Abstract
Background/Objectives: Human milk is the optimal source of nutrition and protection against infection for infants. If breastfeeding is not possible, standard and hydrolyzed infant formulas (IF) are an alternative. Extensively hydrolyzed IFs (eHFs) contain bioactive peptides, but their activities have rarely been evaluated. The aim of this study was to characterize and compare the bioactive peptide profiles of different eHFs and standard IFs before and after in vitro digestion. Methods: Two forms, liquid and powder, of intact protein formula (iPF) and eHF were subjected to in vitro gastrointestinal digestion, mimicking a young infant’s gut (age 0–4 months) and an older infant’s gut (>6 months). Bioactive peptides of in vitro digested and undigested formulas were analysed with Liquid Chromatography–Mass Spectrometry (LC–MS). Results: In all samples, a variety of peptides with potential bioactive properties were found. Immuno-regulatory peptides, followed by antimicrobial and antioxidative peptides were most frequent, as were peptides promoting wound healing, increasing mucin secretion, regulating cholesterol metabolism, and preventing bacterial infection. Peptides typically found in yoghurt and colostrum were identified in some formula samples. Conclusions: The high amounts of bioactive peptides with various properties in eHFs and iPFs indicate a possible contribution to infection protection, healthy gut microbiomes, and immunological development of infants. eHFs showed similar compositions of bioactive peptides to iPFs, with intermittently increased peptide variety and quantity.
1. Introduction
Human milk is the optimal source of nutrition for infants. It contributes protective effects against infection [] and promotes immunological development as well as a healthy gut microbiome [,]. Among several components, human milk provides a large variety of bioactive peptides derived from major human milk proteins such as caseins, whey proteins, and mucins []. Caseins and whey proteins (e.g., α-lactalbumin and lactoferrin) constitute the largest part, whereas mucins constitute only a small percentage of total protein []. Small amounts of bioactive peptides may already be present in human milk itself due to endogenous proteases []. Larger quantities of bioactive peptides are released during digestion in an infant’s stomach and small intestine [,]. This has been investigated in various in vitro digestion systems [,] and in animal models []. These peptides can be absorbed, can exert bioactivities, and can furthermore be found in the stool of breast-fed infants []. Bioactive peptides play an important role in the maintenance of the gut epithelial barrier, especially together with secretory IgA (SIgA)—an antibody found in human milk that has been shown to exert an important gut mucosa shielding effect [].
If breastfeeding is not possible, besides infant formulas (IFs) with intact proteins, hydrolyzed formulas are also an alternative for infant nutrition []. Bioactive peptides in infant formulas are likewise formed from bovine milk proteins in cow’s milk formula [], and hydrolyzed formulas originally contain a larger proportion of peptides as they have been exposed to proteases during the manufacturing process. Following gastrointestinal digestion, a variety of small peptides are detected, some of which are also biologically active. It has previously been shown that in vitro digestion of an extensively hydrolyzed IF (eHF) resulted in fewer bioactive peptides as compared to a standard IF. However, several other bioactive peptides, such as fragments of β-lactoglobulin and residues 60–70 of β-casein, were preferentially released by a combination of industrial hydrolysis and in vitro digestion []. Thus, standard IFs and eHFs can be expected to result in the formation of different peptides with varying bioactivities.
There have been several clinical studies on eHFs; however, besides the findings of those focusing on the treatment or prevention of allergies, information on the characteristics and properties of various types of eHFs is limited.
Interestingly, eHFs contain a variety of bioactive peptides, but outcomes based on the activities of such peptides have rarely been evaluated. Further, the quantity of bioactive peptides in IFs is essential to know in order to elicit a measurable biological effect []. According to recent studies, eHFs can reduce allergic sensitization to cow’s milk [,,] and the development of allergic diseases in the first two years of life [], compared to standard IFs. Some eHFs are approved for managing cow’s milk allergy [].
Hydrolyzed and standard infant formulas are available in liquid and powdered forms, each being exposed to varying degrees of heat treatment during the manufacturing process. Heat treatment can affect digestion and the formation of bioactive peptides []; therefore, one aim of this study was to investigate whether the manufacturing processes of liquid and powdered IFs might affect the formation of bioactive peptides. The physical form of an IF is not only a matter of the preference of the caregiver but will also determine whether the IF can be additionally supplemented with pre- or probiotics. Dry-blended powdered IF will allow the addition of both pre- and viable probiotics (i.e., a synbiotic combination), while this is not yet possible in a liquid product, which can only be enriched with prebiotics. Since the presence of pre- or probiotics may affect the digestion of IFs due to bacterial proteases, it is of interest to compare these two types of hydrolyzed formula based on the identical hydrolysate.
The second and most important aim of this study was to characterize and compare the bioactive peptide profiles of the two different formula types: eHF, based on extensively hydrolyzed protein, vs. standard IF, based on intact cow’s milk protein (intact protein formula (iPF)). Therefore, both the variety of bioactive peptides and the overall peptide quantity were compared. In particular, two formula types (powder and liquid) of each eHF and iPF were used. All four formulas were exposed to three in vitro digestion states: undigested and two conditions mimicking the gut of a young infant (stomach pH of 4, for 15 min) [] and of an older infant (stomach pH of 2, for 30 min). The results were then compared to databases of cow’s milk bioactive peptides to identify peptides with known physiological activities.
2. Materials and Methods
2.1. Materials
Standard cow’s milk IF (HiPP Pre Bio COMBIOTIK®) and eHF (HiPP Pre HA COMBIOTIK®) were provided by HiPP GmbH & Co. Vertrieb KG (Pfaffenhofen, Germany). For each of the two formulas, one liquid (L) and one powdered (P) product were analyzed. The formulas were subjected to in vitro gastrointestinal digestion mimicking the physiological gastrointestinal conditions of a young infant (0–4 months of age). Additionally, comparisons to gastrointestinal conditions in older infants (>6 months of age) were performed. A description of the investigated materials is presented in Table 1.

Table 1.
Description of investigated materials.
2.2. Peptide Samples Preparation
Sample preparations and analyses were carried out as biological duplicates. In vitro digestion was performed according to our previously published work []. Formulas were acidified to pH 4 (young infant stomach pH) or pH 2 (older infant stomach pH) with 1 mM HCl. Pepsin (Sigma, St. Louis, MO, USA) was added as 2% pepsin in 1 mM HCl in PBS, at a ratio of 22 µL per 25 mL of sample, to achieve a protein/pepsin ratio of 12.5:1. Pepsin activity was 56 U/mL of sample. After pepsin addition, samples were incubated at 37 °C for 15 min (young infant) and 30 min (older infant). Following incubation for 15 and 30 min with pepsin, pH was adjusted back to 7.0 using NaHCO3, after which pancreatin (Sigma, St. Louis, MO, USA) as a 0.4% solution in 1 mM NaHCO3 was added to the samples at a ratio of 4 µL pancreatin per 25 mL of formula, to achieve a protein:pancreatin ratio of 62.5:1. After pancreatin addition, samples were incubated for 15 and 30 min, respectively, and enzymes were then inactivated by placing the samples in a water bath at 85 °C for one min.
Peptidomic analysis of the undigested and digested formulas was performed by the Proteomics Core Facility (UC Davis, CA, USA). Undigested eHF and iPF, and the digested formulas were spun on 10 kDa filters (Millipore, Burlington, MA, USA) for 5 min to separate peptides from proteins larger than 10 kDa, obtaining two fractions: one for peptidomic analysis and another for proteomics analysis. The proteins fraction and any peptides above 10 kDa were subjected to trypsinization in order to obtain peptide sizes that were compatible with typical Liquid Chromatography–Mass Spectrometry (LC–MS) analyses. Both the gastrointestinal digestion under 10 kDa peptides and the above 10 kDa trypsinized peptides were then desalted and subjected to LC–MS.
2.3. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis
Nano-liquid chromatography (nLC) separation was performed on an Easy-nLC II High-Pressure Liquid Chromatography system (HPLC) (Thermo Fisher Scientific, Waltham, MA, USA). The trypsinized peptides were reconstituted in 2% acetonitrile/0.1% trifluoroacetic acid and 1 µg of each sample was loaded onto the LC–MS system. For the LC part, samples were first sent onto a 100 µm × 25 mm Magic C18, 100 Å, 5 µm reverse phase trap where they were desalted on-line, then directly eluted onto an analytical column for on-line separation. The analytical column is a 75 µm × 150 mm Magic C18, 200 Å, 3 µm reverse phase column. Peptides were then eluted into the mass spectrometer directly from this analytical column using a 2-buffer gradient: buffer A being 0.1% formic acid in water, and buffer B 0.1% formic acid in 100% acetonitrile, at a flow rate of 300 nL/min. The 95 min LC gradient is as follows: 0–80 min goes from 5% to 40% acetonitrile; 80–85 min goes from 35% to 80% acetonitrile; stays at 80% acetonitrile for 3 min; then goes from 80% to 5% acetonitrile over 2 min; and finally held at 5% acetonitrile for 5 min.
Mass spectra were collected on an Orbitrap Q Exactive mass spectrometer (Thermo Fisher Scientific) in a data-dependent mode with one MS precursor scan, resolution 70,000, followed by 15 MS/MS scans, resolution 17,500.
2.4. Data Analysis
The LC–MS raw files were processed with Proteome Discoverer 2.5 (Thermo Fisher) using the integrated SEQUEST engine. Precursor mass tolerance was set to 10 ppm. Fragment ion tolerance was 0.6 Da when using the ion trap analyzer. Trypsin, pepsin, chymotrypsin, and elastase (pancreatin) were specified as proteases and a maximum of two missed cleavages was allowed. Carbamidomethylation on cysteine (+57.021 Da) was set as static modification. Dynamic modifications included oxidation on methionine (+15.995 Da), deamidation of asparagine and glutamine (0.985 Da) and phosphorylation on serine, threonine, and tyrosine (+79.966 Da). All data were searched against the Bos Taurus Uniprot UP0009136 reference proteome without isoforms (37,511 entries), and a database of 112 common laboratory contaminants []. A false discovery rate of 1% was set at the PSM level as well as at 1% at the protein level. We accepted identifications with at least one unique peptide and XCorr scores of above 2 for doubly charged and of above 3 for triply charged tryptic peptides. The ptmRS node was used to localize phosphorylation sites, with a probability of 90% or higher considered to be a confident indicator of a phosphorylation site.
For label-free relative quantitation, peptides contributing to the protein abundance values were defined as those with over four points under the curve. This ensured a robust quantitation value.
Bioactive peptides were identified by manually examining the Proteome Discoverer 2.5 (Thermo Fisher)-generated peptides list. To expand, identification was performed against the appropriate databases (according to the provenience of milk samples), plus an equal number of reverse sequences and 60 common laboratory contaminant proteins, assuming a non-specific digestion enzyme. Peptides derived from major bovine milk proteins of interest were submitted to the Milk Bioactive Peptide Database [,,]. This database classifies milk peptides based on biological functions that the database creators have gathered from over 260 primary research articles on milk from several mammalian species, such as cows, humans, camels, goats, and more. Several other milk databases were also searched for any peptides not found using the literature search. The search results were further corroborated by browsing reference articles.
3. Results
Different bioactive peptides derived from major bovine milk proteins were found in the 12 formula samples during in vitro gastrointestinal digestion, including caseins and whey proteins (such as β-lactoglobulin, and lactoferrin). In all 12 formulas, casein peptides were the most commonly found peptides, specifically from α-S1-casein, β-casein, α-S2-casein, and κ-casein (overall > 97% of all peptides). Among these, β-casein derived peptides were the most common peptides (see Figure 1).
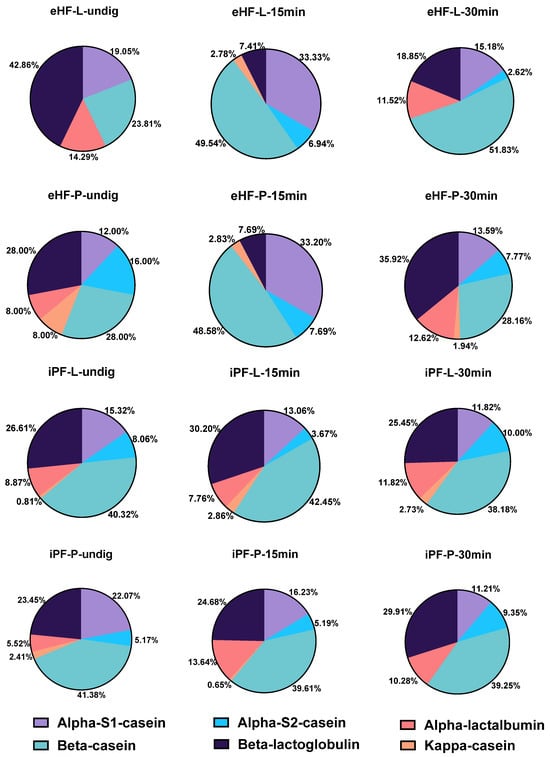
Figure 1.
Percentage of milk protein-derived peptides found in the 12 formula samples.
Overall, the most commonly found bioactive peptides by their associated biological functions across all samples were immuno-regulatory peptides, as well as peptides with antimicrobial, antioxidative, wound healing, increasing mucin secretion, cholesterol regulation, and Salmonella antivirulence activities. Additionally, peptides known from yoghurt and colostrum were found. An overview of the most commonly found bioactive peptides in formula samples after 15 min of in vitro digestion is presented in Table 2.

Table 2.
Most common (top 25%) bioactive peptides identified in samples of IFs after 15 min of in vitro digestion that showed sequence homology with a known bioactive milk peptide from the BIOPEP-UWM database.
The quantity of bioactive peptides of the nine most relevant peptide groups (as per their associated function or source), as well as the sum of unique bioactive peptides were analyzed per sample and are depicted in Figure 2. For the peptide quantities, the integral of the respective peptide curve was calculated (i.e., the area under the curve), which represents the number of peptides present in a sample. Since this value is a unitless value, in order to present and discuss the peptide quantity values, the description ‘quant value’ will be used in the following sections.
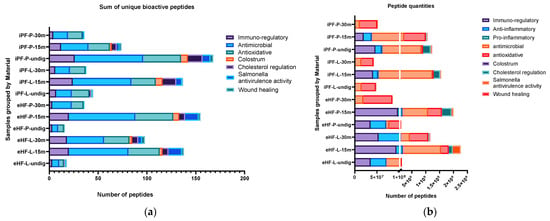
Figure 2.
(a) Sum of unique bioactive peptides per peptide group found in the 12 formula samples. (b) Number of bioactive peptides per peptide group found in the 12 formula samples.
The largest sum of unique bioactive peptides was found in the undigested iPF-P sample (170 unique bioactive peptides), followed by the samples of eHF-P (156), iPF-L (149), and eHF-L (143), all after 15 min of in vitro digestion (Figure 2a). Antimicrobial and antioxidative peptides were the most common bioactive peptides found, with antimicrobial peptides having the highest overall absolute quantity of peptides (antimicrobial: total of >4.8 × 109, quant value; antioxidative: total of >2.5 × 109 quant value) and antioxidative peptides being the most common peptide group in eight of 12 samples (Figure 2b). Other peptides found with high quantities were cholesterol metabolism regulation (>1 × 109 quant value), Salmonella antivirulence activity (>477 × 106 quant value), immuno-regulatory (including osteopontin and peptides with functions related to T cell response) (>426 × 106 quant value), anti-inflammatory (>318 × 106 quant value), and wound healing (>66 × 106 quant value).
Furthermore, peptides known from colostrum were found, albeit in lower amounts, in six of the 12 samples (>39 × 106 quant value). Small amounts of pro-inflammatory peptides were found in eight of the 12 samples (>6 × 106 quant value).
The following sections focus on the analysis of each peptide group across all formula samples.
3.1. Immuno-Regulatory Peptides
3.1.1. Overview of Immuno-Regulatory Peptides
The sums of unique immuno-regulatory peptides (i.e., variety of peptides) per sample and digestion status are presented in Figure 3a. The highest sum of unique immuno-regulatory peptides was identified in the undigested iPF-P sample (26 unique immuno-regulatory peptides), followed by iPF-L after 15 min of in vitro digestion (24 peptides), eHF-L and eHF-P, each after 15 min of in vitro digestion (each 20 peptides), and eHF-L after 30 min of in vitro digestion (18 peptides).
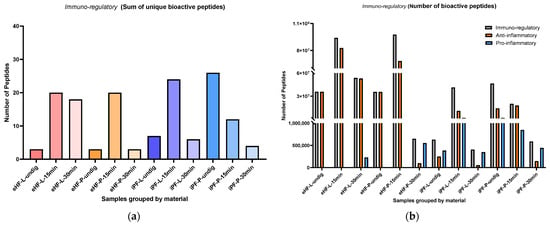
Figure 3.
(a) Sum of unique bioactive peptides found among immuno-regulatory peptides per formula sample and digestion status; (b) Number of bioactive peptides found among immuno-regulatory peptides per formula sample and digestion status.
Across all the samples, the highest sums of unique immuno-regulatory peptides were found in samples after 15 min of in vitro digestion; except for iPF-P, where the highest sum was found in the undigested state. In contrast, the lowest sums of unique immuno-regulatory peptides were found in samples after 30 min of in vitro digestion; except for eHF-L, where the undigested state showed the lowest variety of peptides.
Overall, there were no distinct differences between the powder and liquid samples for both formula types (eHFs and iPFs) except for the eHF samples after 30 min of in vitro digestion, where eHF-L showed a higher variety of peptides.
The numbers of immuno-regulatory peptides (i.e., quantity) per sample and digestion status are presented in Figure 3b. The highest quantities of immuno-regulatory peptides were found in the eHF-P sample (>97 × 106 quant value of immuno-regulatory peptides) and the eHF-L sample (>93 × 106 quant value), each after 15 min of in vitro digestion, followed by the eHF-L sample after 30 min of in vitro digestion (>53 × 106 quant value). Across the iPFs, the highest quantities of immuno-regulatory peptides were found in the undigested iPF-P sample (>46 × 106 quant value) and in the iPF-L sample after 15 min of in vitro digestion (>41 × 106 quant value).
For the anti-inflammatory peptides, the highest quantity was found in the eHF-L sample (>82 × 106 peptides), followed by eHF-P sample (>68 × 106 peptides), each after 15 min of in vitro digestion, and in the eHF-L sample after 30 min of in vitro digestion (>53 × 106 quant value). Across the iPFs, the highest quantities of anti-inflammatory peptides were found in the iPF-P sample after 15 min of in vitro digestion (>17 × 106 quant value) and in the undigested iPF-P sample (>14 × 106 quant value).
Pro-inflammatory peptides were found in all iPF samples, with the highest quantities in undigested iPF-P and iPF-L after 15 min of in vitro digestion (each >1 × 106 quant value). In the eHFs, pro-inflammatory peptides were only found after 30 min of in vitro digestion for both powder and liquid samples.
After 15 min of in vitro digestion, the quantities of immuno-regulatory and anti-inflammatory peptides were at their highest for all samples; except for iPF-P, where the immuno-regulatory peptides were higher in its undigested state. Pro-inflammatory peptides were not detected in eHFs after 15 min of in vitro digestion.
Overall, there were no pronounced differences between the powder and liquid samples for both formula types (eHFs and iPFs) except for eHF samples after 30 min of in vitro digestion, where eHF-L showed a higher quantity of peptides.
Additionally, some immuno-regulatory peptides related to allergic diseases or allergic sensitization were found. The peptide Asn-Pro-Trp-Asp-Gln (NPWDQ, amino acids 107–111 of αs2-casein), which is known to inhibit intestinal allergen permeation [], was found in several samples of iPF. It was found in all digestion statuses of the iPF-L formula and in the undigested iPF-P formula.
Further, the peptide LAYFYPE (amino acids 142–149 of αs1-casein), which is known to secrete IFN-γ, a potent inhibitor of Th2-dependent events, including IgE production [], was found in two samples of iPF. It was found in the undigested iPF-P formula and in the iPF-L formula after 15 min of in vitro digestion.
3.1.2. Lactoferrin
The numbers of lactoferrin peptides per sample and digestion status are presented in Figure 4. The highest quantity of lactoferrin was found in the undigested eHF-L sample (>1.3 × 106 quant value for lactoferrin peptides), followed by iPF-L (>1.2 × 106 quant value) after 30 min of in vitro digestion, undigested iPF-L (>1.2 × 106 quant value), eHF-P (>1.1 × 106 quant value) after 30 min of in vitro digestion, and undigested eHF-P (>1 × 106 quant value). No lactoferrin peptides were found in the eHF-P sample after 15 min of in vitro digestion.
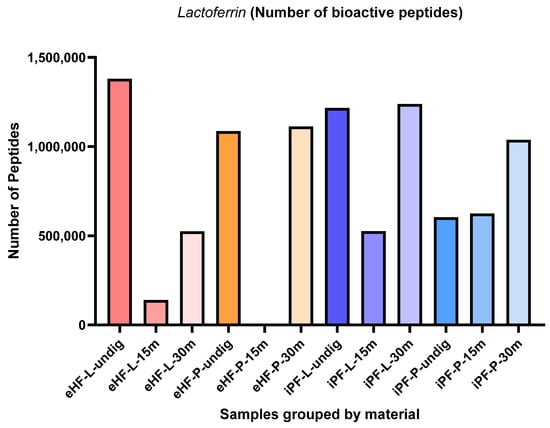
Figure 4.
Number of lactoferrin peptides per formula sample and digestion status.
Across all samples, the highest quantities of lactoferrin peptides were found in samples after 30 min of in vitro digestion; except for eHF-L, where the highest quantity was found in the undigested state.
After 15 min of in vitro digestion, the quantity of lactoferrin peptides was lowest for all samples; except iPF-P, where the lactoferrin peptide quantity was slightly lower in its undigested state.
Overall, the quantities of lactoferrin peptides in the powder and liquid samples of both formula types were somewhat similar. In the eHF samples, eHF-L showed a higher quantity of lactoferrin peptides in the undigested state and after 15 min of in vitro digestion, although eHF-P dominated in quantity after 30 min of in vitro digestion. In the iPF samples, iPF-L also showed a higher quantity of lactoferrin peptides in the undigested state and after 30 min of in vitro digestion, although iPF-P was higher after 15 min of in vitro digestion.
3.1.3. T Cell Response
Across all samples, two bioactive peptides with functions related to T cell response were found: polymeric immunoglobulin receptor (pIgR) [] and glycosylation-dependent cell adhesion molecule 1 (GlyCAM1) [] peptides. PIgR is a 100–120 kDa type 1 transmembrane protein and is involved in the transport of IgM and IgA in the gut epithelium. GlyCAM1 is a mucin-like endothelial glycoprotein and is part of the milk mucin complex.
The numbers of pIgR and GlyCAM1 peptides per sample and digestion status are presented in Figure 5. The highest quantity of pIgR peptides was found in the eHF-L sample (>9.9 × 106 pIgR peptides quant value) after 30 min of in vitro digestion, followed by iPF-P (>4.8 × 106 quant value) and eHF-L (>4.0 × 106 quant value), each after 15 min of in vitro digestion, and undigested eHF-L (>2.8 × 106 quant value).
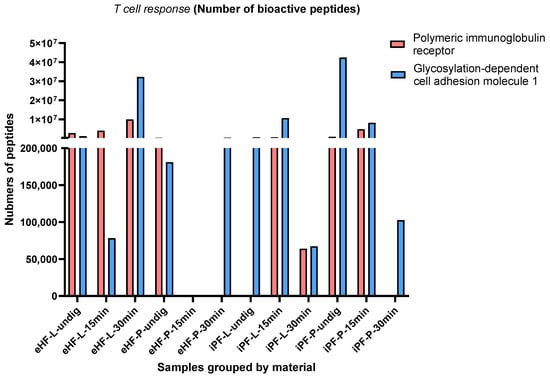
Figure 5.
Number of the two peptides with functions related to T cells’ polymeric immunoglobulin receptor (pIgR) and glycosylation-dependent cell adhesion molecule 1 (GlyCAM1) per formula sample and digestion status.
The highest quantity of GlyCAM1 peptides was found in the undigested iPF-P sample (>42 × 106 quant value for GlyCAM1 peptides), followed by eHF-L (>32 × 106 quant value) after 30 min of in vitro digestion, and iPF-L (>10 × 106 quant value) and iPF-P (>8 × 106 quant value), each after 15 min of in vitro digestion.
No pIgR peptides were found in undigested iPF-L, iPF-P, and eHF-P after 30 min of in vitro digestion. Neither pIgR nor GlyCAM1 peptides were found in the eHF-P formula after 15 min of in vitro digestion. No clear patterns in terms of digestion status were observed.
After 15 min of in vitro digestion, the quantity of pIgR peptides was highest in iPF-P and for GlyCAM1 peptides it was highest in iPF-L.
Of the eHF samples, eHF-L showed higher quantities of both pIgR and GlyCAM1 peptides, while the opposite was found for the iPF samples.
3.1.4. Osteopontin
The numbers of osteopontin peptides per sample and digestion status are presented in Figure 6. The highest quantity of osteopontin peptides was found in the eHF-L sample after 30 min of in vitro digestion (>77 × 106 osteopontin peptides’ quant value), while the lowest quantities were found in undigested iPF-L (<56 × 103 quant value) and iPF-L after 30 min of in vitro digestion (<83 × 103 quant value).
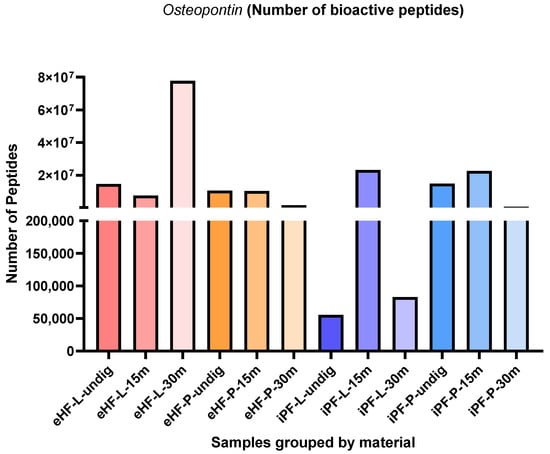
Figure 6.
Number of osteopontin peptides per formula sample and digestion status.
In the iPF samples, the highest quantities of osteopontin peptides were found in samples after 15 min of in vitro digestion, while no clear pattern was observed for the eHF samples.
After 15 min of in vitro digestion, the quantities of osteopontin peptides were highest for both iPF-L and iPF-P (each >22 × 106 quant value), whereas the quantities in the respective eHF samples were slightly lower (eHF-L: >7 × 106, eHF-P: >10 × 106 quant value).
Overall, the quantities of osteopontin peptides in the powder and liquid samples of both formula types were somewhat similar. In the eHF samples, eHF-L showed a higher quantity after 30 min of in vitro digestion. In the iPF samples, iPF-L showed a higher quantity after 15 min of in vitro digestion, whereas iPF-P was higher in quantity in the undigested state and after 30 min of in vitro digestion.
3.2. Antimicrobial Peptides
The sums of unique antimicrobial peptides per sample and digestion status are presented in Figure 7a. The highest sum of unique antimicrobial peptides was found in the undigested iPF-P sample (70 unique antimicrobial peptides), followed by eHF-P (68 peptides), eHF-L (61 peptides), and iPF-L (60 peptides), all after 15 min of in vitro digestion.
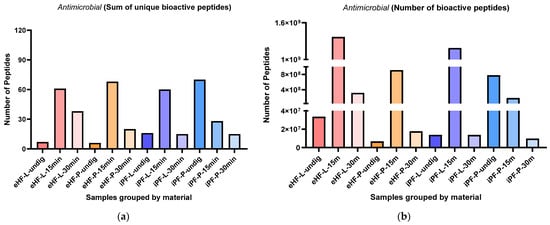
Figure 7.
(a) Sum of unique bioactive peptides found among antimicrobial peptides per formula sample and digestion status. (b) Number of bioactive peptides found among antimicrobial peptides per formula sample and digestion status.
Overall, the highest sums of unique antimicrobial peptides were found after 15 min of in vitro digestion, except for the iPF-P sample, which had the highest number of unique peptides in the undigested state.
In the eHFs, the lowest numbers of unique antimicrobial peptides were found in the undigested states. In the iPFs, the lowest sums were found after 30 min of in vitro digestion; however, for iPF-L the number of peptides was similar to that in the undigested state.
Overall, there were no pronounced differences between the powder and liquid samples for both formula types (eHFs and iPFs).
The numbers of antimicrobial peptides per sample are presented in Figure 7b. The highest quantity of antimicrobial peptides was found in eHF-L after 15 min of in vitro digestion (>13 × 106 antimicrobial peptides’ quant value), followed by iPF-L (>11 × 106 peptides’ quant value), and eHF-P (>913 × 106 quant value), both after 15 min of in vitro digestion, and undigested iPF-P (>780 × 106 quant value).
Across all samples, the highest quantities of antimicrobial peptides were found after 15 min of in vitro digestion; except for iPF-P, where the highest amount was found in the undigested state.
In the eHF samples, the lowest quantities of antimicrobial peptides per sample were found in the undigested state. In the iPFs, the lowest quantities were found after 30 min of in vitro digestion, but for iPF-L the amount was similar to that in the undigested state.
Overall, there were no distinct differences between the powder and liquid samples of both formula types (eHFs and iPFs).
3.3. Antioxidative Peptides
The sums of unique antioxidative peptides per sample and digestion status are presented in Figure 8a. The highest sum of unique antioxidative peptides was found in eHF-P after 15 min of in vitro digestion and in undigested iPF-P (each with 39 unique antioxidative peptides), followed by eHF-L after 15 min of in vitro digestion (32 peptides), eHF-L after 30 min of in vitro digestion (26 peptides), and iPF-L (25 peptides) and iPF-P (22 peptides), both after 15 min of in vitro digestion.
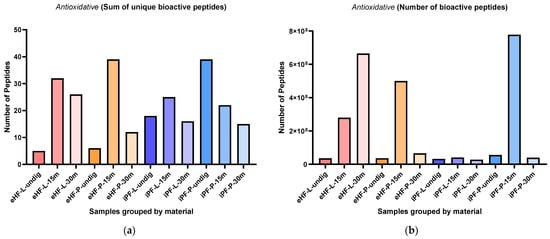
Figure 8.
(a) Sum of unique bioactive peptides found among antioxidative peptides per formula sample and digestion status. (b) Number of bioactive peptides found among antioxidative peptides per formula sample and digestion status.
Overall, the highest sums of unique antioxidative peptides were found after 15 min of in vitro digestion, except for the iPF-P sample, where the undigested state had the highest number of unique peptides.
In the eHFs, the lowest sums of unique antioxidative peptides were found in the undigested state. In the iPFs, the lowest sums were found after 30 min of in vitro digestion, but for iPF-L the sum was similar to the undigested state.
Overall, there were no pronounced differences between the powder and liquid samples for both formula types (eHFs and iPFs).
The numbers of antioxidative peptides per sample and digestion status are presented in Figure 8b. The highest quantity of antioxidative peptides was found in the iPF-P sample after 15 min of in vitro digestion (>1.3 × 109 antioxidative peptides’ quant value), followed by eHF-L after 30 min of in vitro digestion (>665 × 106 quant value), and eHF-P (>499 × 106 quant value) and eHF-L (>280 × 106 quant value), both after 15 min of in vitro digestion.
Across all samples, the highest quantities of antioxidative peptides were found after 15 min of in vitro digestion; except for eHF-L, where the highest amount was found after 30 min of in vitro digestion.
In the eHFs, the lowest quantities of antioxidative peptides were found in the undigested state, and in the iPFs after 30 min of in vitro digestion.
Overall, there were no distinct differences between the powder and liquid samples for both formula types (eHFs and iPFs) except for eHF samples after 30 min of in vitro digestion, where eHF-L showed a higher quantity of peptides and for iPF samples after 15 min of in vitro digestion, where iPF-P showed a higher quantity of peptides.
Additionally, a high amount of the peptide TQTPVVVPPFLQPE (amino acids 93–106 of β-casein) was found in eHF-L after 30 min of in vitro digestion (>585 × 106 for quant value) and in iPF-P after 15 min of in vitro digestion (>746 × 106 as quant value).
3.4. Wound Healing Peptides
The sums of unique wound healing peptides per sample and digestion status are presented in Figure 9a. The highest sum of unique wound healing peptides was found in eHF-L after 30 min of in vitro digestion and in undigested iPF-P (each with three unique wound healing peptides). No wound healing peptides were found in the samples of eHF-P, iPF-L, and iPF-P, all after 30 min of in vitro digestion.
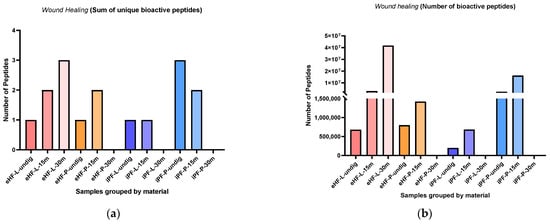
Figure 9.
(a) Sum of unique bioactive peptides found among wound healing peptides per formula sample and digestion status. (b) Number of bioactive peptides found among wound healing peptides per formula sample and digestion status.
Overall, the sum of unique wound healing peptides in the powder and liquid samples of both formula types was somewhat similar. In the eHF samples, eHF-L showed a higher quantity after 30 min of in vitro digestion. In the iPF samples, iPF-P showed a higher quantity in the undigested state and after 15 min of in vitro digestion.
The numbers of wound healing peptides per sample and digestion status are presented in Figure 9b. The highest quantity of wound healing peptides was found in eHF-L after 30 min of in vitro digestion (>41 × 106 wound healing peptides’ total quant value), followed by iPF-P after 15 min of in vitro digestion (>16 × 106 quant value).
Across all samples, the highest quantities of wound healing peptides were found after 15 min of in vitro digestion; except for eHF-L, where the highest amount was found after 30 min of in vitro digestion.
In the eHF samples, the liquid form showed a higher quantity of wound healing peptides than the powder form, except for the undigested samples which were similar in quantity. In the iPF samples the powder form showed a higher quantity of peptides.
3.5. Cholesterol Metabolism Regulation Peptides
The sums of unique cholesterol metabolism regulation (CMR) peptides per sample and digestion status are presented in Figure 10a. The highest sum of unique CMR peptides was found in the undigested iPF-P sample (15 unique CMR peptides), followed by iPF-L after 15 min of in vitro digestion (14 peptides). No CMR peptides were found in samples of undigested eHF-P, and eHF-P and iPF-P after 30 min of in vitro digestion. All other samples had a maximum sum of six unique peptides.
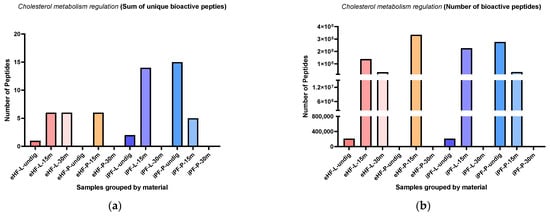
Figure 10.
(a) Sum of unique bioactive peptides found among cholesterol metabolism regulation peptides per formula sample and digestion status. (b) Number of bioactive peptides found among cholesterol metabolism regulation peptides per formula sample and digestion status.
Across all samples, the highest sums of unique CMR peptides were found after 15 min of in vitro digestion; except for iPF-P, where the highest sum was in the undigested state.
The numbers of CMR peptides per sample and digestion status are presented in Figure 10b. The highest quantities of CMR peptides were found in eHF-P after 15 min of in vitro digestion (>334 × 106 cholesterol regulation peptides’ quant value), followed by undigested iPF-P (>276 × 106 peptides), and iPF-L after 15 min of in vitro digestion (>225 × 106 as quant value). No CMR peptides were found in the undigested eHF-P sample, nor in eHF-P, iPF-L, and iPF-P, all after 30 min of in vitro digestion.
Overall, there were no marked differences between the powder and liquid samples for both formula types (eHFs and iPFs).
3.6. Peptides Affecting Salmonella Antivirulence Activity
The sums of unique Salmonella antivirulence activity (SAA) peptides per sample and digestion status are presented in Figure 11a. The highest sums of unique SAA peptides were found in eHF-L and eHF-P (14 unique SAA peptides each), both after 15 min of in vitro digestion. No SAA peptides were found in undigested eHF-P. All other samples had a maximum sum of under eight unique peptides.
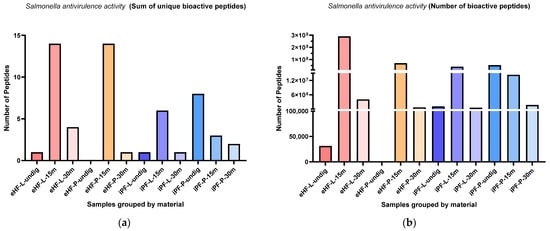
Figure 11.
(a) Sum of unique bioactive peptides found among Salmonella antivirulence activity peptides per formula sample and digestion status. (b) Number of bioactive peptides found among Salmonella antivirulence activity peptides per formula sample and digestion status.
Across all samples, the highest sums of unique SAA peptides were found after 15 min of in vitro digestion; except for iPF-P, where the highest sum was in the undigested state.
The numbers of SAA peptides per sample and digestion status are presented in Figure 11b. The highest quantities of SAA peptides were found in eHF-L after 15 min of in vitro digestion (>289 × 106 SAA peptides’ quant value). All other samples with SAA peptides had quantities below 71 × 106.
Across all samples, the highest quantities of SAA peptides were found after 15 min of in vitro digestion; except for iPF-P, where the highest amount was found in the undigested state.
In the eHF samples, eHF-L showed a higher quantity of SAA peptides across all digestion states. In the iPF samples, there were no distinct differences between the powder and liquid forms.
3.7. Peptides Known from Colostrum
The sums of unique peptides known from colostrum per sample and digestion status are presented in Figure 12a. The highest sums of unique peptides known from colostrum were found in undigested iPF-P and iPF-L after 15 min of in vitro digestion (each with eight unique peptides known from colostrum), followed by eHF-P after 15 min of in vitro digestion (six peptides). No peptides known from colostrum were found in undigested eHF-L, eHF-P, and iPF-L, nor in eHF-P, iPF-L, and iPF-P, all after 30 min of in vitro digestion. All other samples had a maximum sum of three unique peptides.
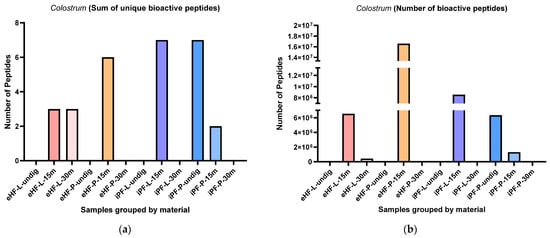
Figure 12.
(a) Sum of unique bioactive peptides found among peptides known from colostrum per formula sample and digestion status. (b) Number of bioactive peptides found among peptides known from colostrum per formula sample and digestion status.
Overall, the highest sums of unique peptides known from colostrum were found in samples after 15 min of in vitro digestion; except for the iPF-P, which had the highest number of unique peptides in the undigested state.
Overall, there were no marked differences between the powder and liquid samples for both formula types (eHFs) and (iPFs); except for eHF and iPF samples after 15 min of in vitro digestion, where eHF-P and iPF-L showed a higher variety of peptides.
The numbers of peptides known from colostrum per sample and digestion status are presented in Figure 12b. The highest quantity of peptides known from colostrum was found in eHF-P after 15 min of in vitro digestion (>16 × 106 peptides known from colostrum quant value), followed by iPF-L after 15 min of in vitro digestion (>8 × 106 quant value).
Across all samples, the highest quantities of peptides known from colostrum were found after 15 min of in vitro digestion; except for iPF-P, where the highest amount was found in the undigested state.
Overall, there were no pronounced differences between the powder and liquid forms for both formula types (eHFs and iPFs).
4. Discussion
Bioactive peptides result from the digestion of proteins in human milk and play an important role in the maintenance of gut health in infants. Formula-fed infants also receive—and benefit from—bioactive peptides, which stem from bovine milk proteins []. Their quantity and quality are impacted by the degree of processing the IF has undergone [].
In this study, two types of IF, eHF and iPF (in both liquid and powder forms), were subjected to in vitro gastrointestinal digestion and analyses of bioactive peptides were performed at the undigested stage and after 15 and 30 min of in vitro digestion (mimicking the gut of a young infant and of an older infant). Overall, the form of IF (liquid vs. powder) had little effect on the number of bioactive peptides, but the effect of the type of IF (extensively hydrolyzed vs. intact protein) was more decisive. While in a previous study only very few bioactive peptides were found in undigested and digested extensively hydrolyzed formula [], in this study, a variety of peptides with potential bioactive properties were found in all samples. This suggests that different properties of the hydrolyzed proteins are retained depending on the production process of the hydrolysate and IF. Across all samples in this study immuno-regulatory peptides, followed by antimicrobial and antioxidative peptides, were the most frequently found, as were peptides known for their roles in promoting wound healing, increasing mucin secretion, regulating cholesterol metabolism, and preventing bacterial infection. Peptides typically found in yoghurt and colostrum were also identified in some formula samples. Similar results were observed in other studies for antimicrobial [] and antioxidative peptides [], and peptides known from colostrum [].
The most common peptide type across all samples was casein peptides, especially β-casein. Other types found were from whey proteins. Since all whey protein concentrates are either produced by acidification or the result of industrial cheese processes, they typically also contain small quantities of casein proteins [,], which correlates with the results in this study. Casein peptides will also be present in whey protein concentrates before hydrolysis (derived partly from enzymatic activity in the udder and partly from rennet activity during cheese production) [,]. Further, this is similar to what has been found for whey proteins and casein in human milk [,]. Notably, as bioactive peptides are broken down into protein fragments, they do not lead to allergic sensitization; on the contrary, they can act as immuno-modulators [].
The variety of immuno-regulatory peptides was comparable in eHF and iPF samples; however, the quantity of immuno-regulatory, anti-inflammatory and osteopontin peptides was higher in the eHF samples. Osteopontin is a natural component of human milk [], acts as a Th1 cytokine, and is known to have a role in setting up a tolerogenic milieu []. Furthermore, Lactoferrin is one of the major human milk proteins [,]. It is an ion-binding glycoprotein enhancing intestinal iron absorption, promotes gut health, and plays a crucial role in modulating the development of infants’ immune systems [,]. Lactoferrin peptides were found in almost all the samples analysed. Interestingly, Lactoferrin peptides were not found in all stages of digestion in eHF-P samples. Lactoferrin peptides were not found at 15 min of in vitro digestion but were found in undigested samples and after 30 min of in vitro digestion. This may be attributed to the release or degradation of varying bioactive peptides at different stages during the digestive process. The quantities of peptides with functions related to T cell response were also comparable in eHF and iPF samples, whereas the quantity of pro-inflammatory peptides was lower in eHF samples. Pro-inflammatory peptides in eHF samples were only found after 30 min of in vitro digestion (for liquid and powder). These findings suggest that eHFs, in both liquid and powder form, are more likely to trigger an anti-inflammatory response than iPFs.
The variety and quantity of antimicrobial peptides was comparable in eHF and iPF samples, with the overall highest amounts being found in three samples after 15 min of in vitro digestion (eHF-L, eHF-P, and iPF-L) and in the undigested iPF-P sample. It is therefore assumed that the 15 min digestion time of IF samples exposed their ‘encrypted’ bioactive peptides [,], which were detected in eHF-L, eHF-P, and iPF-L. While a longer digestion time (30 min) can lead to such strong cleavage that no more peptides are detectable. Further studies are required to clarify why the peptide digestion pattern of iPF-P differs in this regard.
The variety of antioxidative peptides was comparable in eHF and iPF samples; however, the overall quantity was higher across the eHF samples, although the highest quantity was found in an iPF-P sample after 15 min of in vitro digestion. A high amount of the peptide TQTPVVVPPFLQPE (amino acids 93–106 of β-casein) was found in two samples: eHF-L after 30 min and iPF-P after 15 min of in vitro digestion. This bioactive peptide is linked to several functions: antioxidative, anti-hypertensive, or ACE-inhibitory, which consequently can aid in lowering blood pressure [].
The varieties of wound healing peptides were somewhat comparable in eHF and iPF samples; however, more iPF samples showed no traces of wound healing peptides (iPF-L and iPF-P, both after 30 min of in vitro digestion) than eHF samples (only eHF-P after 30 min of in vitro digestion). The overall quantities were, however, higher in the eHF samples.
Peptide assays for wound healing can be carried out using fibroblast cells in culture []. Fibroblasts are known to be involved in chronic gut inflammation, and thus, the ability to improve wound healing in the gut with bioactive peptides may contribute to gut homeostasis and gut health []. Intestinal injury accompanied by impaired mucosal wound healing as it is seen, e.g., in preterm infants with necrotizing enterocolitis (NEC) [], or in patients with chronic inflammatory bowel disease (IBD) [,], or Crohn’s disease (CD) [,], may also be attenuated by such wound healing bioactive peptides. However, this hypothesis has yet to be confirmed.
The variety of peptides regulating cholesterol metabolism was higher in the iPF samples than the eHFs; however, no peptides were found in two samples each (undigested eHF-P after 30 min of in vitro digestion and iPF-L and iPF-P, both after 30 min of in vitro digestion). Interestingly, the overall quantities of cholesterol metabolism regulation peptides were comparable in the eHF and iPF samples and highest in the eHF-P sample after 15 min of in vitro digestion. This result is in line with findings from the ongoing PADI study []. In this study, the blood cholesterol levels in infants fed with IFs similar to eHF-P and iPF-P at one year of age were compared. Infants fed eHF-P had significantly lower blood cholesterol levels than infants fed iPF-P (p < 0.05) [] (unpublished data), which was associated with a higher quantity of bioactive peptides for cholesterol metabolism regulation in eHF-P compared with iPF-P after in vitro infant digestion.
The variety of Salmonella antivirulence activity (SAA) peptides was higher in the eHF samples than in the iPF samples; however, no peptides were found in the undigested eHF-P sample. Interestingly, the overall quantities of SAA peptides were comparable in the eHF and iPF samples, with the highest quantity found in the eHF-L sample after 15 min of in vitro digestion. Salmonella infection (salmonellosis) is a common bacterial disease caused by Salmonella typhimurium and characterized by inflammation of the intestinal tract [,]. Consequently, the higher levels of SAA peptides in the eHF samples could benefit infants fed eHF, resulting in a lower susceptibility to Salmonella infections and outbreaks.
Peptides known from bovine colostrum were found in three of the six IF samples, for each of the eHFs and iPFs. The variety of peptides known from colostrum was higher in the iPF samples than the eHFs, but the maximum number of unique peptides in the iPFs was seven compared with the maximum of six unique peptides in the eHFs. In contrast, the overall quantities of peptides known from colostrum were higher in the eHF samples. Colostrum is the initial milk secreted by mammals during the first few days after the birth of their offspring and is a source of biologically active peptides with health-promoting effects [].
An overall interesting result is the generally high amounts and varieties of bioactive peptides found in the undigested iPF-P samples. Across all the peptide types described above, undigested iPF-P had the highest variety for six of the described nine bioactivities and the highest quantity for two of the 15 quantity analyses, therefore often scoring higher than the respective digested samples. One possible explanation for this finding is the methodology used for sample preparation, whereby all samples were further subjected to trypsinization, thus creating smaller peptide sizes which were detectable in LC–MS analyses. The less a sample is processed from one container to another at room temperature, the fewer peptides will be lost during preparation. Protein degradation already begins above −80 °C; therefore, peptides resulting from this degradation are less likely to be identified by the software. This would be the case, even if the mass spectrometer detects them. Further, the resulting peptides can have such small sizes that as a consequence they might get washed away during the process.
This study had both strengths and limitations. A series of different IFs were analysed as biological duplicates, but no statistics were compiled, and the analysis was limited to a descriptive comparison of the peptides found in the different samples. Samples were analyzed as total formula, which is in contrast to analyzing the protein itself, and additionally included fat molecules. This could potentially have affected the extraction of proteins in the formulas, due to the fat moieties in the aqueous buffer used for the tryptic digestion, which consisted of 50 mM triethyl ammonium bicarbonate in H2O. As a consequence, the proteins in the formulas would have reacted with a heightened stickiness and thus would have been less detectable in the LC–MS analysis. Samples were analyzed by LC–MS, which provides a relatively unbiased global view of the IFs’ bioactive peptide composition. Further, this method can be used to analyze all samples in the same manner; thus, analytical bias towards the various formulations is limited. However, by using LC–MS, certain hydrophilic or small peptides (<approximately four amino acids) may not have been registered during the 10 kDa molecular weight separation. This may also have affected the tryptic peptides produced from the above 10 kDa peptide fraction. Consequently, the probability of the formula samples containing even higher numbers of bioactive peptides is plausible. Due to these limitations in using the LC–MS system, an analysis of potential biologically active peptides with other methods is recommended in future studies.
In summary, the overall highest quantities and varieties of bioactive peptides were found in the samples of eHF-L after 15 min of in vitro digestion, eHF-L after 30 min of in vitro digestion, iPF-L after 15 min of in vitro digestion, and undigested iPF-P. In contrast, the lowest overall quantities and varieties were found in three of the samples digested for 30 min (eHF-P, iPF-L, and iPF-P), as well as in the undigested eHF-P samples. Interestingly, in contrast to results from a previous study, eHF samples showed similar compositions of bioactive peptides overall as iPF samples, with a tendency to higher variety and quantity across peptide types. The high amounts of immuno-regulatory, antimicrobial, and antioxidative peptides across most samples further indicate possible contributions to protection against infection, to healthy gut microbiomes, and to immunological development in infants.
5. Conclusions
In this study two types of IF, eHF and iPF (in both liquid and powder forms), were subjected to in vitro gastrointestinal digestion, and analyses of bioactive peptides were performed. In all samples, varieties of peptides with potential bioactive properties were found. Immuno-regulatory peptides, followed by antimicrobial and antioxidative peptides, were the most frequently found, as were peptides known for their roles in promoting wound healing, increasing mucin secretion, regulating cholesterol metabolism, and preventing bacterial infection. Peptides typically found in yoghurt and colostrum were also identified in some formula samples. The overall highest quantities and varieties of bioactive peptides were found in two eHF and in two iPF samples.
Author Contributions
Conceptualization, X.D., E.M.H. and B.L.; methodology, G.G., X.D. and B.L.; software, G.G. and R.K.; validation, G.G., X.D., E.M.H. and B.L.; formal analysis, G.G. and E.M.H.; investigation, G.G., X.D. and B.L.; resources, B.L.; data curation, G.G., X.D. and E.M.H.; writing—original draft preparation, G.G. and B.L., supported by Oracle Life Sciences, Munich, Germany; writing—review and editing, G.G., X.D., E.M.H. and B.L., supported by Oracle Life Sciences; visualization, X.D.; supervision, B.L.; project administration, G.G., X.D., E.M.H. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research (A20-3367) was funded by HiPP GmbH & Co. Vertrieb KG, Pfaffenhofen, Germany.
Institutional Review Board Statement
The PADI study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the independent ethics committees, as well as the local ethics committee in all countries: Bulgaria (Medical University Sofia, 31/05/2018, 3175/27.04.2018); Czech Republic (Ethics Committee of the Institute for Clinical and Experimental Medicine & Thomayer Hospital Praha, 13/06/2018, A-18-06); Finland (Joint Municipal Board of the Hospital District of Southwest Finland Turku, 20/03/2018, 27/1800/2018); France (CPP Ile de France XI St-Germain-en-Laye, 29/08/2018, EC/NJ18-0245); Germany (Charité, Berlin, 06/08/2018, EA2/098/18; Münster, 10/09/2018, 2018-518-b-S; Nordrhein, 28/09/2018, 2018265; Bochum, 19/09/2018, 18-6429; Hannover, 27/08/2018, 8035_BO_K_2018; Frankfurt, 28/01/2019, 302/18; Greifswald, 07/05/2019, 88 063/19); Italy (Università degli studi della Campania “Luigi Vanvitelli” Naples, 07/09/2018, 14166/18; Area Vasta Emilia Nord Parma, 11/09/2018, 34922/ 42019; Bambino Gesù Rome, 03/10/2018, 1028; Università Federico II Naples, 24/10/2018, 192/18; Milan, 24/10/2018, 43894/2018; Padova, 24/01/2019, 4610/AO/18; Turin, 27/09/2018, 0095880); Poland (University Bioethics Committee Poznan, 14/06/2018, 608/18); Portugal (Comissao de Etica Hospital Braga, 27/06/2018, 46/2018; Comissao de Etica Centro Hospitalar de Entre o Douro e Vouga, EPE, Hospital de Sao Sebastiao, 14/08/2018, EC-05-18; Comissao de Etica, Hospital Beatriz Angelo, 10/09/2018, 0342; Comissao de Etica para a Saude do Hospital Prof Dr Fernando Fonseca, 04/10/2018, 57/2018; Comissao de Etica da ULS Matosinhos, 28/09/2018, 116/ 18 / RS; Comissao de Etica Centro Hospitalar Lieria; 25/10/2018, 16/18; Comissao de Etica de Tras os Montes e Alto Douro, 09/11/2018, 438/2018—C.A.; Comissao de Etica Hospital de Santarem, 20/02/2019, HDS-134A.05); Serbia (KBC “DR DRAGISA MISOVIé-DEDINJE” Eticki odbor Belgrade, 22/06/2018, 01-6524/12); Spain (Fundacio Unio Catalana Hospitals Tarragona, 24/04/2018, PADI; CEIC Hospital Clinico San Carlos Madrid, 03/05/2018, 4.2/18; Xunta de Galicia A Coruna, 21/06/2018, 2018/209; Junta de Andalucia Sevilla, 27/05/2018, PADI; CEIC IDIAP Jordi Gol i Gurina Barcelona, 03/06/2018, 5OB18/005).
Informed Consent Statement
Informed consent was obtained from parents/legal Guardians involved in the PADI study according to the regulatory and legal requirements of the corresponding country.
Data Availability Statement
The data supporting the findings of this study will be provided by the corresponding author upon reasonable request.
Acknowledgments
The authors thank David Dallas for providing advice regarding the use of the bioactive milk peptide databases. The authors thank Tamara Nutz (medical writing, i.e., drafting of the manuscript based on author’s instructions, editing, and preparing figures and tables), Oracle Life Sciences, Munich, Germany; for their services and support. These contributions (as well as study presented in this publication) were funded by HiPP GmbH & Co. Vertrieb KG, Pfaffenhofen, Germany.
Conflicts of Interest
E.M.H. is employed at HiPP GmbH & Co. Vertrieb KG, Pfaffenhofen, Germany. G.G. and B.L. received honoraries from HiPP GmbH & Co. Vertrieb KG as Scientific Advisors/Consultants.
References
- Davisse-Paturet, C.; Adel-Patient, K.; Forhan, A.; Lioret, S.; Annesi-Maesano, I.; Heude, B.; Charles, M.A.; de Lauzon-Guillain, B. Breastfeeding initiation or duration and longitudinal patterns of infections up to 2 years and skin rash and respiratory symptoms up to 8 years in the EDEN mother-child cohort. Matern. Child. Nutr. 2020, 16, e12935. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. J. Nutr. 2005, 135, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.-Y.; Tan, L.T.-H.; Law, J.W.-F.; Hong, K.-W.; Ratnasingam, V.; Ab Mutalib, N.-S.; Lee, L.-H.; Letchumanan, V. Exploring the potential of human milk and formula milk on infants’ gut and health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins—Mechanisms of action. J. Nutr. Biochem. 2014, 25, 503–514. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537s–1543s. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Dallas, D.C. Milk proteins are predigested within the human mammary gland. J. Mammary Gland. Biol. Neoplasia 2017, 22, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Beverly, R.L.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Dallas, D.C. Differences in human milk peptide release along the gastrointestinal tract between preterm and term infants. Clin. Nutr. 2021, 40, 1214–1223. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides released by in vitro digestion of standard and hydrolyzed infant formulas. Peptides 2015, 73, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Lönnerdal, B. Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Pediatr. Res. 2015, 77, 546–553. [Google Scholar] [CrossRef]
- Wada, Y.; Phinney, B.S.; Weber, D.; Lönnerdal, B. In vivo digestomics of milk proteins in human milk and infant formula using a suckling rat pup model. Peptides 2017, 88, 18–31. [Google Scholar] [CrossRef]
- Beverly, R.L.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Dallas, D.C. Milk peptides survive in vivo gastrointestinal digestion and are excreted in the tool of infants. J. Nutr. 2020, 150, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. The gut as communicator between environment and host: Immunological consequences. Eur. J. Pharmacol. 2011, 668, S16–S32. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd-Markwick, K.J. Food proteins as a source of bioactive peptides with diverse functions. Br. J. Nutr. 2012, 108 (Suppl. S2), S149–S157. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy. Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef]
- Freidl, R.; Garib, V.; Linhart, B.; Haberl, E.M.; Mader, I.; Szépfalusi, Z.; Schmidthaler, K.; Douladiris, N.; Pampura, A.; Varlamov, E.; et al. Extensively hydrolyzed hypoallergenic infant formula with retained T cell reactivity. Nutrients 2023, 15, 111. [Google Scholar] [CrossRef]
- Golkar, A.; Milani, J.M.; Vasiljevic, T. Altering allergenicity of cow’s milk by food processing for applications in infant formula. Crit. Rev. Food Sci. Nutr. 2019, 59, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, I.; Pazdirkova, A.; Lokaj, J.; Szepfalusi, Z.; Hrstkova, H. Early feeding in infancy and atopic dermatitis—A prospective observational study. Klin. Padiatr. 2009, 221, 78–82. [Google Scholar] [CrossRef]
- Strozyk, A.; Horvath, A.; Meyer, R.; Szajewska, H. Efficacy and safety of hydrolyzed formulas for cow’s milk allergy management: A systematic review of randomized controlled trials. Clin. Exp. Allergy 2020, 50, 766–779. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Effects of different industrial heating processes of milk on site-specific protein modifications and their relationship to in vitro and in vivo digestibility. J. Agric. Food Chem. 2014, 62, 4175–4185. [Google Scholar] [CrossRef]
- The Global Proteome Machine. 2012. Available online: https://www.thegpm.org/crap/ (accessed on 31 July 2024).
- Milk Bioactive Peptide Database. 2024. Available online: https://mbpdb.nws.oregonstate.edu/ (accessed on 31 July 2024).
- Nielsen, S.D.-H.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.-J.; Dallas, D.C. Bioactive milk peptides: An updated comprehensive overview and database. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Suzuki, M.; Oda, M.; Tanabe, S. Enzyme-modified cheese exerts inhibitory effects on allergen permeation in rats suffering from indomethacin-induced intestinal inflammation. Biosci. Biotechnol. Biochem. 2008, 72, 1740–1745. [Google Scholar] [CrossRef]
- Totsuka, M.; Kakehi, M.; Kohyama, M.; Hachimura, S.; Hisatsune, T.; Kaminogawa, S. Enhancement of antigen-specific IFN-γ production from CD8+T cells by a single amino acid-substituted peptide derived from bovine αs1-Casein. Clin. Immunol. Immunopathol. 1998, 88, 277–286. [Google Scholar] [CrossRef]
- Kaetzel, C.S.; Mostov, K. Immunoglobulin transport and the polymeric immunoglobulin receptor. Mucosal Immunol. 2005, 211–250. [Google Scholar] [CrossRef]
- Dowbenko, D.; Kikuta, A.; Fennie, C.; Gillett, N.; Lasky, L.A. Glycosylation-dependent cell adhesion molecule 1 (GlyCAM 1) mucin is expressed by lactating mammary gland epithelial cells and is present in milk. J. Clin. Investig. 1993, 92, 952–960. [Google Scholar] [CrossRef]
- Raikos, V.; Dassios, T. Health-promoting properties of bioactive peptides derived from milk proteins in infant food: A review. Dairy. Sci. Technol. 2014, 94, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Li, F.; Dhordain, P.; Hearn, M.T.W.; Martin, L.L.; Bennett, L.E. Comparative yields of antimicrobial peptides released from human and cow milk proteins under infant digestion conditions predicted by in silico methodology. Food Funct. 2023, 14, 5442–5452. [Google Scholar] [CrossRef]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates—A review. Int. Dairy. J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- Gomes, R.D.S.; Anaya, K.; Galdino, A.B.S.; Oliveira, J.P.F.; Gama, M.A.S.; Medeiros, C.A.C.X.; Gavioli, E.C.; Porto, A.L.F.; Rangel, A.H.N. Bovine colostrum: A source of bioactive compounds for prevention and treatment of gastrointestinal disorders. NFS J. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Silva, S.V.; Malcata, F.X. Caseins as source of bioactive peptides. Int. Dairy. J. 2005, 15, 1–15. [Google Scholar] [CrossRef]
- Minorova, A.; Romanchuk, I.; Zhukova, Y.; Krushelnytska, N.; Vezhlivtseva, S. Protein composition and technological properties of milk whey concentrates. Agric. Sci. Pract. 2017, 4, 52–58. [Google Scholar] [CrossRef]
- Montone, C.M.; Aita, S.E.; Cavaliere, C.; Cerrato, A.; Laganà, A.; Piovesana, S.; Capriotti, A.L. High-resolution mass spectrometry and chemometrics for the detailed characterization of short endogenous peptides in milk by-products. Molecules 2021, 26, 6472. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Laganà, A. Recent trends in the analysis of bioactive peptides in milk and dairy products. Anal. Bioanal. Chem. 2016, 408, 2677–2685. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; López-Fandiño, R. Immunomodulating peptides for food allergy prevention and treatment. Crit. Rev. Food Sci. Nutr. 2017, 58, 1629–1649. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lönnerdal, B. Osteopontin in human milk and infant formula affects infant plasma osteopontin concentrations. Pediatr. Res. 2019, 85, 502–505. [Google Scholar] [CrossRef]
- Alissafi, T.; Kourepini, E.; Simoes, D.C.M.; Paschalidis, N.; Aggelakopoulou, M.; Sparwasser, T.; Boon, L.; Hammad, H.; Lambrecht, B.N.; Panoutsakopoulou, V. Osteopontin promotes protective antigenic tolerance against experimental allergic airway disease. J. Immunol. 2018, 200, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Kulesza-Brończyk, B.; Bień, A.; Sobieraj, P.; Orczyk-Pawiłowicz, M.; Lis-Kuberka, J.; Czosnykowska-Łukacka, M.; Bzikowska-Jura, A. Factors affecting total protein and lactoferrin in human milk. Sci. Rep. 2023, 13, 22434. [Google Scholar] [CrossRef]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The role of lactoferrin in intestinal health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef]
- Dierick, M.; Vanrompay, D.; Devriendt, B.; Cox, E. Lactoferrin, a versatile natural antimicrobial glycoprotein that modulates the host’s innate immunity. Biochem. Cell Biol. 2021, 99, 61–65. [Google Scholar] [CrossRef]
- Meisel, H.; Bockelmann, W. Bioactive peptides encrypted in milk proteins: Proteolytic activation and thropho-functional properties. Antonie Van Leeuwenhoek 1999, 76, 207–215. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins: An update. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Reddi, S.; Devi, S.; Mada, S.B.; Kapila, R.; Kapila, S. Nrf2 dependent antiaging effect of milk-derived bioactive peptide in old fibroblasts. J. Cell Biochem. 2019, 120, 9677–9691. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast pathology in inflammatory diseases. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.J.; Leaphart, C.L.; Mollen, K.P.; Hackam, D.J. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock 2007, 27, 124–133. [Google Scholar] [CrossRef]
- Liu, C.Y.; Cham, C.M.; Chang, E.B. Epithelial wound healing in inflammatory bowel diseases: The next therapeutic frontier. Transl. Res. 2021, 236, 35–51. [Google Scholar] [CrossRef]
- Sommer, K.; Wiendl, M.; Müller, T.M.; Heidbreder, K.; Voskens, C.; Neurath, M.F.; Zundler, S. Intestinal mucosal wound healing and barrier integrity in IBD–crosstalk and trafficking of cellular players. Front. Med. 2021, 8, 643973. [Google Scholar] [CrossRef]
- Pearson, A.D.; Eastham, E.J.; Laker, M.F.; Craft, A.W.; Nelson, R. Intestinal permeability in children with Crohn’s disease and coeliac disease. Br. Med. J. Clin. Res. Ed. 1982, 285, 20–21. [Google Scholar] [CrossRef]
- The Effect of Low Protein, Extensively Hydrolyzed Infant Formula on Allergy Prevention in at-Risk Infants up to 1 Year of Age: A Randomized, Double-Blind, Controlled Intervention Study and the Long-Term Effect on Allergy Prevention of Early Nutrition Given in the First 120 Days of Life in at-Risk Infants until the Child Is 6 Years of Age. 2024; to be submitted. Available online: https://clinicaltrials.gov/study/NCT03489733?cond=Prevention%20of%20Allergic%20Diseases%20in%20Infants&rank=1 (accessed on 14 September 2024).
- Galán, J.E. Salmonella Typhimurium and inflammation: A pathogen-centric affair. Nat. Rev. Microbiol. 2021, 19, 716–725. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Korhonen, H.J. Production and properties of health-promoting proteins and peptides from bovine colostrum and milk. Cell Mol. Biol. 2013, 59, 12–24. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).