., Topa, M., Sorge, A., Nandi, N., Pugliese, D., Macaluso, F. S., Orlando, A., Saibeni, S., Costantino, A., Stalla, F., Zadro, V., Scaramella, L., Vecchi, M., Caprioli, F., & Elli, L. (2024). The Epidemiology and Clinical Management of Short Bowel Syndrome and Chronic Intestinal Failure in Crohn’s Disease in Italy: An IG-IBD Survey. Nutrients, 16(19), 3311. https://doi.org/10.3390/nu16193311









