Association between Serum Zinc and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: The Osaka Dialysis Complication Study (ODCS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants of This Study
2.2. Ethical Considerations
2.3. Measurement of Serum Zinc
2.4. Mortality Data Collection
2.5. Covariates
2.6. Statistics
3. Results
3.1. Characteristics of Study Participants
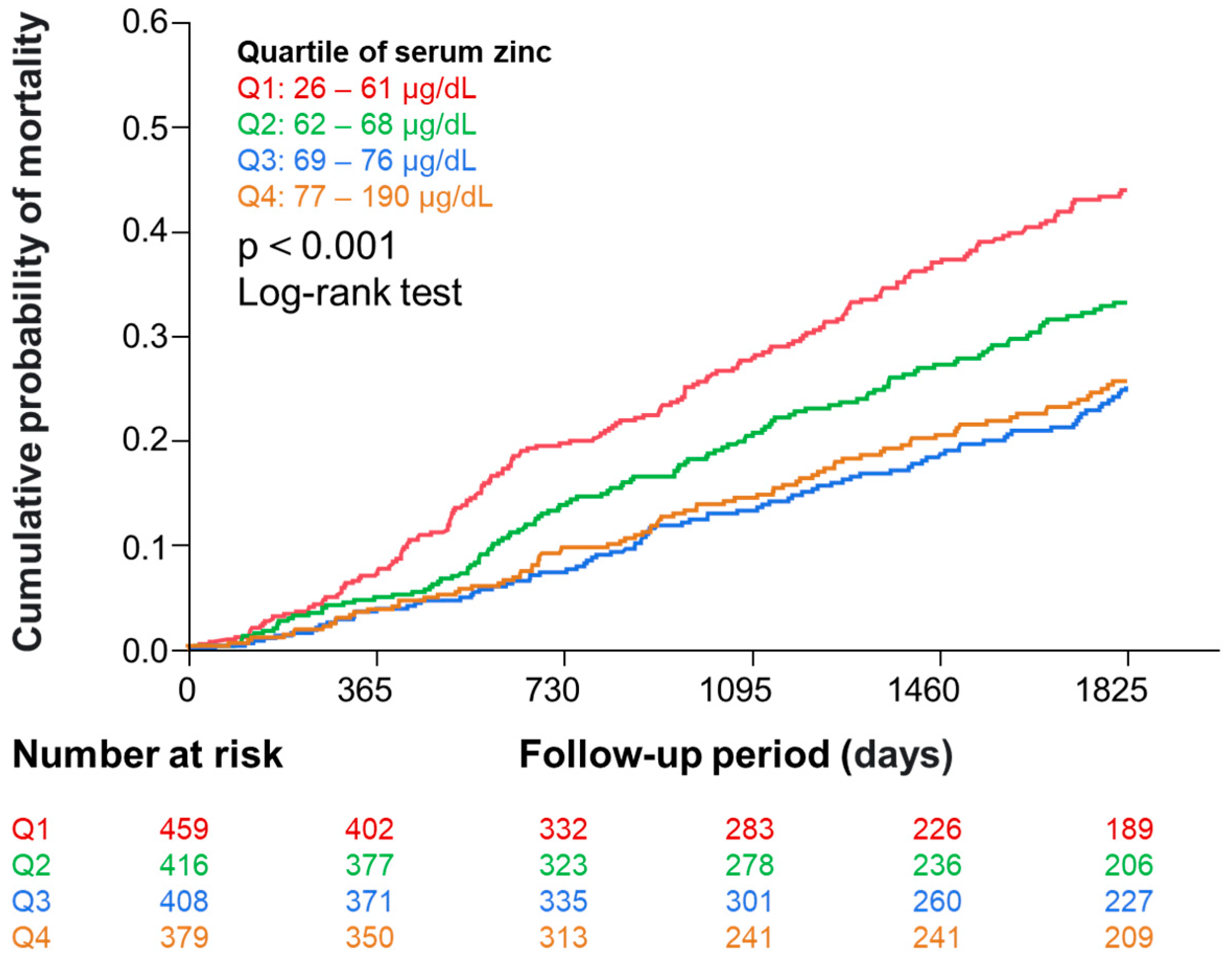
3.2. Serum Zinc Levels and Mortality
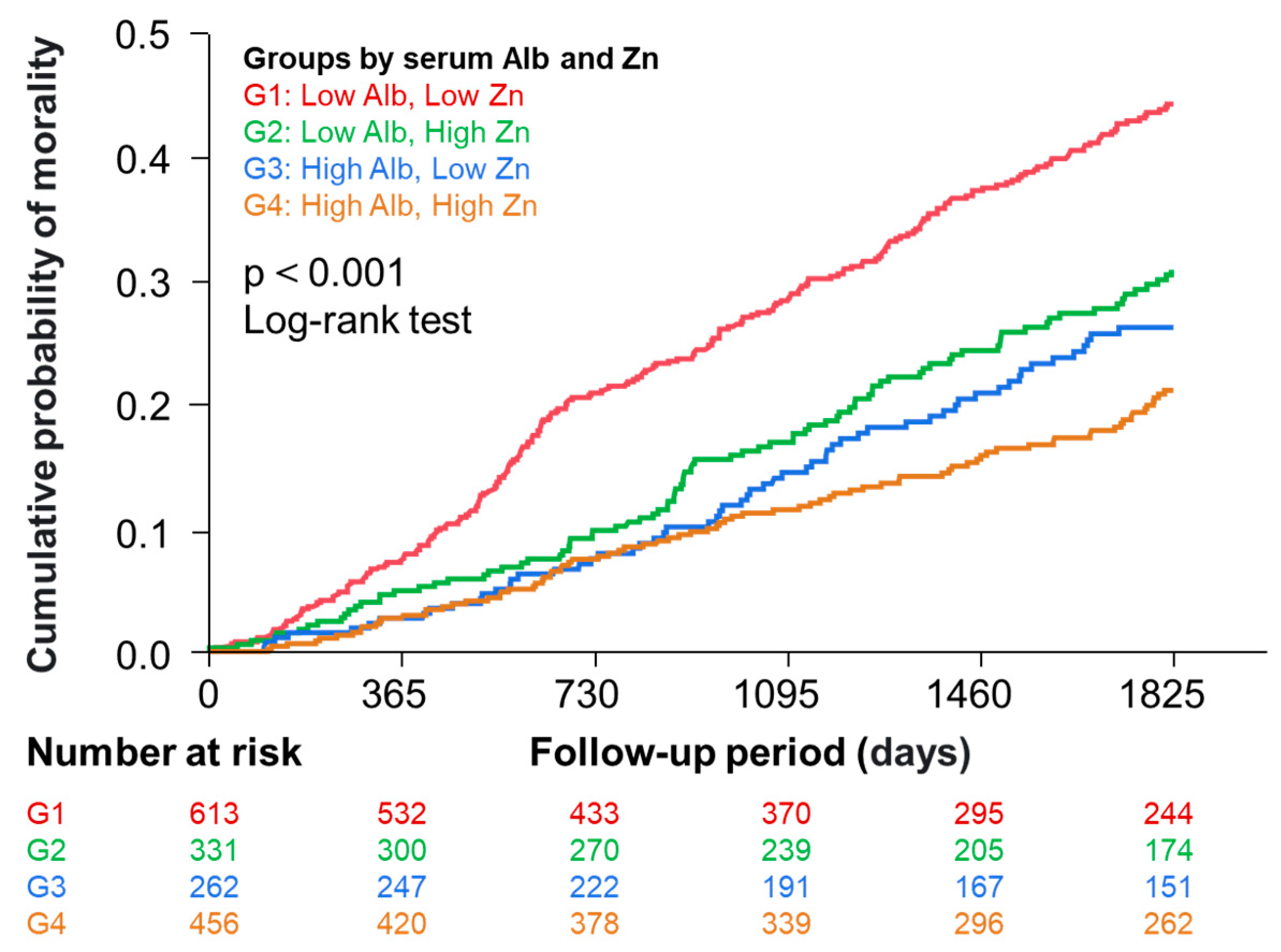
3.3. Relationship between Zinc with Mortality in Four Groups Categorized by Median Serum Albumin and Zinc Levels
3.4. Association between Zinc with Mortality in the Low and High Albumin Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, J.C.; Shames, D.M.; Woodhouse, L.R. Zinc homeostasis in humans. J. Nutr. 2000, 130, 1360S–1366S. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F. Overview of zinc absorption and excretion in the human gastrointestinal tract. J. Nutr. 2000, 130, 1374S–1377S. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.; Mafra, D. Don’t forget the zinc. Nephrol. Dial. Transplant. 2020, 35, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.K.; Bowersox, E.M.; Rye, D.L.; Abu-Hamdan, D.K.; Prasad, A.S.; McDonald, F.D.; Biersack, K.L. Factors underlying abnormal zinc metabolism in uremia. Kidney Int. Suppl. 1989, 27, S269–S273. [Google Scholar] [PubMed]
- Damianaki, K.; Lourenco, J.M.; Braconnier, P.; Ghobril, J.P.; Devuyst, O.; Burnier, M.; Lenglet, S.; Augsburger, M.; Thomas, A.; Pruijm, M. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol. Dial. Transplant. 2020, 35, 1163–1170. [Google Scholar] [CrossRef]
- Fukasawa, H.; Furuya, R.; Kaneko, M.; Nakagami, D.; Ishino, Y.; Kitamoto, S.; Omata, K.; Yasuda, H. Clinical Significance of Trace Element Zinc in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 1667. [Google Scholar] [CrossRef]
- Hou, R.; He, Y.; Yan, G.; Hou, S.; Xie, Z.; Liao, C. Zinc enzymes in medicinal chemistry. Eur. J. Med. Chem. 2021, 226, 113877. [Google Scholar] [CrossRef]
- Ume, A.C.; Wenegieme, T.Y.; Adams, D.N.; Adesina, S.E.; Williams, C.R. Zinc Deficiency: A Potential Hidden Driver of the Detrimental Cycle of Chronic Kidney Disease and Hypertension. Kidney360 2023, 4, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Mistry, M.; Cheriyan, A.M.; Williams, J.M.; Naraine, M.K.; Ellis, C.L.; Mallick, R.; Mistry, A.C.; Gooch, J.L.; Ko, B.; et al. Zinc deficiency induces hypertension by promoting renal Na(+) reabsorption. Am. J. Physiol. Renal. Physiol. 2019, 316, F646–F653. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc in human health: Effect of zinc on immune cells. Mol. Med. 2008, 14, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro, O.M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-kappaB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Wu, M.L.; Chou, Y.Y.; Li, S.Y.; Deng, J.F.; Yang, W.C.; Ng, Y.Y. Essential trace element status and clinical outcomes in long-term dialysis patients: A two-year prospective observational cohort study. Clin. Nutr. 2012, 31, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Knehtl, M.; Piko, N.; Ekart, R.; Hojs, R.; Bevc, S. Serum zinc values, ankle brachial index and mortality in hemodialysis patients. BMC Nephrol. 2022, 23, 355. [Google Scholar] [CrossRef]
- Toida, T.; Toida, R.; Ebihara, S.; Takahashi, R.; Komatsu, H.; Uezono, S.; Sato, Y.; Fujimoto, S. Association between Serum Zinc Levels and Clinical Index or the Body Composition in Incident Hemodialysis Patients. Nutrients 2020, 12, 3187. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Bello, A.; Field, C.J.; Gill, J.S.; Hemmelgarn, B.R.; Holmes, D.T.; Jindal, K.; Klarenbach, S.W.; Manns, B.J.; et al. Concentrations of Trace Elements and Clinical Outcomes in Hemodialysis Patients: A Prospective Cohort Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Shoji, T.; Fujii, H.; Mori, K.; Nakatani, S.; Nagata, Y.; Morioka, T.; Inaba, M.; Emoto, M. Associations of cardiovascular disease and blood pressure with cognition in hemodialysis patients: The Osaka Dialysis Complication Study. Nephrol. Dial. Transplant. 2022, 37, 1758–1767. [Google Scholar] [CrossRef]
- Matsufuji, S.; Shoji, T.; Lee, S.; Yamaguchi, M.; Nishimura, M.; Tsujimoto, Y.; Nakatani, S.; Morioka, T.; Mori, K.; Emoto, M. Association between Levocarnitine Treatment and the Change in Knee Extensor Strength in Patients Undergoing Hemodialysis: A Post-Hoc Analysis of the Osaka Dialysis Complication Study (ODCS). Nutrients 2022, 14, 343. [Google Scholar] [CrossRef]
- Inoue, S.; Kondo, Y.; Yoshida, H. Aensokuteishiyaku Esupa ZnII no seinouhyouka (Evaluation of the reagent for measurement of Zinc ESPA _ Zn II). Iryou to Kensakiki Shiyaku (J. Clin. Lab. Instrum. Reag.) 2018, 41, 283–287. (In Japanese) [Google Scholar]
- Takahashi, A. Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis. Nutrients 2023, 15, 4887. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A. Zinc Supplementation Enhances the Hematopoietic Activity of Erythropoiesis-Stimulating Agents but Not Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors. Nutrients 2024, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef]
- Okamura, T.; Tsukamoto, K.; Arai, H.; Fujioka, Y.; Ishigaki, Y.; Koba, S.; Ohmura, H.; Shoji, T.; Yokote, K.; Yoshida, H.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022. J. Atheroscler. Thromb. 2024, 31, 641–853. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Oberleas, D. Binding of zinc to amino acids and serum proteins in vitro. J. Lab. Clin. Med. 1970, 76, 416–425. [Google Scholar]
- Henkin, R.I. Metal-albumin-amino acid interactions: Chemical and physiological interrelationships. Adv. Exp. Med. Biol. 1974, 48, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Wang, M.Q.; Hu, R.; Yang, Y.; Huang, Y.S.; Xian, S.X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled. Trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef]
- Guo, C.H.; Wang, C.L. Effects of zinc supplementation on plasma copper/zinc ratios, oxidative stress, and immunological status in hemodialysis patients. Int. J. Med. Sci. 2013, 10, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Argani, H.; Mahdavi, R.; Ghorbani-haghjo, A.; Razzaghi, R.; Nikniaz, L.; Gaemmaghami, S.J. Effects of zinc supplementation on serum zinc and leptin levels, BMI, and body composition in hemodialysis patients. J. Trace Elem. Med. Biol. 2014, 28, 35–38. [Google Scholar] [CrossRef]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s Practical Guidelines for Zinc Deficiency with a Particular Focus on Taste Disorders, Inflammatory Bowel Disease, and Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, W.; Ukeda, K.; Machida, S.; Tsuboi, I. Basic study of Zn measurement using the reagent ‘Espa-Zn II’: Performance evaluation of zinc measurement reagent. Jpn. J. Med. Technol. 2021, 70, 80–85. (In Japanese) [Google Scholar]
- Garagarza, C.; Valente, A.; Caetano, C.; Ramos, I.; Sebastião, J.; Pinto, M.; Oliveira, T.; Ferreira, A.; Sousa Guerreiro, C. Zinc deficient intake in hemodialysis patients. A path to a high mortality risk. J. Ren. Nutr. 2022, 32, 87–93. [Google Scholar] [CrossRef] [PubMed]
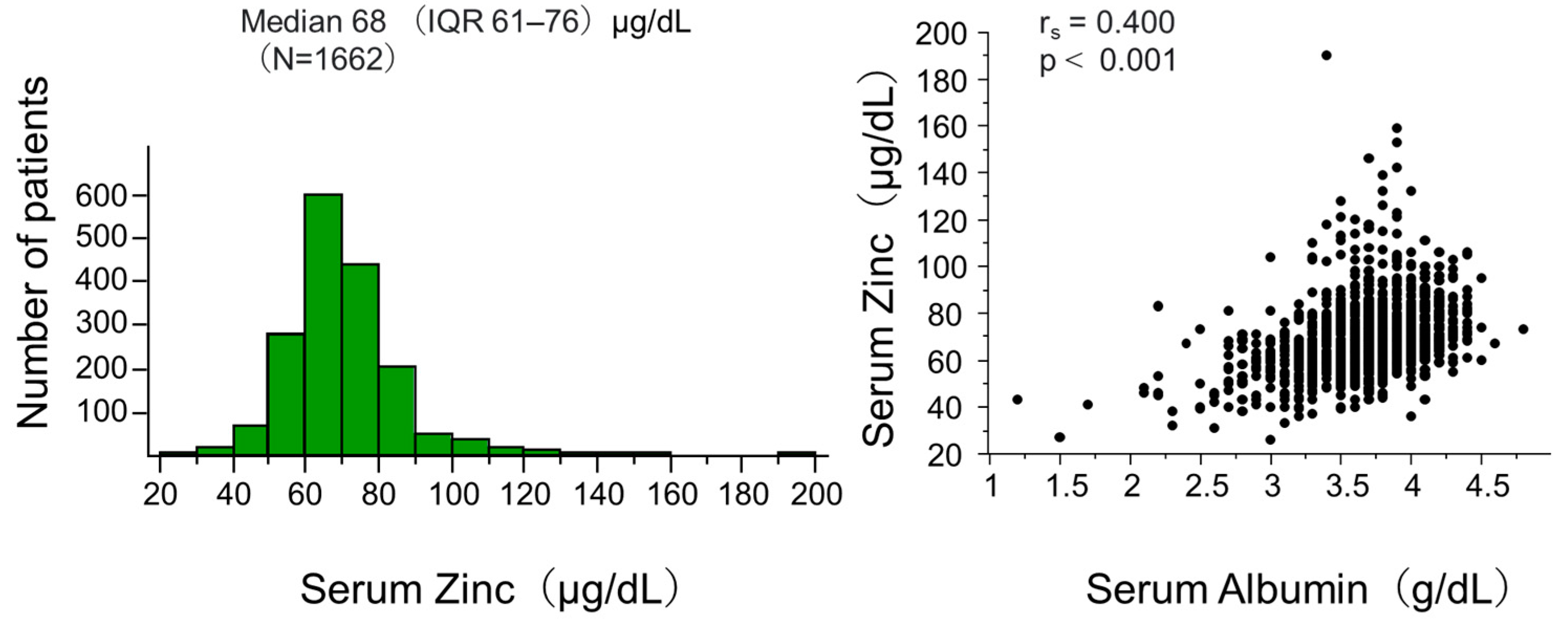
| Variable | Unit | Overall (n = 1662) | Serum Zinc Quartile | p Values across Quartiles | |||
|---|---|---|---|---|---|---|---|
| Q1 (n =459) | Q2 (n =416) | Q3 (n = 408) | Q4 (n =379) | ||||
| Zinc | μg/dL | 68 (61, 76) | 57 (52, 59) | 65 (63, 67) | 72 (70, 74) | 83 (80, 90) | <0.001 |
| Age | year | 68 (60, 75) | 71 (64, 81) | 69 (61, 75) | 65 (59, 73) | 64 (55, 71) | <0.001 |
| Male sex | N (%) | 1048 (63.1) | 295 (65.4) | 262 (37.0) | 247 (60.5) | 244 (64.4) | 0.640 |
| Body mass index | kg/m2 | 21.1 (19, 23.6) | 20.6 (18.7, 22.9) | 21.3 (18.9, 23.7) | 21.3 (19.3, 23.6) | 21.5 (19.84, 21.2) | <0.001 |
| Dialysis vintage | month | 64 (29, 133) | 54 (24, 118) | 73 (29, 141) | 66 (32, 132) | 68 (29, 138) | 0.046 |
| Hypertension | N (%) | 1498 (90.3) | 405 (88.2) | 375 (90.3) | 374 (91.9) | 344 (91.0) | 0.305 |
| Dyslipidemia | N (%) | 1029 (61.9) | 265 (57.7) | 272 (65.4) | 254 (62.3) | 238 (62.8) | 0.129 |
| Diabetic kidney disease | N (%) | 676 (40.7) | 170 (37.0) | 162 (38.4) | 182 (44.6) | 162 (42.7) | 0.097 |
| Prior CVD | N (%) | 634 (38.1) | 190 (41.4) | 143 (34.8) | 149 (36.5) | 152 (40.1) | 0.131 |
| Smoker | N (%) | 254 (15.3) | 59 (12.9) | 60 (14.4) | 60 (14.7) | 75 (19.8) | 0.149 |
| Hemoglobin | g/dL | 10.6 (10.0, 11.3) | 10.6 (9.8, 11.2) | 10.7 (10.0, 11.2) | 10.6 (10.0, 11.4) | 10.6 (10.0, 11.3) | 0.222 |
| Use of IV iron | N (%) | 443 (26.7) | 120 (26.1) | 111 (26.7) | 112 (27.5) | 100 (26.4) | 0.976 |
| Use of ESA | N (%) | 1369 (82.4) | 394 (85.8) | 320 (76.9) | 343 (95.1) | 312 (82.3) | 0.004 |
| Calcium | mg/dL | 8.9 (8.5, 9.4) | 8.8 (8.3, 9.2) | 8.9 (8.4, 9.5) | 9.0 (8.6, 9.5) | 9.0 (8.6, 9.5) | <0.001 |
| Phosphate | mg/dL | 5.1 (4.3, 5.9) | 4.8 (4.0, 5.8) | 5.0 (4.3, 5.7) | 5.1 (4.3 5.8) | 5.5 (4.5, 6.3) | <0.001 |
| intact PTH | pg/mL | 115 (60, 187) | 115 (56, 182) | 122 (64, 195) | 118 (72, 202) | 107 (54, 178) | 0.066 |
| Use of VDRAs | N (%) | 1113 (68.2) | 307 (66.9) | 272 (65.4) | 292 (71.6) | 262 (69.1) | 0.244 |
| Use of Calcimimetics | N (%) | 334 (20.0) | 90 (19.6) | 99 (23.8) | 70 (17.2) | 75 (19.8) | 0.120 |
| C-reactive protein | mg/dL | 0.1 (0.05, 0.32) | 0.16 (0.07, 0.64) | 0.10 (0.06, 0.31) | 0.10 (0.05, 0.24) | 0.10 (0.05, 0.21) | <0.001 |
| Albumin | g/dL | 3.7 (3.5, 3.9) | 3.5 (3.3, 3.7) | 3.7 (3.3, 3.7) | 3.8 (3.6, 4.0) | 3.8 (3.6, 4.0) | <0.001 |
| Variable | Unadjusted | Adjusted Model 1 | Adjusted Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | |
| Zinc | 0.77 | 0.71–0.84 | <0.001 | 0.88 | 0.81–0.96 | 0.006 | 0.95 | 0.87–1.04 | 0.300 |
| Age | 1.07 | 1.06–1.08 | <0.001 | 1.07 | 1.05–1.08 | <0.001 | 1.06 | 1.05–1.07 | <0.001 |
| Male sex | 1.13 | 0.94–1.37 | 0.197 | 1.15 | 0.94–1.42 | 0.158 | 1.20 | 0.98–1.47 | 0.085 |
| Body mass index | 0.91 | 0.88–0.93 | <0.001 | 0.93 | 0.90–0.96 | <0.001 | 0.94 | 0.91–0.97 | <0.001 |
| Dialysis vintage | 0.99 | 1.00–1.00 | 0.183 | 1.00 | 1.00–1.00 | 0.071 | 1.00 | 1.00–1.00 | 0.164 |
| Hypertension | 0.82 | 0.61–1.09 | 0.171 | 0.66 | 0.48–0.90 | 0.008 | 0.70 | 0.51–0.95 | 0.021 |
| Dyslipidemia | 1.08 | 0.90–1.30 | 0.421 | 1.00 | 0.83–1.22 | 0.957 | 0.98 | 0.80–1.19 | 0.815 |
| Diabetic kidney disease | 1.47 | 1.22–1.76 | <0.001 | 1.65 | 1.35–2.02 | <0.001 | 1.68 | 1.37–2.05 | <0.001 |
| Prior CVD | 2.29 | 1.91–2.75 | <0.001 | 1.87 | 1.55–2.27 | <0.001 | 1.83 | 1.51–2.22 | <0.001 |
| Smoker | 0.76 | 0.57–1.00 | 0.051 | 1.16 | 0.87–1.56 | 0.317 | 1.19 | 0.89–1.60 | 0.236 |
| Hemoglobin | 0.91 | 0.84–0.98 | 0.019 | 1.00 | 0.92–1.09 | 0.947 | 1.06 | 0.97–1.15 | 0.215 |
| Use of IV iron | 0.94 | 0.76–1.16 | 0.565 | 0.93 | 0.75–1.15 | 0.509 | 0.94 | 0.76–1.17 | 0.604 |
| Use of ESA | 1.08 | 0.85–1.38 | 0.525 | 1.11 | 0.86–1.43 | 0.433 | 1.09 | 0.84–1.41 | 0.520 |
| Calcium | 0.78 | 0.69–0.88 | <0.001 | 0.93 | 0.81–1.06 | 0.275 | 1.05 | 0.91–1.21 | 0.491 |
| Phosphate | 0.84 | 0.78–0.91 | <0.001 | 0.97 | 0.90–1.05 | 0.470 | 1.00 | 0.92–1.08 | 0.938 |
| intact PTH | 1.00 | 1.00–1.00 | 0.943 | 1.00 | 1.00–1.00 | 0.999 | 1.00 | 1.00–1.00 | 0.999 |
| Use of VDRAs | 0.81 | 0.67–0.98 | 0.027 | 1.00 | 0.82–1.22 | 0.978 | 1.03 | 0.84–1.26 | 0.794 |
| Use of Calcimimetics | 0.63 | 0.49–0.82 | <0.001 | 0.88 | 0.66–1.17 | 0.387 | 0.95 | 0.71–1.26 | 0.721 |
| C-reactive protein | 1.11 | 1.07–1.15 | <0.001 | 1.10 | 1.04–1.15 | <0.001 | 1.06 | 1.01–1.12 | 0.031 |
| Albumin | 0.26 | 0.21–0.32 | <0.001 | — | — | — | 0.42 | 0.31–0.56 | <0.001 |
| Patient Groups | N | Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|---|---|
| Serum Albumin (g/dL) | Serum Zinc (μg/dL) | HR | 95%(CI) | p | HR | 95%(CI) | p | |
| ≤3.7 | ≤68 | 613 | 1.64 | 1.29–2.10 | <0.001 | 1.31 | 1.02–1.69 | 0.036 |
| >68 | 331 | 1.00 | Ref. | — | 1.00 | Ref. | — | |
| >3.7 | ≤68 | 262 | 1.27 | 0.91–1.77 | 0.157 | 1.15 | 0.82–1.61 | 0.429 |
| >68 | 456 | 1.00 | Ref. | — | 1.00 | Ref. | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, S.; Shoji, T.; Morioka, F.; Nakaya, R.; Ueda, M.; Uedono, H.; Tsuda, A.; Morioka, T.; Fujii, H.; Yoshida, H.; et al. Association between Serum Zinc and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: The Osaka Dialysis Complication Study (ODCS). Nutrients 2024, 16, 3270. https://doi.org/10.3390/nu16193270
Nakatani S, Shoji T, Morioka F, Nakaya R, Ueda M, Uedono H, Tsuda A, Morioka T, Fujii H, Yoshida H, et al. Association between Serum Zinc and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: The Osaka Dialysis Complication Study (ODCS). Nutrients. 2024; 16(19):3270. https://doi.org/10.3390/nu16193270
Chicago/Turabian StyleNakatani, Shinya, Tetsuo Shoji, Fumiyuki Morioka, Rino Nakaya, Mayuko Ueda, Hideki Uedono, Akihiro Tsuda, Tomoaki Morioka, Hisako Fujii, Hisako Yoshida, and et al. 2024. "Association between Serum Zinc and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: The Osaka Dialysis Complication Study (ODCS)" Nutrients 16, no. 19: 3270. https://doi.org/10.3390/nu16193270
APA StyleNakatani, S., Shoji, T., Morioka, F., Nakaya, R., Ueda, M., Uedono, H., Tsuda, A., Morioka, T., Fujii, H., Yoshida, H., Mori, K., & Emoto, M. (2024). Association between Serum Zinc and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis: The Osaka Dialysis Complication Study (ODCS). Nutrients, 16(19), 3270. https://doi.org/10.3390/nu16193270







