Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review
Abstract
1. Introduction
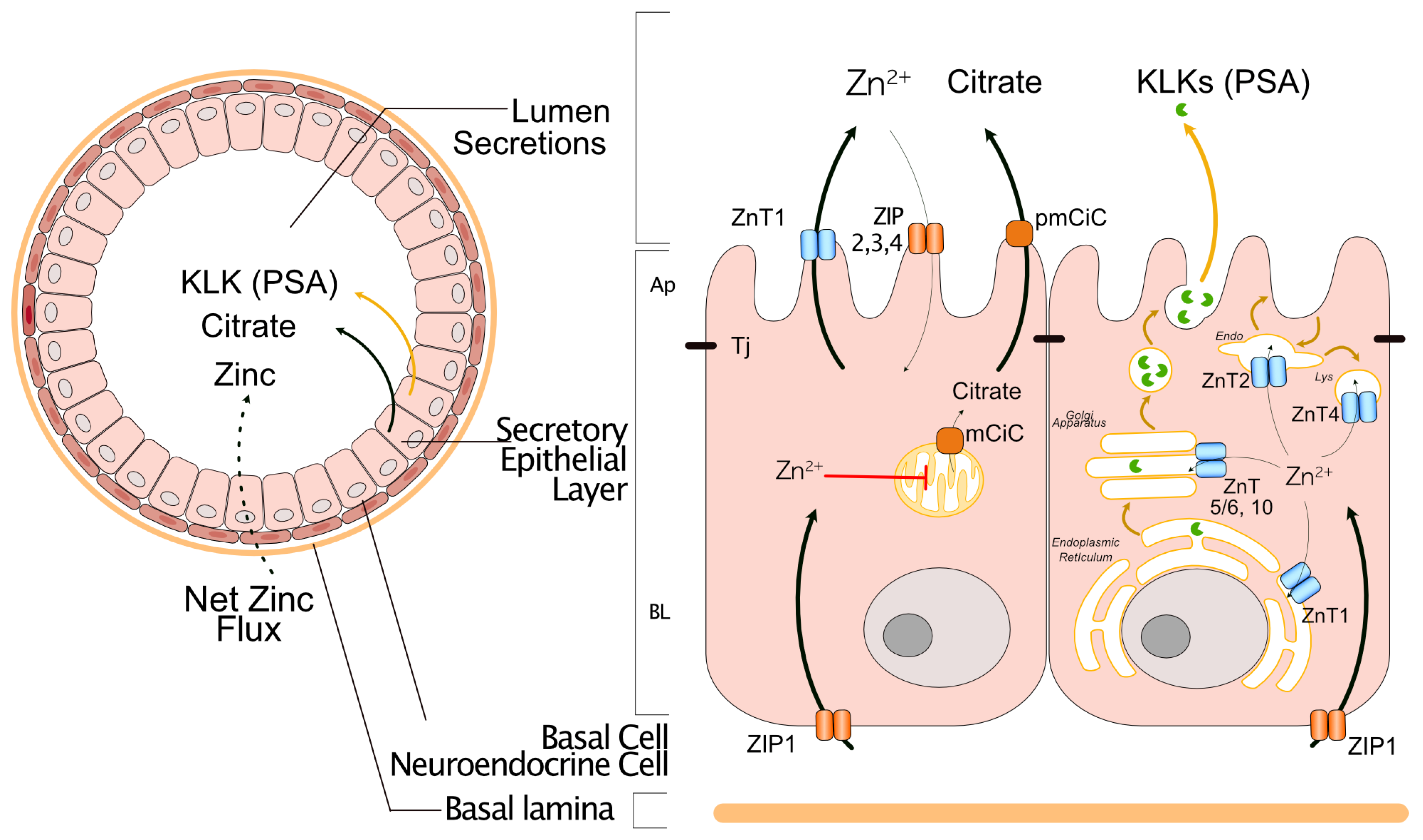
2. Zinc Homeostasis in the Normal Prostate Gland
3. Dysregulation of Zinc and Zinc Transporters Contributes to Pathological Mechanisms in Prostate Cancer
| ZIPs (Gene Name) | Human Prostate Cancer | Prostate Cancer Cell Lines/Prostate Cancer Transgenic Mouse or Other Cancer | References |
|---|---|---|---|
| ZIP1 (SLC39A1) | DOWN, adenocarcinomatous glands negative in 15 of 22 samples, 68% a,b. | DOWN, ZIP1 protein in TRAMP mouse model b,c. | [3,80] |
| DOWN in TMPRSS2-ERG.Pten and Hi-Myc | [64] | ||
| ZIP2 (SLC9A2) | DOWN, adenocarcinomatous glands negative in 21 of 24 samples, 87.5% b | DOWN in TMPRSS2-ERG.Pten and Hi-Myc | [39,80] |
| ZIP3 (Slc3SLC9A3) | DOWN, adenocarcinomatous glands negative in 21 of 24 samples, 87.5% b | DOWN in TMPRSS2-ERG.Pten and Hi-Myc | [39,80] |
| ZIP4 (SLC39A4) | DOWN in prostate cancer tissue sample (n = 14) compared with BPH (n = 20), samples collected in China Medical University a. Downregulated in 3 of 4 adenocarcinoma samples, compared with BPH (n = 4) c | UP, in prostate cancer cell lines 22RV1 and PC3, compared to DU145 a. | [40] |
| DOWN, in prostate cancer cell line DU145 c | |||
| ZIP5, 6, 7 (SLC39A5, 6 and 7) | Unknown | Downregulated after incubation with zinc for 6 h | [70] |
| ZIP8 (SLC39A8) | N/A | UP, in prostate cancer cell lines, BPH-1, DU145, C4-2B, LNCaP and PC3 compared to normal prostate cancer cell lines (RWPE-1, RWPE-W99, CF-91, MLC8891) c | [76] |
| ZIP9 (SLC39A9) | UP, in human prostate cancer tissues (acinar adenocarcinoma) paired (n = 4) a. | N/A | [71,72,73] |
| ZIP10, 11, 12 and 13 (SLC39A10, 11, 12 and 13). | Unknown in prostate cancer tissue. They were upregulated in other cancer tissues, ZIP10 in human hepatocellular carcinoma compared to normal liver samples (n = 20) a and (n= 70 out of 95 HCC tissues) b, samples collected in China, Guangdong Provincial People’s Hospital. | Downregulated in PC3 after incubation with zinc for 6 h | [70,81,82,83,84] |
| ZIP14 (SLC39A14) | DOWN, in prostate cancer (n = 150) compared with benign (n = 29) a. Down, ZIP14 protein level (n = 98) compared with benign (n = 81) c. | N/A | [85] |
| Zinc Transporters Gene/Protein | Human Prostate Cancer | Evidence from Prostate Cancer Cell lines/Prostate Cancer Transgenic Mouse or Other Cancer | References |
|---|---|---|---|
| ZnT1 (SLC30A1) | UP, in prostate tumor from European American (n = 13). Non-change, in prostate tumor from African American (n = 12) a. | DOWN, in prostate cancer cell line * a. UP, in TMPRSS2-ERG.Pten and Hi-Myc | [57,80] |
| ZnT2 (SLC 30A2) | No change or non-detected in human prostate cancer tissue samples (n = 25) a. | DOWN, in human prostate cell line * except C4-2 a. UP, in TMPRSS2-ERG.Pten and Hi-Myc | [57] |
| ZnT4 (SLC30A4) | No changes in prostate tumor from European American or African American a. Upregulated in cancer grade group 3 a. | UP, LNCaP, C4-2, and MDA PCa 2b are known to be derived from a metastatic site a. | [57,86] |
| DOWN, with weak staining in 112 (75%) and moderate or strong staining in only 37 (25%) cases. | DOWN, in most prostate cancer cell line DU145, 22Rν 1, PC3 a. | ||
| ZnT5 (SLC30A5) | DOWN, PCa tissue in both EA and AA populations (n total= 25) a. | DOWN, in all tested prostate cancer cell lines * except MDA PCa 2b a. | [57] |
| ZnT6 (SLC30A6) | DOWN, PCa tissue in both EA and AA populations (n total= 25) a. | DOWN, in all human PCa cell lines * a. | [57] |
| ZnT7 (SLC30A7) | Non-significative changes or non-detected in human prostate cancer tissue sample analyses (n = 25) a. | DOWN, in all human PCa cell lines * a. Knockout mice of SLC30A7 accelerate prostate tumor formation in TRAMP mice (65% in 2 weeks), suggesting insufficient SLC30A7 activity may contribute to PCa progression. | [57,87] |
| ZnT9 (SLC30A9) | UP, in combined analysis, samples from European and African Americans (n = 25) a. | UP, in all human PCa cell lines * except in PC3 and 22Rv1 a. UP, in TMPRSS2-ERG.Pten and Hi-Myc | [57,80] |
| ZnT10 (SLC30A10) | UP, in prostate tumors from European Americans (n= 13) and in prostate tumors from African Americans (n = 12) a. | UP, in prostate cancer cell line 22Rν 1, LNCaP, C4-2, and MDA PCa 2b a. UP, in TMPRSS2-ERG.Pten and Hi-Myc | [57,80] |
| DOWN, in prostate cancer cell line DU145, PC3, E006AA-Par, and E006AA-HT a. |
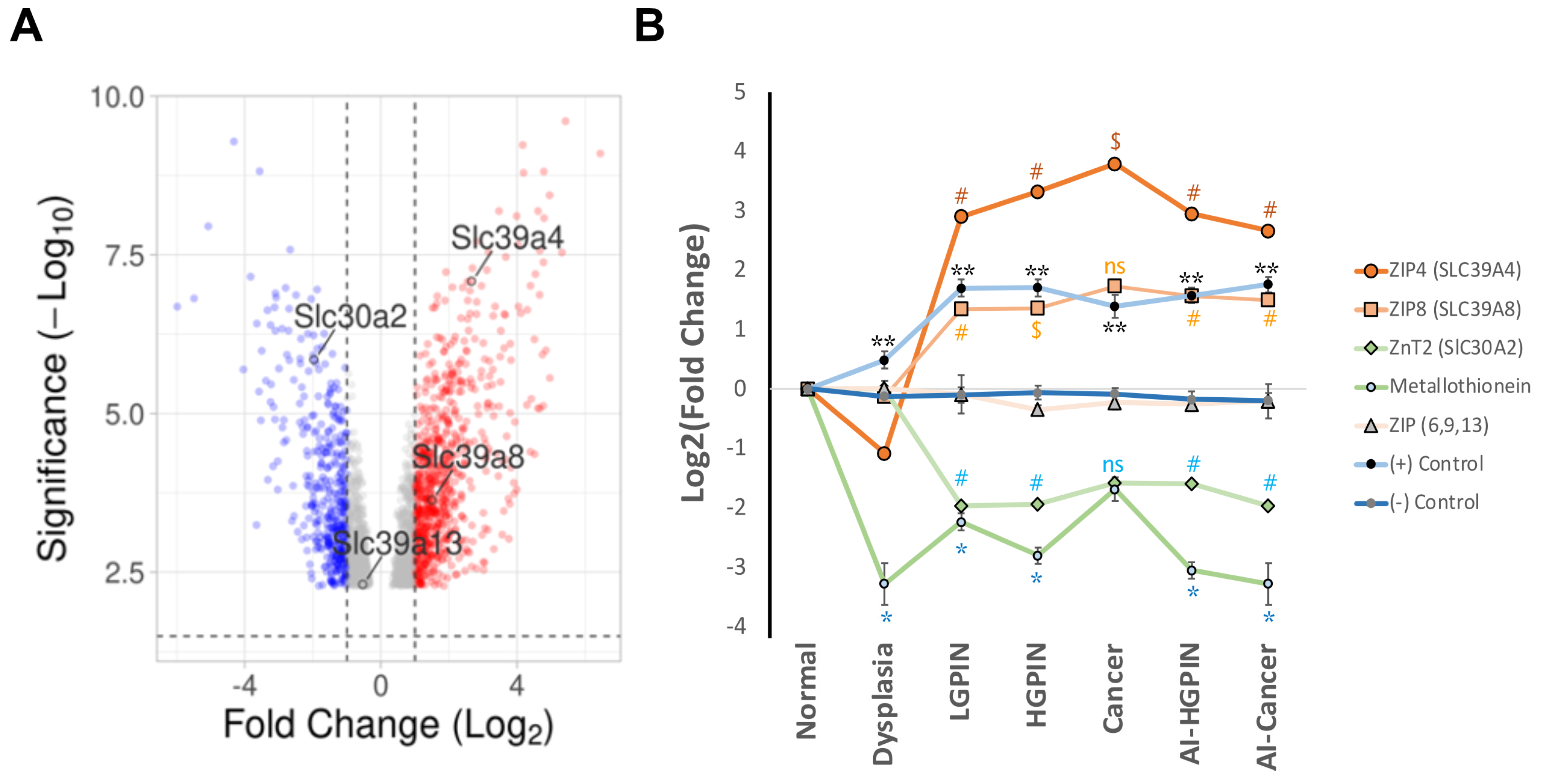
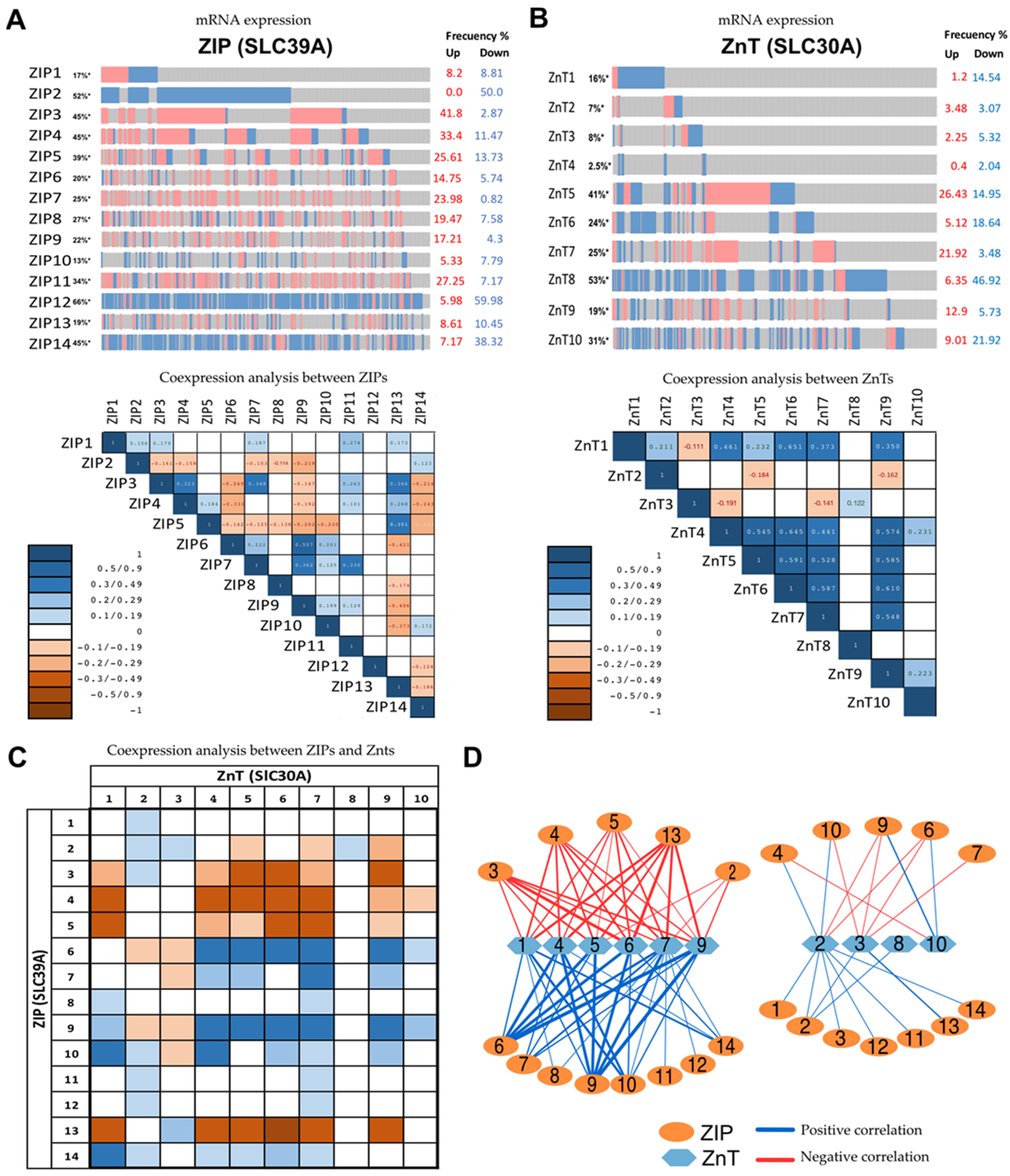
4. Exploring Different Patterns of Expressions of Zinc Transporter by In Silico Analysis of Available mRNAs Databases
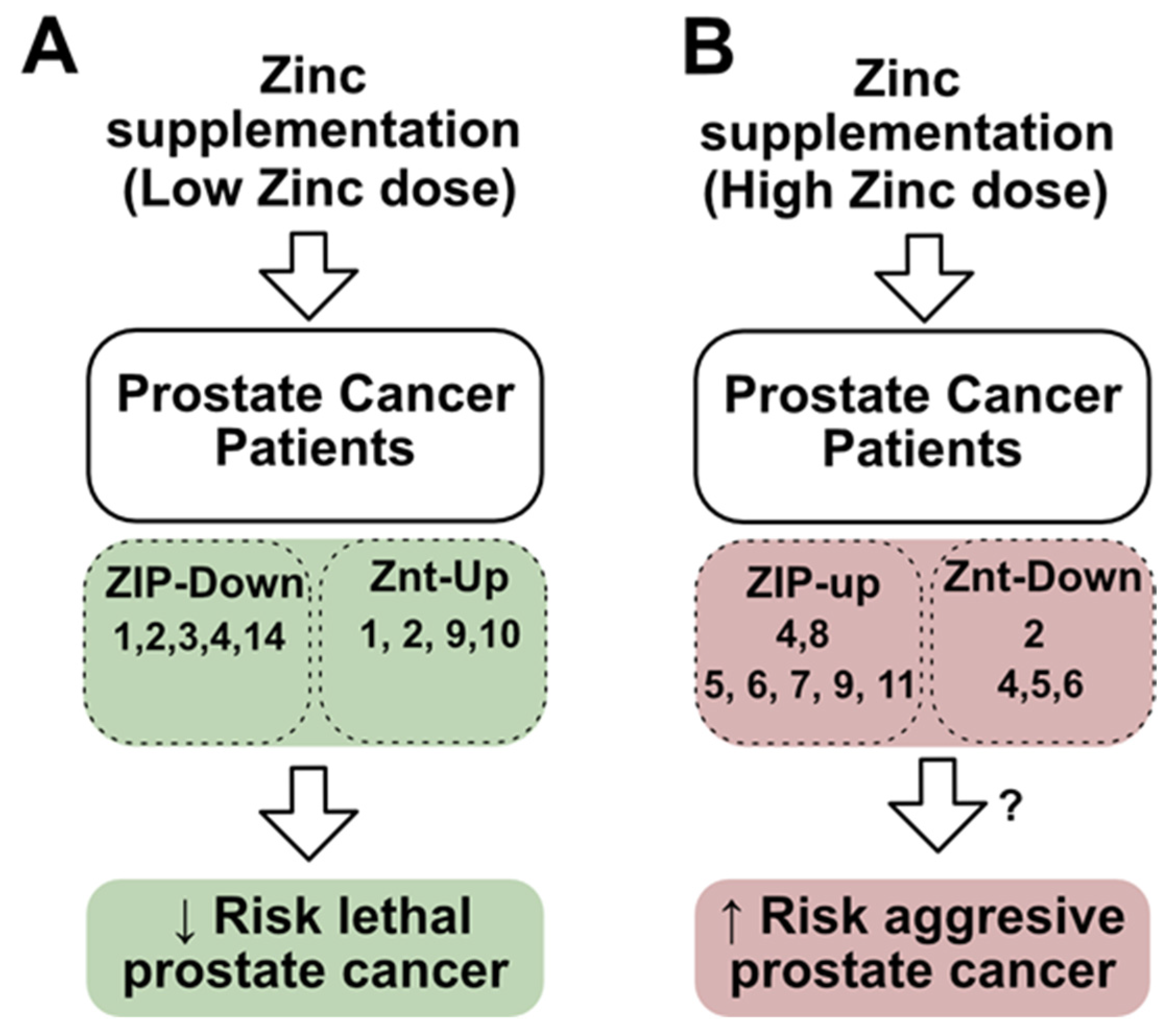
5. Could the Dysregulation of Zinc Transporters Affect Zinc Supplementation Treatments?
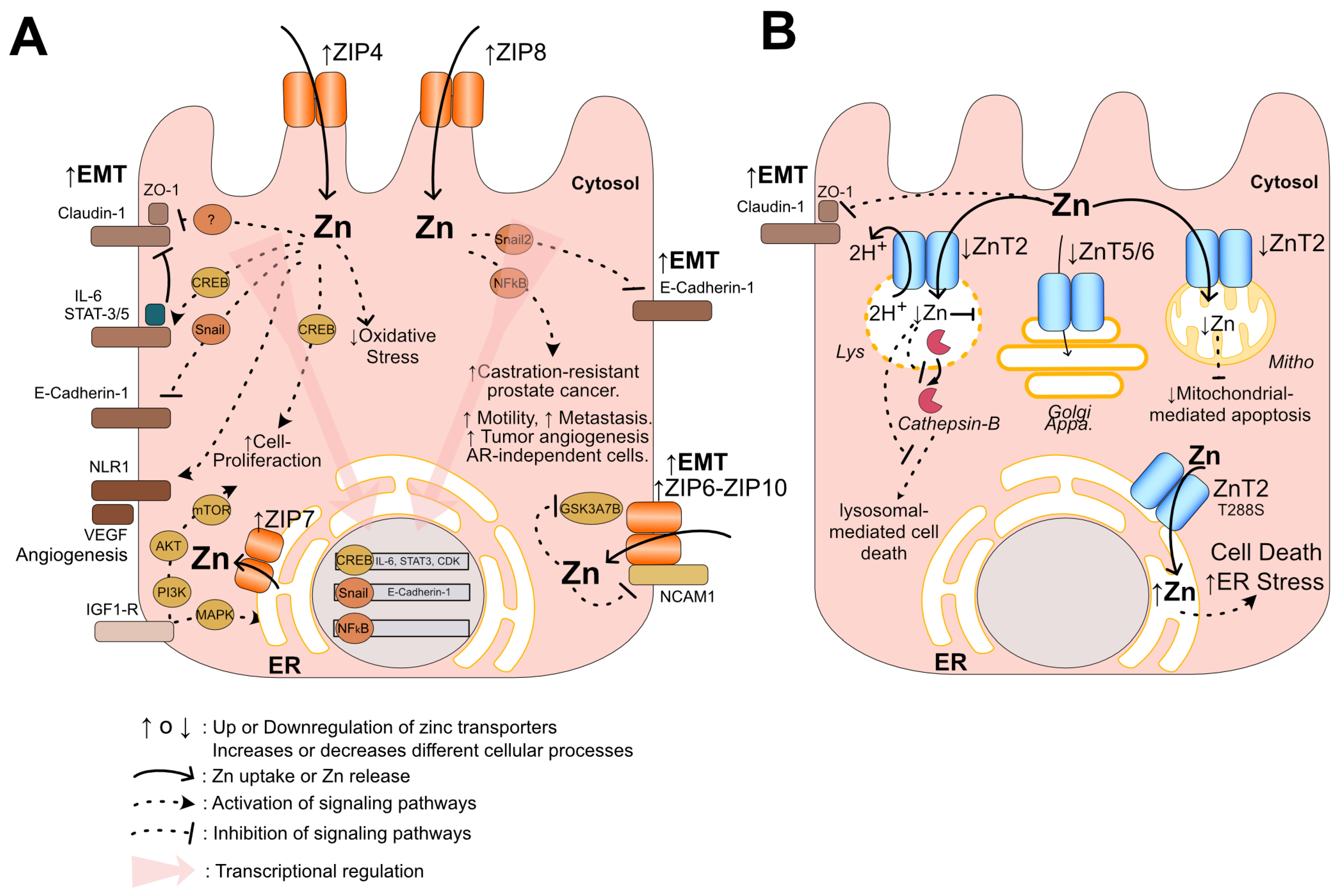
6. Potential Mechanisms of Pattern ZIP-Up/ZnT-Down in Prostate Cancer Progression
7. Summary and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI cancer | Androgen-independent cancer |
| AR | Androgen receptor |
| AKT | Akt serine/threonine kinase family |
| AP2 | Adaptor protein complex 2 |
| ALP | Alkaline phosphatase |
| ATF | Activating transcription factor |
| ATX | Autotaxin |
| Axn2 | Axis inhibitor protein 2 |
| BAFFR | B-cell activating factor receptor |
| BPH | Benign prostate dysplasia |
| CDC42 | Cell division cycle 42 protein |
| CHOP | C/EBP homologous protein |
| CREB | cAMP responsive element binding protein |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ERG | ETS-related gene |
| ER | Endoplasmic reticulum |
| Erp44 | Endoplasmic Reticulum Protein 44 |
| ETS | Erythroblast transformation-specific |
| GEO2R | Gene expression omnibus |
| GSK3A | Glycogen Synthase Kinase 3 Alpha |
| HGPIN | High prostatic intraepithelial neoplasia |
| IHC | Inmunohistochemistry |
| IGF-1 | Insulin-like growth factor 1 |
| IGF1-R | Insulin-like growth factor 1-receptor |
| IL-1R | Interleukin-1 receptor |
| IL-6 | Interleukin 6 |
| KLK | Kallikrein-related peptidase |
| LCD | Lysosomal-mediated cell death |
| LMP | Lysosomal membrane permeabilization |
| LGPIN | Low prostatic intraepithelial neoplasia |
| MAPK | Mitogen-activated protein kinase |
| MEC | Mammary epithelium |
| MEF | Mouse fibroblast cells |
| MMP-9 | Metalloproteinase |
| MT | Metallothioneins |
| MTF-1 | Metal regulatory transcription factor 1 |
| mTOR | Mechanistic Target of Rapamycin |
| MYC | Myelocytomatosis oncogene |
| NCAM1 | Neural cell adhesion molecule 1 |
| NCBI | National Center for Biotechnology Information |
| NF-kB | Nuclear factor kappa B |
| NKX3.1 | NK3 homeobox 1 |
| NLR-1 | Receptor neuropilin-1 |
| PAR | Partitioning defective protein |
| PI3K | Phosphatidylinositol 3-kinase |
| PSA | Prostate-specific antigen |
| PTEN | Phosphatase and tensin homolog |
| PTP1B | protein-tyrosine phosphatase 1B |
| RREB-1 | Ras responsive element binding protein 1 |
| SNAIL | Snail family transcriptional repressor 1 |
| STAT-3 | Signal transducer and activator of transcription 3 |
| TGFb | Transforming growth factor-beta |
| TMPRSS2 | Transmembrane protease serine 2 gene |
| TNF-α | Tumor necrosis factor-alpha |
| TNFR | Tumor necrosis factor receptor |
| TRAMP | Transgenic adenocarcinoma of the mouse prostate |
| TP53 | Tumor protein p53 |
| UPR | Unfolded protein response |
| VEGF | Vascular endothelial growth factor |
| ZIPs | Zn-regulated, Iron-regulated transporter-like proteins (Zn influx proteins) |
| ZnTs | Zinc efflux transporter proteins |
| ZO-1 | Zonula occludens-1 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. HZIP1 Zinc Uptake Transporter down Regulation and Zinc Depletion in Prostate Cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. A Comprehensive Review of the Role of Zinc in Normal Prostate Function and Metabolism; and Its Implications in Prostate Cancer. Arch. Biochem. Biophys. 2016, 611, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Zinc Is Decreased in Prostate Cancer: An Established Relationship of Prostate Cancer! JBIC J. Biol. Inorg. Chem. 2011, 16, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Stanford, J.L.; Cohen, J.H.; Wicklund, K.; Patterson, R.E. Vitamin and Mineral Supplement Use Is Associated with Reduced Risk of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 1999, 8, 887–892. [Google Scholar]
- Gonzalez, A.; Peters, U.; Lampe, J.W.; White, E. Zinc Intake from Supplements and Diet and Prostate Cancer. Nutr. Cancer 2009, 61, 206–215. [Google Scholar] [CrossRef]
- Feng, P.; Li, T.L.; Guan, Z.X.; Franklin, R.B.; Costello, L.C. Effect of Zinc on Prostatic Tumorigenicity in Nude Mice. Ann. N. Y. Acad. Sci. 2003, 1010, 316–320. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Liu, Y.-Y.; Zou, J.; Franklin, R.B.; Costello, L.C.; Feng, P. Inhibitory Effect of Zinc on Human Prostatic Carcinoma Cell Growth. Prostate 1999, 40, 200–207. [Google Scholar] [CrossRef]
- Epstein, M.M.; Kasperzyk, J.L.; Andrén, O.; Giovannucci, E.L.; Wolk, A.; Håkansson, N.; Andersson, S.O.; Johansson, J.E.; Fall, K.; Mucci, L.A. Dietary Zinc and Prostate Cancer Survival in a Swedish Cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Mukhtar, H.; Beck, F.W.J.; Adhami, V.M.; Siddiqui, I.A.; Din, M.; Hafeez, B.B.; Kucuk, O. Dietary Zinc and Prostate Cancer in the TRAMP Mouse Model. J. Med. Food 2010, 13, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Siddiqui, I.A.; Panackal, J.E.; Mintie, C.A.; Ahmad, N. Quercetin–Resveratrol Combination for Prostate Cancer Management in TRAMP Mice. Cancers 2020, 12, 2141. [Google Scholar] [CrossRef] [PubMed]
- Gallus, S.; Foschi, R.; Negri, E.; Talamini, R.; Franceschi, S.; Montella, M.; Ramazzotti, V.; Tavani, A.; Dal Maso, L.; La Vecchia, C. Dietary Zinc and Prostate Cancer Risk: A Case-Control Study from Italy. Eur. Urol. 2007, 52, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Kolonel, L.N.; Yoshizawa, C.N.; Hankin, J.H. Diet and Prostatic Cancer: A Case-Control Study in Hawaii. Am. J. Epidemiol. 1988, 127, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Coogan, P.; Palmer, J.R.; Strom, B.L.; Rosenberg, L. Vitamin and Mineral Use and Risk of Prostate Cancer: The Case-Control Surveillance Study. Cancer Causes Control 2009, 20, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Zinc Supplement Use and Risk of Aggressive Prostate Cancer: A 30-Year Follow-up Study. Eur. J. Epidemiol. 2022, 37, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and Phenotypic Heterogeneity in Prostate Cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Wu, B.; Lu, X.; Shen, H.; Yuan, X.; Wang, X.; Yin, N.; Sun, L.; Shen, P.; Hu, C.; Jiang, H.; et al. Intratumoral Heterogeneity and Genetic Characteristics of Prostate Cancer. Int. J. Cancer 2020, 146, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V. A Systematic Review of the Zinc Content of the Normal Human Prostate Gland. Biol. Trace Elem. Res. 2021, 199, 3593–3607. [Google Scholar] [CrossRef] [PubMed]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The Emerging Role of Zinc Transporters in Cellular Homeostasis and Cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [PubMed]
- Mosaoa, R.; Kasprzyk-Pawelec, A.; Fernandez, H.R.; Avantaggiati, M.L. The Mitochondrial Citrate Carrier Slc25a1/Cic and the Fundamental Role of Citrate in Cancer, Inflammation and Beyond. Biomolecules 2021, 11, 141. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.P.; Prasad, P.D.; Gopal, E.; Fraser, S.P.; Bolt, L.; Rizaner, N.; Palmer, C.P.; Foster, C.S.; Palmieri, F.; Ganapathy, V.; et al. Molecular Origin of Plasma Membrane Citrate Transporter in Human Prostate Epithelial Cells. EMBO Rep. 2010, 11, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Karunasinghe, N. Zinc in Prostate Health and Disease: A Mini Review. Biomedicines 2022, 10, 3206. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Hellman, J.; Hogg, P.; Lilja, H. Enzymatic Action of Prostate-Specific Antigen (PSA or HK3): Substrate Specificity and Regulation by Zn2+, a Tight-Binding Inhibitor. Prostate 2000, 45, 132–139. [Google Scholar] [CrossRef]
- Anamthathmakula, P.; Winuthayanon, W. Mechanism of Semen Liquefaction and Its Potential for a Novel Non-Hormonal Contraception. Biol. Reprod. 2020, 103, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Verze, P.; Cai, T.; Lorenzetti, S. The Role of the Prostate in Male Fertility, Health and Disease. Nat. Rev. Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Prostatic Fluid Electrolyte Composition for the Screening of Prostate Cancer: A Potential Solution to a Major Problem. Prostate Cancer Prostatic Dis. 2009, 12, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hong, S.K. Kallikreins as Biomarkers for Prostate Cancer. BioMed Res. Int. 2014, 2014, 526341. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Taylor, K.M.; Fu, D. Zinc Transporters and Their Functional Integration in Mammalian Cells. J. Biol. Chem. 2021, 296, 100320. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T. An Overview of a Wide Range of Functions of ZnT and Zip Zinc Transporters in the Secretory Pathway. Biosci. Biotechnol. Biochem. 2011, 75, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc Dysregulation in Cancers and Its Potential as a Therapeutic Target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of Zinc Signaling Studies in Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 667. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.-T.T.; Parrott, D.; Jordan, M.V.C.; Joseph, D.B.; Strand, D.; Lo, U.-G.G.; Lin, H.; Darehshouri, A.; Sherry, A.D. The Roles of ZnT1 and ZnT4 in Glucose-Stimulated Zinc Secretion in Prostate Epithelial Cells. Mol. Imaging Biol. 2021, 23, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Usui, S.; Inoue, T.; Sugimura, Y.; Tatematsu, M.; Hirano, K. High-Level Expression of Zinc Transporter-2 in the Rat Lateral and Dorsal Prostate. J. Androl. 2002, 23, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, T.; Zeng, W.; Jiang, Y.; Bai, X.C. Cryo-EM Structures of Human ZnT8 in Both Outward- and Inward-Facing Conformations. Elife 2020, 9, e58823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal Structures of a ZIP Zinc Transporter Reveal a Binuclear Metal Center in the Transport Pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef] [PubMed]
- Desouki, M.M.; Geradts, J.; Milon, B.; Franklin, R.B.; Costello, L.C. HZip2 and HZip3 Zinc Transporters Are down Regulated in Human Prostate Adenocarcinomatous Glands. Mol. Cancer 2007, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Z.; Yang, Q.; Shan, G.; Yu, X.; Kong, C. The Role of Zinc Transporter ZIP4 in Prostate Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Dufner-Beattie, J.; Wang, F.; Kuo, Y.M.; Gitschier, J.; Eide, D.; Andrews, G.K. The Acrodermatitis Enteropathica Gene ZIP4 Encodes a Tissue-Specific, Zinc-Regulated Zinc Transporter in Mice. J. Biol. Chem. 2003, 278, 33474–33481. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Wang, F.; Dufner-Beattie, J.; Andrews, G.K.; Eide, D.J.; Petris, M.J. Zn2+-Stimulated Endocytosis of the MZIP4 Zinc Transporter Regulates Its Location at the Plasma Membrane. J. Biol. Chem. 2004, 279, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. Zinc Transporter 1 (ZNT1) Expression on the Cell Surface Is Elaborately Controlled by Cellular Zinc Levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Kurokawa, Y.; Chiche, J.; Pouysségur, J.; Sato, H.; Fukuzawa, H.; Nagao, M.; Kambe, T. Dissecting the Process of Activation of Cancer-Promoting Zinc-Requiring Ectoenzymes by Zinc Metalation Mediated by ZNT Transporters. J. Biol. Chem. 2017, 292, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Matsunaga, M.; Takeda, T.A. Understanding the Contribution of Zinc Transporters in the Function of the Early Secretory Pathway. Int. J. Mol. Sci. 2017, 18, 2179. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Findley, S.D. ZnT-2, a Mammalian Protein That Confers Resistance to Zinc by Facilitating Vesicular Sequestration. EMBO J. 1996, 15, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hennigar, S.R.; Alam, S.; Nishida, K.; Kelleher, S.L. Essential Role for Zinc Transporter 2 (ZnT2)-Mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J. Biol. Chem. 2015, 290, 13064–13078. [Google Scholar] [CrossRef] [PubMed]
- Podany, A.B.; Wright, J.; Lamendella, R.; Soybel, D.I.; Kelleher, S.L. ZnT2-Mediated Zinc Import Into Paneth Cell Granules Is Necessary for Coordinated Secretion and Paneth Cell Function in Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Patrushev, N.; Seidel-Rogol, B.; Salazar, G. Angiotensin II Requires Zinc and Downregulation of the Zinc Transporters ZnT3 and ZnT10 to Induce Senescence of Vascular Smooth Muscle Cells. PLoS ONE 2012, 7, e33211. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Juang, H.-H.; Chung, L.-C.; Sung, H.-C.; Feng, T.-H.; Lee, Y.-H.; Chang, P.-L.; Tsui, K.-H. Metallothionein 3: An Androgen-Upregulated Gene Enhances Cell Invasion and Tumorigenesis of Prostate Carcinoma Cells. Prostate 2013, 73, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lichten, L.A.; Ryu, M.-S.; Liuzzi, J.P.; Wang, F.; Cousins, R.J. STAT5-Glucocorticoid Receptor Interaction and MTF-1 Regulate the Expression of ZnT2 (Slc30a2) in Pancreatic Acinar Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Ravindra, R.; Daniels, P.J.; Andrews, G.K. The Transcription Factor MTF-1 Mediates Metal Regulation of the Mouse ZnT1 Gene. J. Biol. Chem. 2000, 275, 34803–34809. [Google Scholar] [CrossRef]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic Reprogramming in Prostate Cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Costello, L.C.; Franklin, R.B. Zinc: The Wonder Drug for the Treatment of Carcinomas. Acta Sci. Cancer Biol. 2020, 4, 33–39. [Google Scholar] [CrossRef]
- Singh, C.K.; Malas, K.M.; Tydrick, C.; Siddiqui, I.A.; Iczkowski, K.A.; Ahmad, N. Analysis of Zinc-Exporters Expression in Prostate Cancer. Sci. Rep. 2016, 6, 36772. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Wang, C.-G.; Zhu, Y.-D.; Chen, W.-H.; Shao, S.-L.; Jiang, F.-N.; Liao, Q.-D. Decreased Expression of SLC 39A14 Is Associated with Tumor Aggressiveness and Biochemical Recurrence of Human Prostate Cancer. OncoTargets Ther. 2016, 9, 4197–4205. [Google Scholar] [CrossRef]
- Rishi, I.; Baidouri, H.; Abbasi, J.A.; Bullard-Dillard, R.; Kajdacsy-Balla, A.; Pestaner, J.P.; Skacel, M.; Tubbs, R.; Bagasra, O. Prostate Cancer in African American Men Is Associated With Downregulation of Zinc Transporters. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Kanak, M.A.; Kajdacsy-Balla, A.; Pestaner, J.P.; Bagasra, O. Differential Zinc Accumulation and Expression of Human Zinc Transporter 1 (HZIP1) in Prostate Glands. Methods 2010, 52, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Milon, B.C.; Agyapong, A.; Bautista, R.; Costello, L.C.; Franklin, R.B. Ras Responsive Element Binding Protein-1 (RREB-1) down-Regulates HZIP1 Expression in Prostate Cancer Cells. Prostate 2010, 70, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Milon, B.C.; Desouki, M.M.; Costello, L.C.; Franklin, R.B. HZIP1 Zinc Transporter Down-Regulation in Prostate Cancer Involves the Overexpression of Ras Responsive Element Binding Protein-1 (RREB-1). Prostate 2011, 71, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.B.; Golovine, K.V.; Kutikov, A.; Canter, D.J.; Rybko, V.A.; Roshchin, D.A.; Matveev, V.B.; Uzzo, R.G.; Kolenko, V.M. Reversal of Epigenetic Silencing of AP-2alpha Results in Increased Zinc Uptake in DU-145 and LNCaP Prostate Cancer Cells. Carcinogenesis 2011, 32, 1773–1781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Costello, L.C.; Franklin, R.B.; Zou, J.; Feng, P.; Bok, R.; Swanson, M.G.; Kurhanewicz, J. Human Prostate Cancer ZIP1/Zinc/Citrate Genetic/Metabolic Relationship in the TRAMP Prostate Cancer Animal Model. Cancer Biol. Ther. 2011, 12, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.M.; DeGraff, D.J.; Yu, X.; Jin, R.J.; Chen, Z.; Borowsky, A.D.; Matusik, R.J. Mouse Models of Prostate Cancer: Picking the Best Model for the Question. Cancer Metastasis Rev. 2014, 33, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.; Jing, R.; Smalley, K.J.; Wang, Z.X.; Taccioli, C.; Fan, S.; Chen, H.; Alder, H.; Huebner, K.; Farber, J.L.; et al. Human-like Hyperplastic Prostate with Low ZIP1 Induced Solely by Zn Deficiency in Rats. Proc. Natl. Acad. Sci. USA 2018, 115, E11091–E11100. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Aydemir, T.B.; Kim, J.; Cousins, R.J. Hepatic ZIP14-Mediated Zinc Transport Is Required for Adaptation to Endoplasmic Reticulum Stress. Proc. Natl. Acad. Sci. USA 2017, 114, E5805–E5814. [Google Scholar] [CrossRef] [PubMed]
- So, A.Y.-L.; de la Fuente, E.; Walter, P.; Shuman, M.; Bernales, S. The Unfolded Protein Response during Prostate Cancer Development. Cancer Metastasis Rev. 2009, 28, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Lessard, L.; Labbé, D.P.; Deblois, G.; Bégin, L.R.; Hardy, S.; Mes-Masson, A.-M.; Saad, F.; Trotman, L.C.; Giguère, V.; Tremblay, M.L. PTP1B Is an Androgen Receptor-Regulated Phosphatase That Promotes the Progression of Prostate Cancer. Cancer Res. 2012, 72, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wei, H.; Maeder, D.; Franklin, R.B.; Feng, P. Profiling of Zinc-Altered Gene Expression in Human Prostate Normal vs. Cancer Cells: A Time Course Study. J. Nutr. Biochem. 2009, 20, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and Characterization of Membrane Androgen Receptors in the ZIP9 Zinc Transporter Subfamily: II. Role of Human ZIP9 in Testosterone-Induced Prostate and Breast Cancer Cell Apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Converse, A.; Berg, H.A. ZIP9, a Novel Membrane Androgen Receptor and Zinc Transporter Protein. Gen. Comp. Endocrinol. 2018, 257, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Dong, J. Membrane Androgen Receptor Characteristics of Human ZIP9 (SLC39A) Zinc Transporter in Prostate Cancer Cells: Androgen-Specific Activation and Involvement of an Inhibitory G Protein in Zinc and MAP Kinase Signaling. Mol. Cell. Endocrinol. 2017, 447, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Dong, J. (-)-Epicatechin Acts as a Potent Agonist of the Membrane Androgen Receptor, ZIP9 (SLC39A9), to Promote Apoptosis of Breast and Prostate Cancer Cells. J. Steroid Biochem. Mol. Biol. 2021, 211, 105906. [Google Scholar] [CrossRef] [PubMed]
- Bulldan, A.; Bartsch, J.-W.; Konrad, L.; Scheiner-Bobis, G. ZIP9 but Not the Androgen Receptor Mediates Testosterone-Induced Migratory Activity of Metastatic Prostate Cancer Cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- McDermott, J.R.; Geng, X.; Jiang, L.; Gálvez-Peralta, M.; Chen, F.; Nebert, D.W.; Liu, Z. Zinc- and Bicarbonate-Dependent ZIP8 Transporter Mediates Selenite Uptake. Oncotarget 2016, 7, 35327–35340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feresin, R.G.; Falcon-Perez, J.M.; Salazar, G. Differential Targeting of SLC30A10/ZnT10 Heterodimers to Endolysosomal Compartments Modulates EGF-Induced MEK/ERK1/2 Activity. Traffic 2016, 17, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, H.J.; Thornton, J.K.; Coneyworth, L.J.; Ford, D.; Valentine, R.A. Efflux Function, Tissue-Specific Expression and Intracellular Trafficking of the Zn Transporter ZnT10 Indicate Roles in Adult Zn Homeostasis. Metallomics 2012, 4, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Guérin, O.; Fischel, J.L.; Ferrero, J.M.; Bozec, A.; Milano, G. EGFR Targeting in Hormone-Refractory Prostate Cancer: Current Appraisal and Prospects for Treatment. Pharmaceuticals 2010, 3, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.R.; Raina, K.; Mishra, N.; Tomar, M.S.; Kumar, R.; Palmer, A.E.; Maroni, P.; Agarwal, R. Stage-specific Differential Expression of Zinc Transporter SLC30A and SLC39A Family Proteins during Prostate Tumorigenesis. Mol. Carcinog. 2022, 61, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, Z.; Wang, S.; Zhou, Q.; Ma, Z.; Liu, C.; Huang, B.; Zheng, Z.; Yang, L.; Zou, Y.; et al. SLC39A10 Upregulation Predicts Poor Prognosis, Promotes Proliferation and Migration, and Correlates with Immune Infiltration in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 899–912. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Huo, R.; Zhi, Q.; Zhan, M.; Chen, X.; Hua, Z.-C. Increased Expression of Zinc Transporter ZIP4, ZIP11, ZnT1, and ZnT6 Predicts Poor Prognosis in Pancreatic Cancer. J. Trace Elem. Med. Biol. 2021, 65, 126734. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Wang, C. Analysis of the Prognostic Significance of Solute Carrier (SLC) Family 39 Genes in Breast Cancer. Biosci. Rep. 2020, 40, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, J.; Liu, C.; Jiang, T.; Yang, N.; Liu, D.; Zhao, H.; Xu, Z. Zinc Transporter SLC39A13/ZIP13 Facilitates the Metastasis of Human Ovarian Cancer Cells via Activating Src/FAK Signaling Pathway. J. Exp. Clin. Cancer Res. 2021, 40, 199. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wallace, M.B.; Yang, J.; Jiang, L.; Zhai, Q.; Zhang, Y.; Hong, C.; Chen, Y.; Frank, T.S.; Stauffer, J.A.; et al. ZIP4 Is a Novel Diagnostic and Prognostic Marker in Human Pancreatic Cancer: A Systemic Comparison Between EUS-FNA and Surgical Specimens. Curr. Mol. Med. 2014, 14, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Henshall, S.M.; Afar, D.E.H.; Rasiah, K.K.; Horvath, L.G.; Gish, K.; Caras, I.; Ramakrishnan, V.; Wong, M.; Jeffry, U.; Kench, J.G.; et al. Expression of the Zinc Transporter ZnT4 Is Decreased in the Progression from Early Prostate Disease to Invasive Prostate Cancer. Oncogene 2003, 22, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Tepaamorndech, S.; Huang, L.; Kirschke, C.P. A Null-Mutation in the Znt7 Gene Accelerates Prostate Tumor Formation in a Transgenic Adenocarcinoma Mouse Prostate Model. Cancer Lett. 2011, 308, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Zhang, W.; Zhang, D.; Zhang, Y.; Pang, C.; Huang, Y.; Wang, M.; Cui, L.; He, L.; Zhang, J.; et al. Spatial Intratumor Genomic Heterogeneity within Localized Prostate Cancer Revealed by Single-Nucleus Sequencing. Eur. Urol. 2018, 74, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Smiley, D.; Dagogo-Jack, S.; Umpierrez, G. Therapy Insight: Metabolic and Endocrine Disorders in Sickle Cell Disease. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Harmon, S.A.; Terrigino, N.T.; Karzai, F.; Pinto, P.A.; Madan, R.A.; VanderWeele, D.J.; Lake, R.; Atway, R.; Bright, J.R.; et al. A Case Report of Multiple Primary Prostate Tumors with Differential Drug Sensitivity. Nat. Commun. 2020, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, B.; Watson, M.A.; Humphrey, P.A.; Lim, H.; Milbrandt, J. Loss of Nkx3.1 Leads to the Activation of Discrete Downstream Target Genes during Prostate Tumorigenesis. Oncogene 2009, 28, 3307–3319. [Google Scholar] [CrossRef] [PubMed]
- Bowen, C.; Bubendorf, L.; Voeller, H.J.; Slack, R.; Willi, N.; Sauter, G.; Gasser, T.C.; Koivisto, P.; Lack, E.E.; Kononen, J.; et al. Loss of NKX3.1 Expression in Human Prostate Cancers Correlates with Tumor Progression. Cancer Res. 2000, 60, 6111–6115. [Google Scholar] [PubMed]
- Valkenburg, K.C.; Williams, B.O. Mouse Models of Prostate Cancer. Prostate Cancer 2011, 2011, 895238. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. Modeling Human Prostate Cancer in Genetically Engineered Mice. Progress. Mol. Biol. Transl. Sci. 2011, 100, 1–49. [Google Scholar]
- Gingrich, J.R.; Barrios, R.J.; Morton, R.A.; Boyce, B.F.; DeMayo, F.J.; Finegold, M.J.; Angelopoulou, R.; Rosen, J.M.; Greenberg, N.M. Metastatic Prostate Cancer in a Transgenic Mouse. Cancer Res. 1996, 56, 4096–4102. [Google Scholar]
- Ouyang, X.; Jessen, W.J.; Al-Ahmadie, H.; Serio, A.M.; Lin, Y.; Shih, W.J.; Reuter, V.E.; Scardino, P.T.; Shen, M.M.; Aronow, B.J.; et al. Activator Protein-1 Transcription Factors Are Associated with Progression and Recurrence of Prostate Cancer. Cancer Res. 2008, 68, 2132–2144. [Google Scholar] [CrossRef]
- Chiaverotti, T.; Couto, S.S.; Donjacour, A.; Mao, J.-H.; Nagase, H.; Cardiff, R.D.; Cunha, G.R.; Balmain, A. Dissociation of Epithelial and Neuroendocrine Carcinoma Lineages in the Transgenic Adenocarcinoma of Mouse Prostate Model of Prostate Cancer. Am. J. Pathol. 2008, 172, 236–246. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR Is a Web App for Creating, Exploring, Labeling and Sharing Volcano Plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Edgar, R. Gene Expression Omnibus: NCBI Gene Expression and Hybridization Array Data Repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, E.; Levanon, E.Y. Human Housekeeping Genes, Revisited. Trends Genet. 2013, 29, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Levy, B.A.; Zou, J.; Hanna, N.; Desouki, M.M.; Bagasra, O.; Johnson, L.A.; Costello, L.C. ZIP14 Zinc Transporter Downregulation and Zinc Depletion in the Development and Progression of Hepatocellular Cancer. J. Gastrointest. Cancer 2012, 43, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Weksler, M.; Simon, A.; Lenkinski, R.E.; Landsman, H.; Matzkin, H.; Mabjeesh, N.; Leibovitch, I. A Novel Modality Enables New Evidence-Based Individual Risk Stratification That Can Potentially Lead to Decisive Management and Treatment Decisions in Prostate Cancer. Diagnostics 2023, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Costello, L.C. Zinc as an Anti-Tumor Agent in Prostate Cancer and in Other Cancers. Arch. Biochem. Biophys. 2007, 463, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Nejad, S.S.; Golzari, Z.; Zangiabadian, M.; Khozani, A.A.S.A.; Ebrahimi, R.; Nejadghaderi, S.A.; Aletaha, A. The Association between Zinc and Prostate Cancer Development: A Systematic Review and Meta-Analysis. PLoS ONE 2024, 19, e0299398. [Google Scholar] [CrossRef]
- Zhang, Y.; Stopsack, K.H.; Wu, K.; Song, M.; Mucci, L.A.; Giovannucci, E. Post-Diagnostic Zinc Supplement Use and Prostate Cancer Survival among Men with Nonmetastatic Prostate Cancer. J. Urol. 2023, 209, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-González, E.; Castelló, A.; Fernández-Navarro, P.; Castaño-Vinyals, G.; Llorca, J.; Salas, D.; Salcedo-Bellido, I.; Aragonés, N.; Fernández-Tardón, G.; Alguacil, J.; et al. Dietary Zinc and Risk of Prostate Cancer in Spain: MCC-Spain Study. Nutrients 2018, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Kondo, Y.; Himeno, S.; Suzuki, Y.; Hara, S.; Akimoto, M.; Imura, N. Modulation of Telomerase Activity by Zinc in Human Prostatic and Renal Cancer Cells. Biochem. Pharmacol. 2000, 59, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.K.; Meeker, A. Telomeres and Telomerase in Prostate Cancer Development and Therapy. Nat. Rev. Urol. 2017, 14, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Pollak, M.N.; Willett, W.C.; Hankinson, S.E. Dietary Correlates of Plasma Insulin-like Growth Factor I and Insulin-like Growth Factor Binding Protein 3 Concentrations. Cancer Epidemiol. Biomark. Prev. 2002, 11, 852–861. [Google Scholar]
- Lefebvre, D.; Beckers, F.; Ketelslegers, J.M.; Thissen, J.P. Zinc Regulation of Insulin-like Growth Factor-I (IGF-I), Growth Hormone Receptor (GHR) and Binding Protein (GHBP) Gene Expression in Rat Cultured Hepatocytes1Presented in Part at the 10th International Congress of Endocrinology, San Francisco, CA, 1996.1. Mol. Cell. Endocrinol. 1998, 138, 127–136. [Google Scholar] [CrossRef]
- Siech, C.; Rutz, J.; Maxeiner, S.; Grein, T.; Sonnenburg, M.; Tsaur, I.; Chun, F.K.-H.; Blaheta, R.A. Insulin-like Growth Factor-1 Influences Prostate Cancer Cell Growth and Invasion through an Integrin A3, A5, AV, and Β1 Dependent Mechanism. Cancers 2022, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, Y. Comprehensive Analysis of the Expression of SLC30A Family Genes and Prognosis in Human Gastric Cancer. Sci. Rep. 2020, 10, 18352. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Liu, Z.; Bharadwaj, U.; Wang, H.; Wang, X.; Zhang, S.; Liuzzi, J.P.; Chang, S.M.; Cousins, R.J.; et al. Aberrant Expression of Zinc Transporter ZIP4 (SLC39A4) Significantly Contributes to Human Pancreatic Cancer Pathogenesis and Progression. Proc. Natl. Acad. Sci. USA 2007, 104, 18636–18641. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhang, W.; Emerson, R.E.; Xu, Y. ZIP4 Is a Novel Cancer Stem Cell Marker in High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 3692. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, Y.; Liu, J.; Han, J.; Guo, J.; Lu, S.; Huang, X.; Yi, P.; Lang, J.; Zhang, P.; et al. Inhibition of ZIP4 Reverses Epithelial-to-Mesenchymal Transition and Enhances the Radiosensitivity in Human Nasopharyngeal Carcinoma Cells. Cell Death Dis. 2019, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Gartmann, L.; Wex, T.; Grüngreiff, K.; Reinhold, D.; Kalinski, T.; Malfertheiner, P.; Schütte, K. Expression of Zinc Transporters ZIP4, ZIP14 and ZnT9 in Hepatic Carcinogenesis—An Immunohistochemical Study. J. Trace Elem. Med. Biol. 2018, 49, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, K.; Zheng, B.; Zekria, D.; Suo, T.; Liu, H.; Shen, S.; Liu, H.; Ni, X. Knockdown of Slc39a4 Expression Inhibits the Proliferation and Motility of Gallbladder Cancer Cells and Tumor Formation in Nude Mice. Cancer Manag. Res. 2021, 13, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, H.J.; Xie, H.Y.; Li, J.; Zhuang, R.Z.; Ling, Q.; Zhou, L.; Wei, X.Y.; Liu, Z.K.; Ding, S.M.; et al. ZIP4, a Novel Determinant of Tumor Invasion in Hepatocellular Carcinoma, Contributes to Tumor Recurrence after Liver Transplantation. Int. J. Biol. Sci. 2014, 10, 245–256. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Wang, Y.; Yang, J.; Zhu, V.F.; Liu, Y.; Cui, X.; Chen, L.; Yan, W.; Jiang, T.; et al. ZIP4 Is a Novel Molecular Marker for Glioma. Neuro Oncol. 2013, 15, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, Y.; Yang, J.; Sun, X.; Hagan, J.P.; Guha, S.; Li, M. ZIP4 Confers Resistance to Zinc Deficiency-Induced Apoptosis in Pancreatic Cancer. Cell Cycle 2014, 13, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharadwaj, U.; Logsdon, C.D.; Chen, C.; Yao, Q.; Li, M. ZIP4 Regulates Pancreatic Cancer Cell Growth by Activating IL-6/STAT3 Pathway through Zinc Finger Transcription Factor CREB. Clin. Cancer Res. 2010, 16, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Polak, K.L.; Chernosky, N.M.; Smigiel, J.M.; Tamagno, I.; Wjackson, M. Balancing STAT Activity as a Therapeutic Strategy. Cancers 2019, 11, 1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Cui, X.; Chen, Y.; Zhu, V.F.; Hagan, J.P.; Wang, H.; Yu, X.; Hodges, S.E.; Fang, J.; et al. A Novel Epigenetic CREB-MiR-373 Axis Mediates ZIP4-Induced Pancreatic Cancer Growth. EMBO Mol. Med. 2013, 5, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Talati, P.G.; Gu, L.; Ellsworth, E.M.; Girondo, M.A.; Trerotola, M.; Hoang, D.T.; Leiby, B.; Dagvadorj, A.; McCue, P.A.; Lallas, C.D.; et al. Jak2-Stat5a/b Signaling Induces Epithelial-to-Mesenchymal Transition and Stem-Like Cell Properties in Prostate Cancer. Am. J. Pathol. 2015, 185, 2505–2522. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Zellweger, T.; Alanen, K.; Mirtti, T.; et al. Stat3 Promotes Metastatic Progression of Prostate Cancer. Am. J. Pathol. 2008, 172, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, J.; Zhang, Y.; Zhou, Z.; Cui, X.; Zhang, L.; Fung, K.M.; Zheng, W.; Allard, F.D.; Yee, E.U.; et al. ZIP4 Promotes Pancreatic Cancer Progression by Repressing ZO-1 and Claudin-1 through a ZEB1-Dependent Transcriptional Mechanism. Clin. Cancer Res. 2018, 24, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhan, H.; Zhang, Y.; Yang, J.; Liu, M.; Xu, C.; Fan, X.; Zhang, J.; Zhou, Z.; Shi, X.; et al. ZIP4 Promotes Non-Small Cell Lung Cancer Metastasis by Activating Snail-N-Cadherin Signaling Axis. Cancer Lett. 2021, 521, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Odero-Marah, V.; Hawsawi, O.; Henderson, V.; Sweeney, J. Epithelial-Mesenchymal Transition (EMT) and Prostate Cancer. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1095, pp. 101–110. ISBN 9783319956930. [Google Scholar]
- Li, M.; Zhang, Y.; Bharadwaj, U.; Zhai, Q.; Ahern, C.H.; Fisher, W.E.; Brunicardi, F.C.; Logsdon, C.D.; Chen, C.; Yao, Q. Down-Regulation of ZIP4 by RNA Interference Inhibits Pancreatic Cancer Growth and Increases the Survival of Nude Mice with Pancreatic Cancer Xenografts. Clin. Cancer Res. 2009, 15, 5993–6001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Yao, Q.; Li, M. ZIP4 Upregulates the Expression of Neuropilin-1, Vascular Endothelial Growth Factor, and Matrix Metalloproteases in Pancreatic Cancer Cell Lines and Xenografts. Cancer Biol. Ther. 2010, 9, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Eble, J.A. Neuropilins in the Context of Tumor Vasculature. Int. J. Mol. Sci. 2019, 20, 639. [Google Scholar] [CrossRef] [PubMed]
- Woollard, D.J.; Opeskin, K.; Coso, S.; Wu, D.; Baldwin, M.E.; Williams, E.D. Differential Expression of VEGF Ligands and Receptors in Prostate Cancer. Prostate 2013, 73, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Tse, B.W.C.; Volpert, M.; Ratther, E.; Stylianou, N.; Nouri, M.; McGowan, K.; Lehman, M.L.; McPherson, S.J.; Roshan-Moniri, M.; Butler, M.S.; et al. Neuropilin-1 Is Upregulated in the Adaptive Response of Prostate Tumors to Androgen-Targeted Therapies and Is Prognostic of Metastatic Progression and Patient Mortality. Oncogene 2017, 36, 3417–3427. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Muraina, I.A.; Brethour, D.; Schmitt-Ulms, G.; Nimmanon, T.; Ziliotto, S.; Kille, P.; Hogstrand, C. Zinc Transporter ZIP10 Forms a Heteromer with ZIP6 Which Regulates Embryonic Development and Cell Migration. Biochem. J. 2016, 473, 2531–2544. [Google Scholar] [CrossRef]
- Nimmanon, T.; Ziliotto, S.; Ogle, O.; Burt, A.; Gee, J.M.W.; Andrews, G.K.; Kille, P.; Hogstrand, C.; Maret, W.; Taylor, K.M. The ZIP6/ZIP10 Heteromer Is Essential for the Zinc-Mediated Trigger of Mitosis. Cell. Mol. Life Sci. 2021, 78, 1781–1798. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-Function Analysis of HKE4, a Member of the New LIV-1 Subfamily of Zinc Transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Nimmanon, T.; Ziliotto, S.; Morris, S.; Flanagan, L.; Taylor, K.M. Phosphorylation of Zinc Channel ZIP7 Drives MAPK, PI3K and MTOR Growth and Proliferation Signalling. Metallomics 2017, 9, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, W.; Kimura, S.; Iwanaga, T.; Furusawa, Y.; Irié, T.; Izumi, H.; Watanabe, T.; Hijikata, A.; Hara, T.; Ohara, O.; et al. Zinc Transporter SLC39A7/ZIP7 Promotes Intestinal Epithelial Self-Renewal by Resolving ER Stress. PLoS Genet. 2016, 12, e1006349. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, G.; Bouwkamp, C.G.; de Vrij, F.M.; Lovenberg, T.; Bonaventure, P.; Kushner, S.A.; Harrington, A.W. The Zinc Transporter SLC39A7 (ZIP7) Is Essential for Regulation of Cytosolic Zinc Levels. Mol. Pharmacol. 2018, 94, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.G.; Cowley, M.J.; Gayevskiy, V.; Roscioli, T.; Thorburn, D.R.; Prelog, K.; Bahlo, M.; Sue, C.M.; Balasubramaniam, S.; Christodoulou, J. A SLC39A8 Variant Causes Manganese Deficiency, and Glycosylation and Mitochondrial Disorders. J. Inherit. Metab. Dis. 2017, 40, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hogrebe, M.; Grüneberg, M.; DuChesne, I.; von der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Liuzzi, J.P.; McClellan, S.; Cousins, R.J. Zinc Transporter ZIP8 (SLC39A8) and Zinc Influence IFN-Gamma Expression in Activated Human T Cells. J. Leukoc. Biol. 2009, 86, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Liu, L.; Banes-Berceli, A.; Yang, Z.; Kang, P.; Shen, J.; Tsai, K.J.; Liu, Z. Role of ZIP8 in Regulating Cell Morphology and NF-ΚB/Snail2 Signaling. Metallomics 2018, 10, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Lin, Y.W.; Wen, Y.C.; Yang, Y.C.; Hsiao, M.; Chang, J.L.; Huang, H.C.; Lee, W.J. Targeting the SPOCK1-Snail/Slug Axis-Mediated Epithelial-to-Mesenchymal Transition by Apigenin Contributes to Repression of Prostate Cancer Metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 246. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Jardin, S.E.; Dahl, H.; Nawas, A.F.; Bautista, M.; Delk, N.A. NF-ΚB Signaling Promotes Castration-Resistant Prostate Cancer Initiation and Progression. Pharmacol. Ther. 2020, 211, 107538. [Google Scholar] [CrossRef]
- Golan, Y.; Lehvy, A.; Horev, G.; Assaraf, Y.G. High Proportion of Transient Neonatal Zinc Deficiency Causing Alleles in the General Population. J. Cell. Mol. Med. 2019, 23, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Kambe, T.; Assaraf, Y.G. The Role of the Zinc Transporter: SLC30A2/ZnT2 in Transient Neonatal Zinc Deficiency. Metallomics 2017, 9, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Lopez, V.; Foolad, F.; Kelleher, S.L. ZnT2-Overexpression Represses the Cytotoxic Effects of Zinc Hyper-Accumulation in Malignant Metallothionein-Null T47D Breast Tumor Cells. Cancer Lett. 2011, 304, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.A.; Lopez, V.; Kelleher, S.L. A Histidine-Rich Motif Mediates Mitochondrial Localization of ZnT2 to Modulate Mitochondrial Function. Am. J. Physiol. Cell Physiol. 2011, 300, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhou, Y.; Gill, D.L.; Kelleher, S.L. A Genetic Variant in SLC30A2 Causes Breast Dysfunction during Lactation by Inducing ER Stress, Oxidative Stress and Epithelial Barrier Defects. Sci. Rep. 2018, 8, 3542. [Google Scholar] [CrossRef] [PubMed]
- Golan, Y.; Alhadeff, R.; Warshel, A.; Assaraf, Y.G. Znt2 Is an Electroneutral Proton-Coupled Vesicular Antiporter Displaying an Apparent Stoichiometry of Two Protons per Zinc Ion. PLoS Comput. Biol. 2019, 15, e1006882. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Seo, Y.A.; Sharma, S.; Soybel, D.I.; Kelleher, S.L. ZnT2 Is a Critical Mediator of Lysosomal-Mediated Cell Death during Early Mammary Gland Involution. Sci. Rep. 2015, 5, 8033. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.J.; Lee, S.-J.; Kim, T.-Y.; Cho, J.-H.; Koh, J.-Y. Zinc and 4-Hydroxy-2-Nonenal Mediate Lysosomal Membrane Permeabilization Induced by H2O2 in Cultured Hippocampal Neurons. J. Neurosci. 2008, 28, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Cho, K.S.; Koh, J.-Y. Oxidative Injury Triggers Autophagy in Astrocytes: The Role of Endogenous Zinc. Glia 2009, 57, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal Membrane Permeabilization and Cell Death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rivera, O.C.; Kelleher, S.L. Zinc Transporter 2 Interacts with Vacuolar ATPase and Is Required for Polarization, Vesicle Acidification, and Secretion in Mammary Epithelial Cells. J. Biol. Chem. 2017, 292, 21598–21613. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.; Morino, N.; Wagatsuma, T.; Munekane, M.; Ueda, S.; Matsunaga, M.; Uchida, Y.; Katayama, T.; Katoh, T.; Kambe, T. ZNT5-6 and ZNT7 Play an Integral Role in Protein N-Glycosylation by Supplying Zn2+ to Golgi α-Mannosidase II. J. Biol. Chem. 2024, 300, 107378. [Google Scholar] [CrossRef] [PubMed]
- Amagai, Y.; Yamada, M.; Kowada, T.; Watanabe, T.; Du, Y.; Liu, R.; Naramoto, S.; Watanabe, S.; Kyozuka, J.; Anelli, T.; et al. Zinc Homeostasis Governed by Golgi-Resident ZnT Family Members Regulates ERp44-Mediated Proteostasis at the ER-Golgi Interface. Nat. Commun. 2023, 14, 2683. [Google Scholar] [CrossRef] [PubMed]
- Corradi, J.P.; Cumarasamy, C.W.; Staff, I.; Tortora, J.; Salner, A.; McLaughlin, T.; Wagner, J. Identification of a Five Gene Signature to Predict Time to Biochemical Recurrence after Radical Prostatectomy. Prostate 2021, 81, 694–702. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, C.; Sun, L.; Li, Z.; Li, J.; Hua, Z.-C. Expression Pattern and Prognostic Implication of Zinc Homeostasis-Related Genes in Acute Myeloid Leukemia. Metallomics 2023, 15, mfad022. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo, S.; Segovia, M.F.; de la Fuente-Ortega, E. Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review. Nutrients 2024, 16, 2026. https://doi.org/10.3390/nu16132026
Acevedo S, Segovia MF, de la Fuente-Ortega E. Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review. Nutrients. 2024; 16(13):2026. https://doi.org/10.3390/nu16132026
Chicago/Turabian StyleAcevedo, Samantha, María Fernanda Segovia, and Erwin de la Fuente-Ortega. 2024. "Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review" Nutrients 16, no. 13: 2026. https://doi.org/10.3390/nu16132026
APA StyleAcevedo, S., Segovia, M. F., & de la Fuente-Ortega, E. (2024). Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review. Nutrients, 16(13), 2026. https://doi.org/10.3390/nu16132026







