1. Introduction
The rapid skeletal growth of the developing fetus and the newborn creates a high demand for calcium during pregnancy and lactation. Approximately 2–3% of a mother’s calcium is transferred to the fetus in the later stages of pregnancy, primarily in the second and third trimesters. During lactation, about 300–400 mg of calcium per day is allocated to breastmilk [
1,
2,
3]. Due to this increased demand, the body activates several mechanisms such as enhancing renal calcium retention, boosting intestinal absorption, and increasing bone resorption to meet that demand [
4]. Despite these physiological adaptations, there is typically a decrease in bone mineral density by about 3% during lactation, which is counterbalanced by elevated levels of dihydroxy-vitamin D; fluctuations in parathyroid hormone (PTH), growth hormone, prolactin, and estrogen; and changes in nutrition, body weight, and lifestyle. The current view holds that the regulation of mineral homeostasis during lactation is primarily influenced by PTH-related protein (PTHrP) levels and a hypoestrogenic state [
5].
In brief, the suckling of the baby stimulates the release of oxytocin and prolactin from the pituitary gland and subsequently leads to the release of PTHrP from the mammary glands to promote bone resorption [
6]. Both static and dynamic histomorphometry have demonstrated increased bone turnover during lactation in favor of resorption [
7]. Both osteoclast-driven trabecular resorption and osteocytic osteolysis of cortical bone contribute to calcium release [
8,
9,
10,
11,
12]. The calcium released from the bone enters circulation and is transferred into the breast milk by the mammary glands to support the growth of the newborn. After weaning, the maternal skeleton typically recovers to the pre-pregnancy state in terms of bone mass [
13].
While the effect of the brain–breast–bone axis is well established, the effect of estrogen (E
2) depletion postpartum has not been investigated in detail. E
2 regulates cell activities by multiple mechanisms. E
2 can regulate transcription via binding to its cognate receptors (ESR1 or ESR2) that are members of the nuclear hormone receptor superfamily [
14]. At the organismal level, E
2 is a key component of the reproductive system in women and thus regulates key systems, including the immune system. During pregnancy, as the fetus is allogenic, the maternal immune system must be regulated to prevent immunogenic responses to both the placenta and the fetus while not immunocompromising the mother. Therefore, pregnancy, postpartum, and menopause could be distinct physiological states compared to normal menstrual cycling. Here, we investigated whether this E
2 loss-dependent T
M activation plays a role in osteolysis during lactation.
We previously identified a pathway of how E
2 regulates the immune system. While a hyperestrogenic state promotes tolerance, the depletion of E
2 by ovariectomy (OVX) leads to the production of TNFα and IL-17A by T
M to promote bone loss [
15]. T
M rely on IL-7 and IL-15 for homeostasis. In the bone marrow, a subset of dendritic cells (DCs) produces IL-7 and IL-15 [
15]. E
2 induces the apoptosis of IL-7
+DC by inducing FasL. With the decline of E
2, FasL is no longer induced, leading to increased numbers of DCs and higher concentrations of IL-7 and IL-15. These two cytokines together, but not individually, promote TNFα and IL-17A production in a subset of T
M, which subsequently activate bone erosion [
15]. To validate the role of IL-15 in activating T
M post-OVX, we ablated the IL-15 receptor alpha chain in T-cells by crossing IL15RA
f/f mice to T-cell-specific CRE mice (Lck-Cre) to generate the IL15RA
ΔΤ strain. These mice developed normal levels of T
M, as expected [
15]. Importantly, E
2 loss did not lead to TNFα and IL-17A induction in T
M in the IL15RA
ΔT mice [
15]. Thus, IL15RA
ΔT mice allow us to distinguish between the cell autonomous effect of E
2 loss and T-cell-mediated inflammation on bone cells. We used these IL15RA
ΔT mice in this study to validate the effect of T
M on bone resorption during lactation.
2. Materials and Methods
Animal use statement: All animals used in this study were maintained in the Department of Comparative Medicine at Saint Louis University School of Medicine in accordance with institutional and Public Health Service guidelines. All mice were housed in microisolator cages in specific pathogen-free (SPF) environments. Weanling mice were maintained on rodent chow 5LOB and breeder trios were fed on breeder diet 5058 (both diets are from Lab Diet, Richmond, IA, USA). The Saint Louis University School of Medicine Institutional Animal Care and Use Committee (IACUC) approved all procedures performed on the mice (protocol 2072).
Mice: C57BL/6J (model 000664) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred in-house. Breeding trios of IL-7
CFP reporter [
16] mice were generously provided by Dr. Scott Durum (NIH-NCI, Bethesda, MD, USA) and bred in-house. The IL15RA
ΔT mice were previously generated [
15] by crossing IL15RA-floxed (model 022365) [
17] and Lck-Cre Tg540-I (model 006889) [
18] mice, and were maintained in-house.Breeder trios (two nulligravid females and a male) were set up at 8–10 weeks of age. For the postpartum experiments in this study, the male was removed from the cage when the females were visibly pregnant and prior to delivery. The females were allowed to nurse pups normally. For postpartum harvests, both the nursing dam and her litter were euthanized on d12 postpartum. Nursing females were given 0.05 mg over a 21-day release of 17β-estradiol pellets (E-121, Innovative Research of America, Sarasota, FL, USA) subcutaneously in the loose skin of the scruff neck between d0 and d2 postpartum. Next, 1.5% isoflurane was used to initiate anesthesia and for maintenance. The pellet was placed using a 10G precision trocar, and closed using a single skin staple (3M, Maplewood, MN, USA; cat# DS-15).
Flow cytometry: Antibodies used in this study are listed in
Table 1. For staining, cells were resuspended with 50 μL of BD Horizon
™ Brilliant Stain Buffer (cat# 566349, BD Biosciences, Franklin Lakes, NJ, USA) and then incubated for 30 min at room temperature with fluorophore-conjugated antibodies protected from light. For intracellular staining (ICS), cells were washed, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton-X100, and stained overnight at 4 °C. Cells were washed, fixed with 1% paraformaldehyde, and analyzed on a LSRII instrument with FACS Diva 9.0 (BD Biosciences) software. Gates were determined using a combination of single-color and fluor-minus-one controls. Data analyses were performed with FlowJo software (version 10.8.1; FlowJo, LLC, Ashland, OR, USA).
Immunofluorescence: Femurs and tibias were fixed in 4% paraformaldehyde (PFA) overnight (16–24 h). The bones were washed briefly with PBS and submitted to Washington University Musculoskeletal Histology and Morphometry Core for decalcification and sectioning. Tibias were decalcified for 10–14 days in EDTA embedded in paraffin and sectioned at 5 μm thickness. Immunofluorescence (IF) staining was performed according to established protocol [
19]. Briefly, slides were deparaffinized with Xylene and rehydrated in decreasing concentrations of ethanol (100%, 95%, 70%, 50%, 30%, and DI-H
2O) in Coplin jars. Antigen retrieval was performed at 55 °C in citrate buffer (cat# C9999, Sigma-Aldrich, Saint Louis, MO, USA), and Background-Sniper (cat# BS966, BioCare Medical, Pacheco, CA, USA) was used for blocking. The primary antibodies used were rabbit anti-mouse IL-7 (cat# ab9732, Abcam, Cambridge, MA, USA), rat anti-mouse IL-15 (cat# MAB477, eBioscience, Santa Clara, CA USA), and Armenian hamster anti-mouse CD11c (cat# ab33483, Abcam). The secondary antibodies used in this study were goat anti-rabbit-AF594 (cat# Ab150080, Abcam), donkey anti-Armenian hamster-AF488 (cat# Ab173003, Abcam), and goat anti-rat-AF647 (cat# A21247, Invitrogen, Waltham, MA, USA). The slides were imaged using Leica TCS SP8 confocal microscopy (Leica, Wetzlar, Germany) at 40× magnification. Image analyses were performed using FIJI/ImageJ2 v.2.14.02/1.54f (
https://imagej.net/software/fiji/downloads (accessed on 7 July 2023)) by an operator blinded to the experiments.
TUNEL assay: Formalin-fixed, paraffin-embedded bone sections were TUNEL-stained per the manufacturer’ instructions (in situ cell death detection kit TMR red, cat# 12156792910, Roche Diagnostics, Mannheim, Germany). Quantitation was performed as described for IF.
In vitro DC culture: The spleen was harvested in cold PBS-1, injected with 100 μL of Liberase TL (cat# 05401020001, Roche Diagnostics) at 125 μg/mL, and incubated at 37 °C for 10 min. The spleen was chopped into smaller pieces and further incubated in 3 mL of PBS-1 (PBS + 1% FBS) containing Liberase TL at 125 μg/mL at 37 °C for 30 min, and vortexed for 30 sec every 5 min. The spleens were passed through a 70 μ cell strainer with the aid of a syringe plunger washed with 5 mL of cold PBS-1. RBCs were lysed using BD Pharm Lyse™ (cat# 555899, BD Biosciences), and remaining cells were cultured in DMEM containing 10 nM 17β-estradiol (cat# E8875, Sigma-Aldrich) at 37 °C for 4 h. Cells were then analyzed via FACS.
In vitro T-cell culture: T-cells were isolated from BMC using Anti-CD4(L3T4) and anti-CD8a(Ly-2) microbeads (cat# 130–117-043 and cat# 130-117-044, Miltenyi Biotec, Bergisch Gladbach, Germany). T-cells were cultured in complete T-cell growth media (RPMI supplemented with 10% heat-inactivated FBS, Penicillin, Streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, non-essential amino acids, and 55 μM β-mercaptoethanol; all from Sigma-Aldrich, St. Louis, MO, USA) for 2 days prior to cytokine treatment.
Serum marker assay: Nursing dams were fasted with their offspring overnight prior to blood collection. Blood (50 μL) was obtained via the submandibular vein and allowed to clot for 1 hour at room temperature. Serum was collected by spinning down the cell pellet at 10,000 g for 10 min, flash freezing, and storing at −80 °C. Serum C-terminal telopeptide of type 1 collagen (serum CTX) was measured using a competitive ELISA according to the manufacturer’s instructions (cat# AC-06F1, Immunodiagnostic Systems, Gaithersburg, MD, USA).
Breastmilk Calcium Assay: Breastmilk collection was adapted from previously described methods [
20]. Briefly, nursing dams were separated from their pups in the same cage for 2 to 4 h using a wire rack prior to breastmilk collection. Female mice were anesthetized using 2.5% isoflurane. A total of 1 U of oxytocin (cat# O4375, Sigma-Aldrich) in PBS was given intraperitoneally to induce letdown. Breastmilk was produced through gentle massaging of the teat and collected by a pipette. Approximately 100 μL of breastmilk was collected from each female. The calcium assay was performed according to the manufacturer’s instructions (cat# MAK022, Sigma-Aldrich).
Quantitative PCR (qPCR): Bone marrow cells (BMCs) were collected by cutting the distal end of the femur or tibia and spinning out the bone marrow at 21×
g for 30 s. RBCs were lysed using BD Pharm Lyse™ (cat# 555899, BD Biosciences). RNA was extracted using Agilent Total RNA Isolation Mini Kit (cat#5185-5999, Agilent Technologies, Santa Clara, CA, USA). Gene expression changes were measured by qPCR using KAPA SYBR FAST One-Step Universal (cat#07959613001, Roche Diagnostics). Primers used are shown in
Table 2.
MicroCT data collection and analysis: Femurs were scanned in μCT35 (Scanco Medical USA, Southeastern, PA, USA) at 55 kV, 72 μA, 4 W, at a resolution of 10 μm. Gauss sigma of 0.8, Gauss support of 1, lower threshold of 220 permilles for cancellous bone and 240 permilles for cortical bone, and upper threshold of 1000 permilles were used for all analyses. These thresholds correspond to 468.8, 592.2, and 3000 mg hydroxyapatite (HA)/cm
3. For trabecular analysis, the start of the distal metaphyseal region of the femur was identified and a 1.5 mm region (150 slices) toward the proximal end was selected for evaluation. For cortical analysis, the midpoint of the femur was identified, and a 1.0 mm (100 slices) region was selected for analysis. Bone mineral density was obtained by quantitative μCT using phantoms for calibration [
21].
Statistical analysis: All statistical significance tests were calculated using Prism software v. 9.5.0 (GraphPad Inc., La Jolla, CA, USA). We used non-parametric unpaired tests (e.g., two-tailed Mann–Whitney U and ANOVA) to avoid the assumption of normal distribution in our data.
3. Results
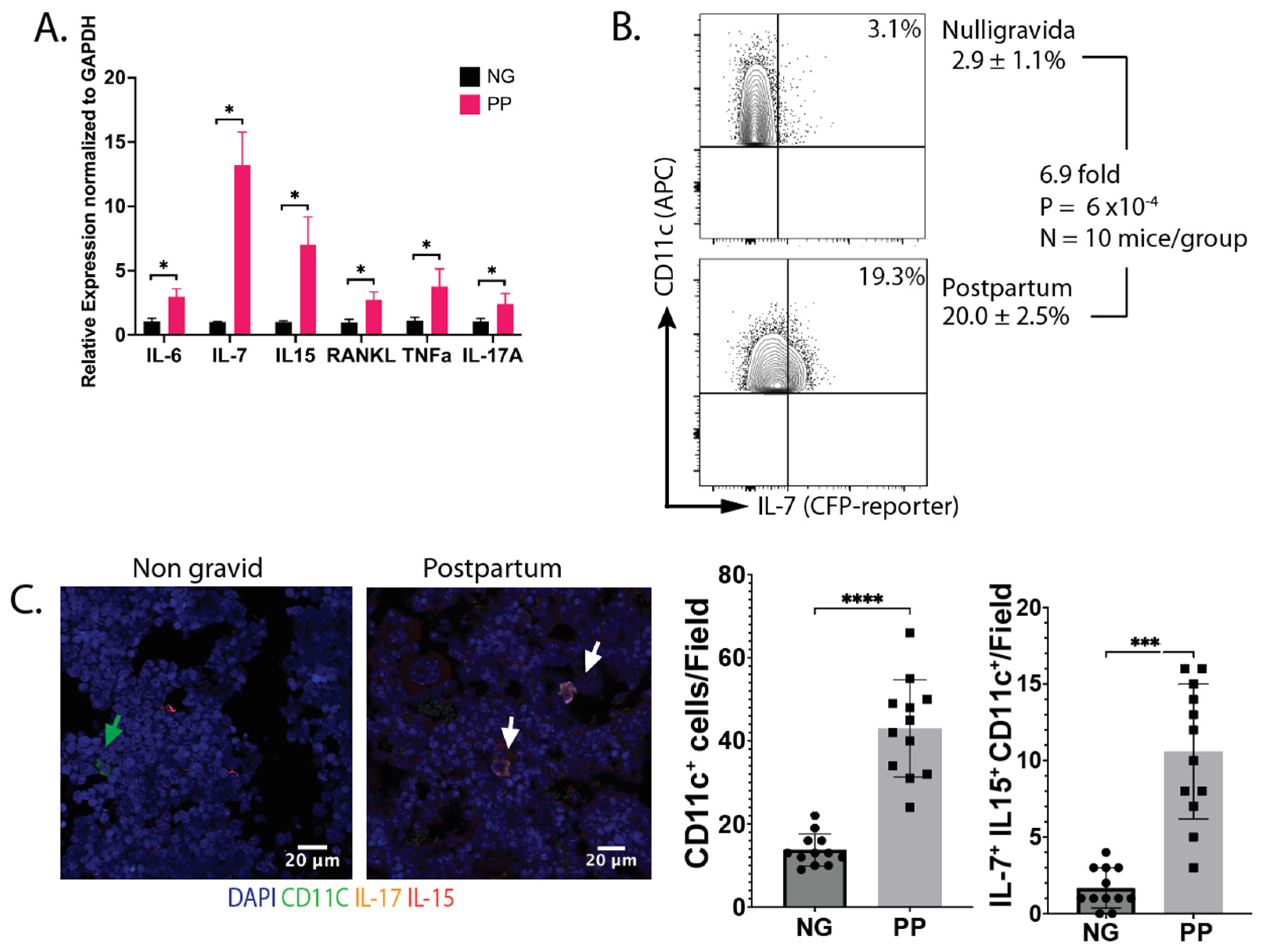
IL-7 and IL-15 increase postpartum: To characterize the state of the immune system, we determined the cytokines expressed by hematopoietically derived cells (CD45
+) in the bone marrow. Cytokine gene expression was analyzed by qRT-qPCR. We found that IL-6, IL-7, IL-15, IL-17A, and TNFα have upregulated expression in lactating dams compared to age-matched nulligravida (NG) females (
Figure 1A). All cytokines were expressed by CD45
+ cells, except for TNFα, which was expressed in both subsets. Next, we investigated the source of IL-7. We harvested bone marrow cells (BMCs) from IL-7
eCFP reporter mice at d12 postpartum (PP), and the cyan fluorescent protein (CFP) expression was analyzed by flow cytometry. We observed a 6.7-fold increase in CFP
+ DCs (
Figure 1B) in PP females compared to NG females. In addition, we used immunofluorescence to assess the expression of IL-7 and IL-15 by CD11c
+ cells in bone sections from virgin and PP mice. An increase in both CD11c
+ cells and CD11c
+ IL-7
+ IL-15
+ triple-positive cells was observed PP (
Figure 1C). In summary, the data in
Figure 1 shows increased levels of BMDCs that express IL-7 and IL-15 PP, consistent with our OVX results [
15].
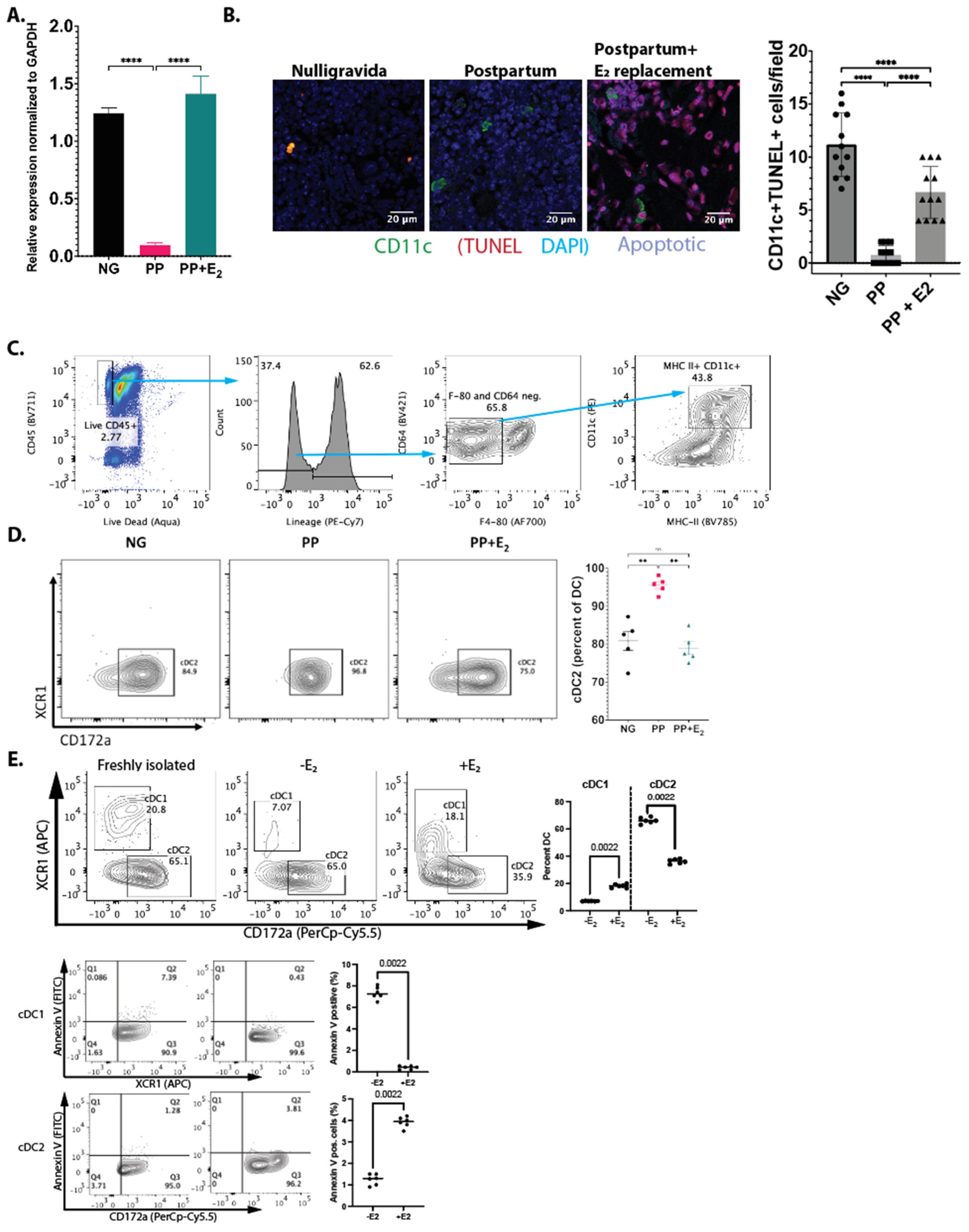
Reduced apoptosis of IL-7+ DCs postpartum: We previously showed that E
2 regulates the levels of IL7
+ DCs post-OVX by inducing apoptosis via Fas ligand (FasL). Here, we determined whether E
2 also induces FasL postpartum. The total RNA was harvested from BMCs, and FasL expression was measured by qRT-qPCR. There was a 6.8-fold reduction in FasL expression in PP females (
Figure 2A). E
2 replacement given shortly after birth (between d0 and d2 postpartum) restored FasL expression back to levels found in NP females at d12. TUNEL staining showed a 10-fold decreased in apoptosis in CD11c
+ DCs in the bone marrow of PP mice. With E
2 replacement, the number of apoptotic cells returned to NG levels (
Figure 2B). Taken together, these data demonstrate that induction of Fas ligand (FasL) expression by E
2 is a conserved mechanism between postpartum and post-OVX [
15].
Differential regulation of dendritic cell subset by estrogen: We also examined the effect of E
2 loss on DC subsets to gain insights into DC dynamics during pregnancy and postpartum. Freshly isolated splenic DCs were cultured in the presence of 10 nM 17β-estradiol, or vehicle, and the cells were characterized via flow cytometry. The results showed that E
2 favors conventional type 1 dendritic cells (cDC1s) and reduces the number of conventional type 2 dendritic cells (cDC2s) (
Figure 2C). To determine whether the change in the levels of the two DC populations is due to apoptosis, we stained for annexin V. Our results show that E
2 reduces the apoptosis of cDC1s but increases the apoptosis of cDC2s (
Figure 2D).
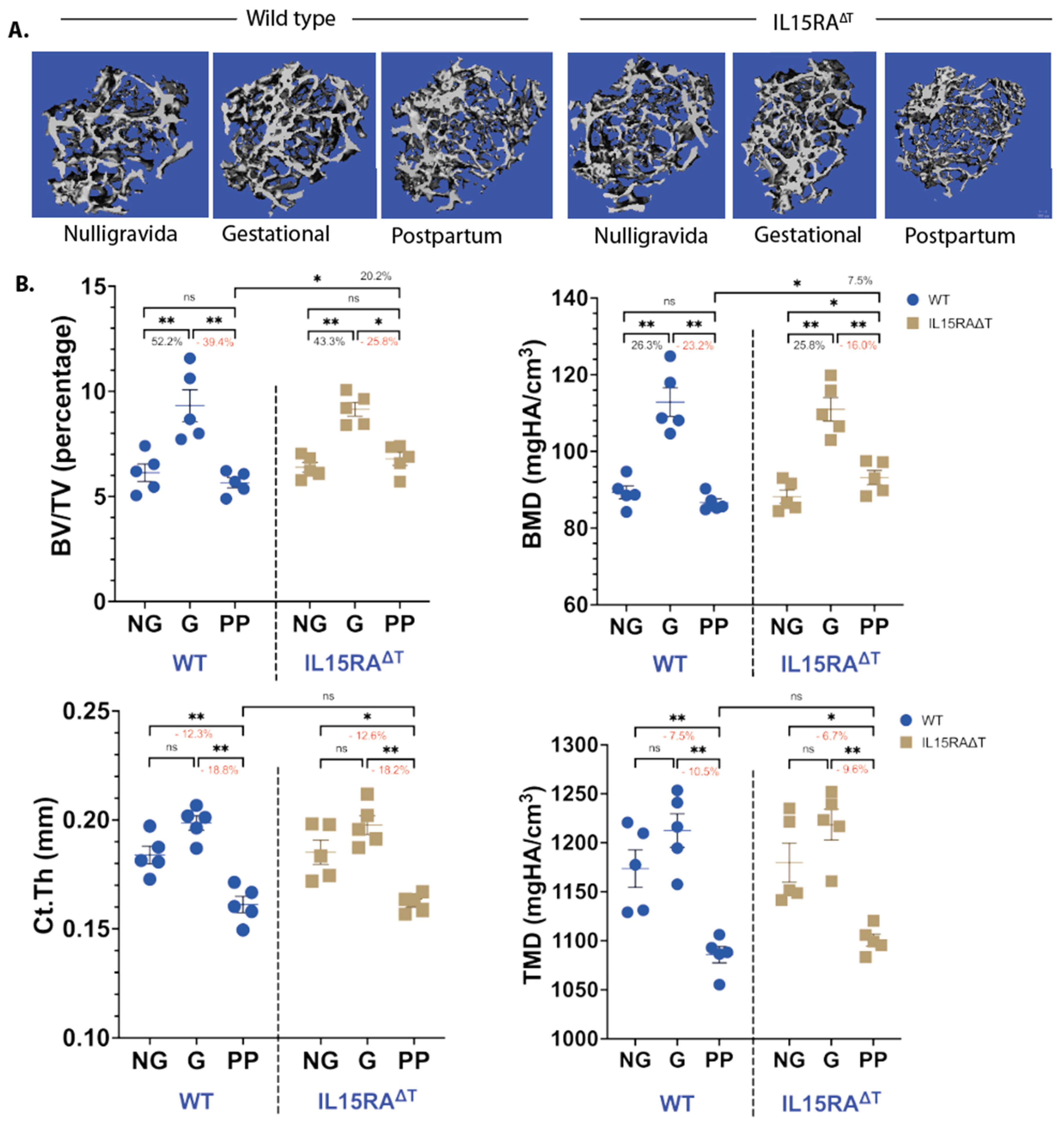
TNFα and IL-17A expression correlates with bone resorption: Our results in
Figure 1 and
Figure 2 show that E
2 loss leads to increased levels of IL-7
+DCs that also produce IL-15. To determine whether T
M activation by IL-15 and the subsequent production of TNFα and IL-17A contribute to bone resorption, we analyzed femurs from IL-15RA
ΔT mice d12 postpartum. Analysis of μCT showed that while both strains gained the same amount of bone during gestation (G), IL-15RA
ΔT mice had approximately 20% less trabecular bone resorption during lactation compared to the WT (
Figure 3A). However, there was no difference in the cortical bone between the two strains (
Figure 3B).
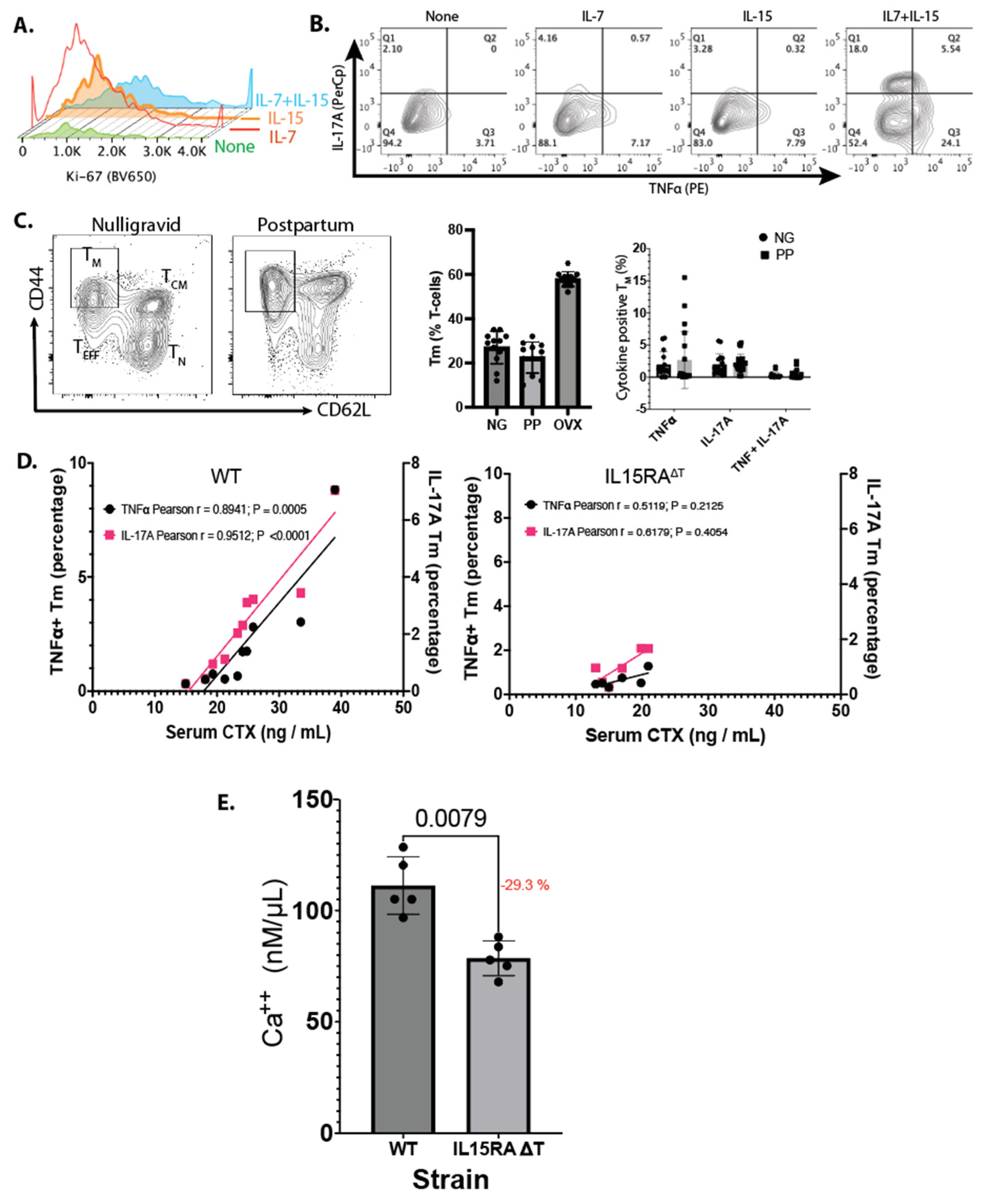
To validate the effect of IL-7 and IL-15 on activating T
M, we purified bone marrow T-cells and cultured them for 48 h in the absence of CD3 and CD28 stimulation, with or without IL-7, IL-15, or both. IL-7 and IL-15 increased Ki-67 staining in T
M, indicating increased proliferation in the presence of IL-7 and IL-15 (
Figure 4A). Importantly, IL-7 and IL-15 lead to the expression of TNFα and IL-17A in the presence of both cytokines, which was not observed when T
M were incubated with either cytokine alone (
Figure 4B). This antigen-independent, cytokine-dependent activation is a unique finding of our study.
To assess whether the low levels of TNFα and IL-17A produced by T
M postpartum promoted bone resorption, we examined the T-cell populations in the bone marrow of both the WT and IL-15RA
ΔT mice during PP using age-matched NG females of the same strain as controls. We observed that the percentage of T
M and the expression of TNFα and IL-17A were highly variable (
Figure 4C) between the PP and control groups and the difference was not statistically significant.
No differences were observed in litter sizes between WT and IL-15RA
ΔT mice. Both strains had litters of 5–6 pups per female. The growth of the pups is not linear—it occurs in spurts. Accordingly, the amount of milk depends on the demand. We hypothesized that TNFα
+ or IL17A
+ T
M may be dependent on the calcium demand. This would contribute to inter-individual sampling variability. To determine whether activated T
M contribute to bone resorption, we correlated serum CTX levels to the percentage of T
M that were positive for TNFα or IL-17A in the same animal. We observed a strong correlation between increased cytokine production and bone resorption in PP. In WT mice, TNFα and IL-17A explained 87.0% and 91.3% of the variance, respectively, with statistical significance (
p < 0.01) (
Figure 4D). In contrast, in IL-15RA
ΔT mice, these cytokines accounted for 22.2% and 16.2% of the variance, respectively, but this was not statistically significant (
p > 0.1). No correlation was found in the NG controls for both strains (
Figure 4D). Finally, the calcium concentration in the breas
tmilk of the IL-15RA
ΔT mice on day 12 postpartum relative to the WT mice was reduced by 28.6% (±2.8%) (
Figure 4E). Together, our results indicate that T
M promote bone resorption postpartum, and in the absence of T
M-mediated TNFα and IL-17, reduces calcium content in breastmilk.
4. Discussion
Previous studies have shown that calcium homeostasis during lactation is maintained by crosstalk between the mammary gland, the pituitary gland, and bone [
22,
23,
24,
25]. The infant triggers an oxytocin burst that leads to the release of PTHrP via prolactin. PTHrP activates bone resorption and the release of calcium into circulation via osteocytic and osteoclastic osteolysis [
9]. The role of the hypoestrogenic state during lactation has not been elucidated. Here, we examined the role of E
2 loss the on the memory T-cell (T
M)-mediated production of TNFα and IL-17A in contributing to bone resorption and the calcium content of breastmilk. Consistent with our previous studies using ovariectomized mice, we observed that the CD45
+ hematopoietic cell lineage is responsible for cytokine production in the bone marrow postpartum (
Figure 1A). IL-7
eCFP reporter mice showed significantly increased CFP-positive cells in the CD45
+ CD11b
+ CD11c
+ population in lactating dams (
Figure 1B). We verified that the increased number of DCs was due to their increased lifespan via FasL expression in the bone marrow postpartum compared to nulliparous controls (
Figure 2A). Reduced FasL expression resulted in decreased apoptosis in DCs (
Figure 2B). E
2 replacement treatment showed increased FasL expression as well as TUNEL
+ cells, demonstrating that E
2 could control DC levels (
Figure 2B).
The role of estrogen in the regulation of DC subsets by controlling their lifespan has not been previously documented. Our findings are consistent with the functional properties of cDC1s, whose main functional role is the cross-presentation of antigens that can also induce tolerance [
26,
27,
28,
29]. During pregnancy, a hyperestrogenic state, cDC1s would be favored as the predominant population to cross-present circulating fetal and placental antigens. In contrast, cDC2s are favored in the low-E
2 state (i.e., postpartum and postmenopause) to promote immune-surveillance against pathogens and to tip the balance toward a proinflammatory environment [
30].
To measure the effect of T
M on bone mass during lactation, we analyzed the femurs of WT and IL-15RA
ΔT mice harvested during gestation (G), during d12 postpartum (PP), and from age-matched NG littermate controls. We observed an increase in trabecular bone mass measured by BV/TV and BMD in both WT and IL-15RA
ΔT mice during gestation, consistent with previous findings [
5,
7]. During lactation, both the BV/TV and BMD in WT mice returned to NG levels. In IL-15RA
ΔT mice, PP mice had a higher BV/TV and BMD compared to the WTs (
Figure 3A). No cortical bone accrual was seen between the G and NG mice for both strains. Cortical bone resorption is the same during lactation between the two strains, both decreasing by about 18% and 10% for Ct.Th and TMD compared to NG, respectively (
Figure 3B). Data in
Figure 3 indicates that IL-15 signaling in T
M contributes to trabecular but not cortical bone resorption during lactation. This is likely because trabecular resorption is predominantly osteoclastic while cortical resorption is osteocytic, which is primarily driven by PTHrP during lactation [
8,
9,
10,
11,
12].
IL-7 and IL-15 together increased T-cell proliferation and cytokine production that is antigen-independent but cytokine-dependent (
Figure 4A,B). While in culture and post-OVX these cytokines promoted TNFα and IL-17A expression in T
M, the level of these cytokines was highly variable postpartum (
Figure 4C). However, the level of TNFα and IL-17A expression correlated with bone resorption in WT mice but not in IL15RA
ΔT mice (
Figure 4D) that do not express TNFα and IL-17A by T
M. Further, a lower calcium concentration was found in the breastmilk of the IL15RA
ΔT mice (
Figure 4E). Together, our data support the notion that T
M-derived TNFα and IL-17A increases bone resorption to release calcium that contributes to the calcium in milk.
Typically, inflammation is considered to be a response to infection and/or pathogens. Our results indicate that low-grade inflammation plays a critical physiological role in releasing calcium from bone for lactation. The T
M-mediated pathway appears to be activated during a hypoestrogenic state, both postpartum as well as postmenopause. Breastfeeding triggers the release of PTHrP from the mammary glands, which stimulates bone resorption to meet the demand for calcium. This fluctuation in serum calcium levels could result in intermittent increases in PTH, which has been shown to induce regulatory T-cells (T
REGs) [
31,
32]. Thus, a potential mechanism of limiting T
M could be due to the induction of T
REGs by iPTH. We attempted to test the suppression of TNFα
+ and IL-17A
+ T
M by administering oxytocin into OVX mice. However, the dosage of oxytocin as well as the time needed to observe phenotypic changes is not well established. These studies are underway and require further optimization.
A limitation of our study is the use of a single timepoint for data collection. We selected day 12 of the 21-day nursing period for mice because it coincided with the peak of milk production and the beginning of the pups’ transition to solid food [
33,
34]. In humans, breastfeeding inhibits the start of estrus cycling. This negative feedback is absent in mice, which can conceive while still nursing. To avoid potential confounding effects from the reactivation of ovarian function, we removed the male mice from the environment. However, differences in estrogen (E
2) production between human and mouse ovaries may still pose a confounding factor in our results.
In summary, our study highlights the physiological role of immune system activation during the postpartum period, particularly through the regulation of calcium homeostasis by memory T-cells in conjunction with the oxytocin-PTHrP pathway. Our findings reveal for the first time the critical involvement of T-cells in reproductive processes in mammals. We propose that the immune-mediated regulation of bone resorption, triggered by E2 loss, likely evolved to ensure an adequate calcium supply in breastmilk. This mechanism ceases once lactation ceases and E2 levels normalize. The PTHrP produced by mammary glands releases Ca++ from the bone, over and above that released by low-grade inflammation, based on demand by feeding. However, the activation of this pathway occurs due to E2 loss postmenopause, but without attenuation, this leads to pathological osteoporosis. Understanding the immune system’s function in physiological contexts—beyond its role in controlling infections or tumors—provides a new insights into the role of the immune system.