The Role of Complementary Feeding Practices in Addressing the Double Burden of Malnutrition among Children Aged 6–23 Months: Insight from the Vietnamese General Nutrition Survey 2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Anthropometric Measurement
2.3. Biochemical Assessment and Definitions of Micronutrient Deficiencies
2.4. Breastfeeding and Complementary Feeding Practices
2.5. Other Demographic and Socioeconomic Determinants
2.6. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Adherence to IYCF Practices
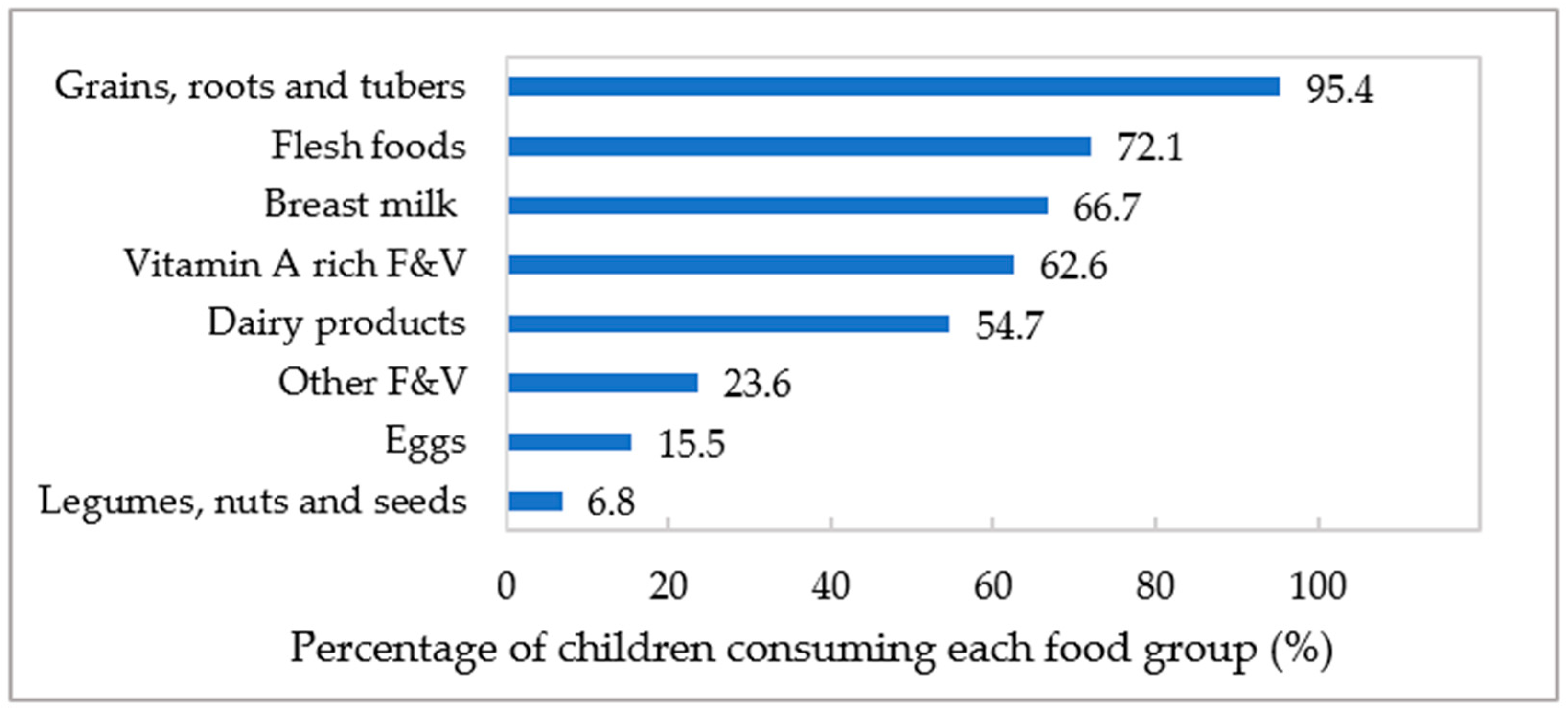
3.3. Consumption of MDD’s Eight Food Groups
3.4. Associations of Feeding Practices with Demographic and Socioeconomic Determinants
3.5. Associations of Feeding Practices with Malnutrition and MNDs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Le Nguyen, B.K.; Le, T.H.; Nguyen, D.V.A.; Tran, T.N.; Nguyen, H.C.; Tran, T.D.; Deurenberg, P.; Khouw, I. Double burden of undernutrition and overnutrition in Vietnam in 2011: Results of the SEANUTS study in 0.5–11-year-old children. Br. J. Nutr. 2013, 110, S45–S56. [Google Scholar] [CrossRef] [PubMed]
- Beal, T.; Le, D.T.; Trinh, T.H.; Burra, D.D.; Huynh, T.; Duong, T.T.; Truong, T.M.; Nguyen, D.S.; Nguyen, K.T.; de Haan, S.; et al. Child stunting is associated with child, maternal, and environmental factors in Vietnam. Matern. Child Nutr. 2019, 15, e12826. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Dominguez, G.; Neves, P.A.R.; Barros, A.J.D.; Victora, C.G. Complementary feeding practices in 80 low- and middle-income countries: Prevalence of and socioeconomic inequalities in dietary diversity, meal frequency, and dietary adequacy. J. Nutr. 2021, 151, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee On Nutrition. Advocacy for improving nutrition in the first 1000 days to support childhood development and adult health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Bedogni, G.; Brambilla, P.; Cianfarani, S.; Nobili, V.; Pietrobelli, A.; Agostoni, C. Epigenetic mechanisms elicited by nutrition in early life. Nutr. Res. Rev. 2011, 24, 198–205. [Google Scholar] [CrossRef]
- De Sanctis, V.; Soliman, A.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N. Early and long-term consequences of nutritional stunting: From childhood to adulthood. Acta Biomed. 2021, 92, e2021168. [Google Scholar] [CrossRef]
- World Health Organization; UNICEF. Indicators for Assessing Infant and Young Child Feeding Practices: Part 2: Measurement; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Hajeebhoy, N.; Nguyen, P.H.; Tran do, T.; de Onis, M. Introducing infant and young child feeding indicators into national nutrition surveillance systems: Lessons from Vietnam. Matern. Child Nutr. 2013, 9 Suppl 2, 131–149. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, P.H.; Hajeebhoy, N.; Nguyen, H.V.; Frongillo, E.A. Infant and young child feeding practices differ by ethnicity of Vietnamese mothers. BMC Pregnancy Childbirth 2016, 16, 214. [Google Scholar] [CrossRef]
- Tan, P.Y.; Som, V.S.; Nguyen, D.S.; Tan, X.; Tran, T.D.; Tran, T.N.; Tran, K.V.; Dye, L.; Moore, J.B.; Caton, S.; et al. Demographic variation and socioeconomic inequalities associated with the triple burden of malnutrition in Vietnamese children aged 6 months to 9 years old: Findings from the Vietnamese General Nutrition Survey 2020. medRxiv 2024. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Vietnam. In Vietnam National Nutrition Strategy 2021-2030 with vision to 2040; Medical Publishing House: Hanoi, Vietnam, 2021.
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- International Zinc Nutrition Consultative Group. Assessing Population Zinc Status with Serum Zinc Concentration; International Zinc Nutrition Consultative Group: Oakland, CA, USA, 2012. [Google Scholar]
- Rutstein, S.O.; Staveteig, S. Making the Demographic and Health Surveys Wealth Index Comparable; DHS Methodological Reports No. 9; ICF International: Rockville, MD, USA, 2013. [Google Scholar]
- von Hippell, P.T. Regression with missing Ys: An improved strategy for analyzing multiply imputed data. Sociol. Methodol. 2007, 37, 83–117. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Menon, P.; Ruel, M.; Hajeebhoy, N. A situational review of infant and young child feeding practices and interventions in Viet Nam. Asia Pac. J. Clin. Nutr. 2011, 20, 359–374. [Google Scholar] [PubMed]
- Roy, A.; Hossain, M.M.; Hanif, A.A.M.; Khan, M.S.A.; Hasan, M.; Hossaine, M.; Shamim, A.A.; Ullah, M.A.; Sarkar, S.K.; Rahman, S.M.M.; et al. Prevalence of infant and young child feeding practices and differences in estimates of minimum dietary diversity using 2008 and 2021 definitions: Evidence from Bangladesh. Curr. Dev. Nutr. 2022, 6, nzac026. [Google Scholar] [CrossRef]
- Harris, J.; Nguyen, P.H.; Tran, L.M.; Huynh, P.N. Nutrition transition in Vietnam: Changing food supply, food prices, household expenditure, diet and nutrition outcomes. Food Secur. 2020, 12, 1141–1155. [Google Scholar] [CrossRef]
- Supthanasup, A.; Cetthakrikul, N.; Kelly, M.; Sarma, H.; Banwell, C. Determinants of complementary feeding indicators: A secondary analysis of Thailand Multiple Indicators Cluster Survey 2019. Nutrients 2022, 14, 4370. [Google Scholar] [CrossRef]
- Damtie, S.B.; Tefera, T.B.; Tegegne, H.M. Dietary diversity practice and associated factors among children aged 6–23 months in Robe Town, Bale Zone, Ethiopia. J. Nutr. Metab. 2020, 2020, 9190458. [Google Scholar] [CrossRef]
- Adhikari, R.K. Food Utilization Practices, Beliefs, and Taboos in Nepal an Overview; United States Agency for International Development: Washington, DC, USA, 2010. [Google Scholar]
- Belew, A.K.; Ali, B.M.; Abebe, Z.; Dachew, B.A. Dietary diversity and meal frequency among infant and young children: A community based study. Ital. J Pediatr. 2017, 43, 73. [Google Scholar] [CrossRef]
- Eicher-Miller, H.A.; Graves, L.; McGowan, B.; Mayfield, B.J.; Connolly, B.A.; Stevens, W.; Abbott, A. A scoping review of household factors contributing to dietary quality and food security in low-income households with school-age children in the United States. Adv. Nutr. 2023, 14, 914–945. [Google Scholar] [CrossRef]
- Ng, C.S.; Dibley, M.J.; Agho, K.E. Complementary feeding indicators and determinants of poor feeding practices in Indonesia: A secondary analysis of 2007 Demographic and Health Survey data. Public Health Nutr. 2012, 15, 827–839. [Google Scholar] [CrossRef]
- Na, M.; Aguayo, V.M.; Arimond, M.; Stewart, C.P. Risk factors of poor complementary feeding practices in Pakistani children aged 6–23 months: A multilevel analysis of the Demographic and Health Survey 2012–2013. Matern. Child Nutr. 2017, 13, e12463. [Google Scholar] [CrossRef] [PubMed]
- Monteith, H.; Checholik, C.; Galloway, T.; Sahak, H.; Shawanda, A.; Liu, C.; Hanley, A.J.G. Infant feeding experiences among Indigenous communities in Canada, the United States, Australia, and Aotearoa: A scoping review of the qualitative literature. BMC Public Health 2024, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Rohit, A.; Tonkin, E.; Maple-Brown, L.; Golley, R.; McCarthy, L.; Brimblecombe, J. Parent feeding practices in the Australian indigenous population within the context of non-indigenous Australians and indigenous populations in other high-income countries-a scoping review. Adv. Nutr. 2019, 10, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.J.; Powell, F.C.; Ali, N.; Penn-Jones, C.P.; Ochieng, B.; Constantinou, G.; Randhawa, G. ‘They are kids, let them eat’: A qualitative investigation into the parental beliefs and practices of providing a healthy diet for young children among a culturally diverse and deprived population in the UK. Int. J. Environ. Res. Public Health 2021, 18, 13087. [Google Scholar] [CrossRef]
- Lundberg, P.C.; Ngoc Thu, T.T. Breast-feeding attitudes and practices among Vietnamese mothers in Ho Chi Minh City. Midwifery 2012, 28, 252–257. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Keithly, S.C.; Nguyen, N.T.; Nguyen, T.T.; Tran, L.M.; Hajeebhoy, N. Prelacteal feeding practices in Vietnam: Challenges and associated factors. BMC Public Health 2013, 13, 932. [Google Scholar] [CrossRef]
- Duong, D.V.; Lee, A.H.; Binns, C.W. Determinants of breast-feeding within the first 6 months post-partum in rural Vietnam. J. Paediatr. Child Health 2005, 41, 338–343. [Google Scholar] [CrossRef]
- Thu, H.N.; Eriksson, B.; Khanh, T.T.; Petzold, M.; Bondjers, G.; Kim, C.N.; Thanh, L.N.; Ascher, H. Breastfeeding practices in urban and rural Vietnam. BMC Public Health 2012, 12, 964. [Google Scholar] [CrossRef]
- Almroth, S.; Arts, M.; Quang, N.D.; Hoa, P.T.; Williams, C. Exclusive breastfeeding in Vietnam: An attainable goal. Acta Paediatr. 2008, 97, 1066–1069. [Google Scholar] [CrossRef]
- Morrow, M. Breastfeeding in Vietnam: Poverty, tradition, and economic transition. J. Hum. Lact. 1996, 12, 97–103. [Google Scholar] [CrossRef]
- Marriott, B.P.; White, A.; Hadden, L.; Davies, J.C.; Wallingford, J.C. World Health Organization (WHO) infant and young child feeding indicators: Associations with growth measures in 14 low-income countries. Matern. Child Nutr. 2012, 8, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Som, S.V.; Van Der Hoeven, M.; Laillou, A.; Poirot, E.; Chan, T.; Polman, K.; Ponce, M.C.; Wieringa, F.T. Adherence to child feeding practices and child growth: A retrospective cohort analysis in Cambodia. Nutrients 2020, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Moursi, M.M.; Arimond, M.; Dewey, K.G.; Treche, S.; Ruel, M.T.; Delpeuch, F. Dietary diversity is a good predictor of the micronutrient density of the diet of 6- to 23-month-old children in Madagascar. J. Nutr. 2008, 138, 2448–2453. [Google Scholar] [CrossRef]
- Obbagy, J.E.; English, L.K.; Psota, T.L.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; et al. Complementary feeding and micronutrient status: A systematic review. Am. J. Clin. Nutr. 2019, 109, 852S–871S. [Google Scholar] [CrossRef]
- Khan, N.C.; Khoi, H.H.; Giay, T.; Nhan, N.T.; Nhan, N.T.; Dung, N.C.; Thang, H.V.; Dien, D.N.; Luy, H.T. Control of vitamin A deficiency in Vietnam: Achievements and future orientation. Food Nutr. Bull. 2002, 23, 133–142. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Z.; Wu, Y.; Wang, Y.; Wang, J.; Zhou, L.; Ni, Z.; Hao, L.; Yang, N.; Yang, X. Early feeding of larger volumes of formula milk is associated with greater body weight or overweight in later infancy. Nutr. J. 2018, 17, 12. [Google Scholar] [CrossRef]
- Le Huerou-Luron, I.; Blat, S.; Boudry, G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and food preference. J. Nutr. 2012, 142, 1142S–1148S. [Google Scholar] [CrossRef]
- Tan, X.; Tan, P.Y.; Gong, Y.Y.; Moore, J.B. Overnutrition is a risk factor for iron, but not for zinc or vitamin A deficiency in children and young people: A systematic review and meta-analysis. BMJ Glob. Health 2024, 9, e015135. [Google Scholar] [CrossRef]
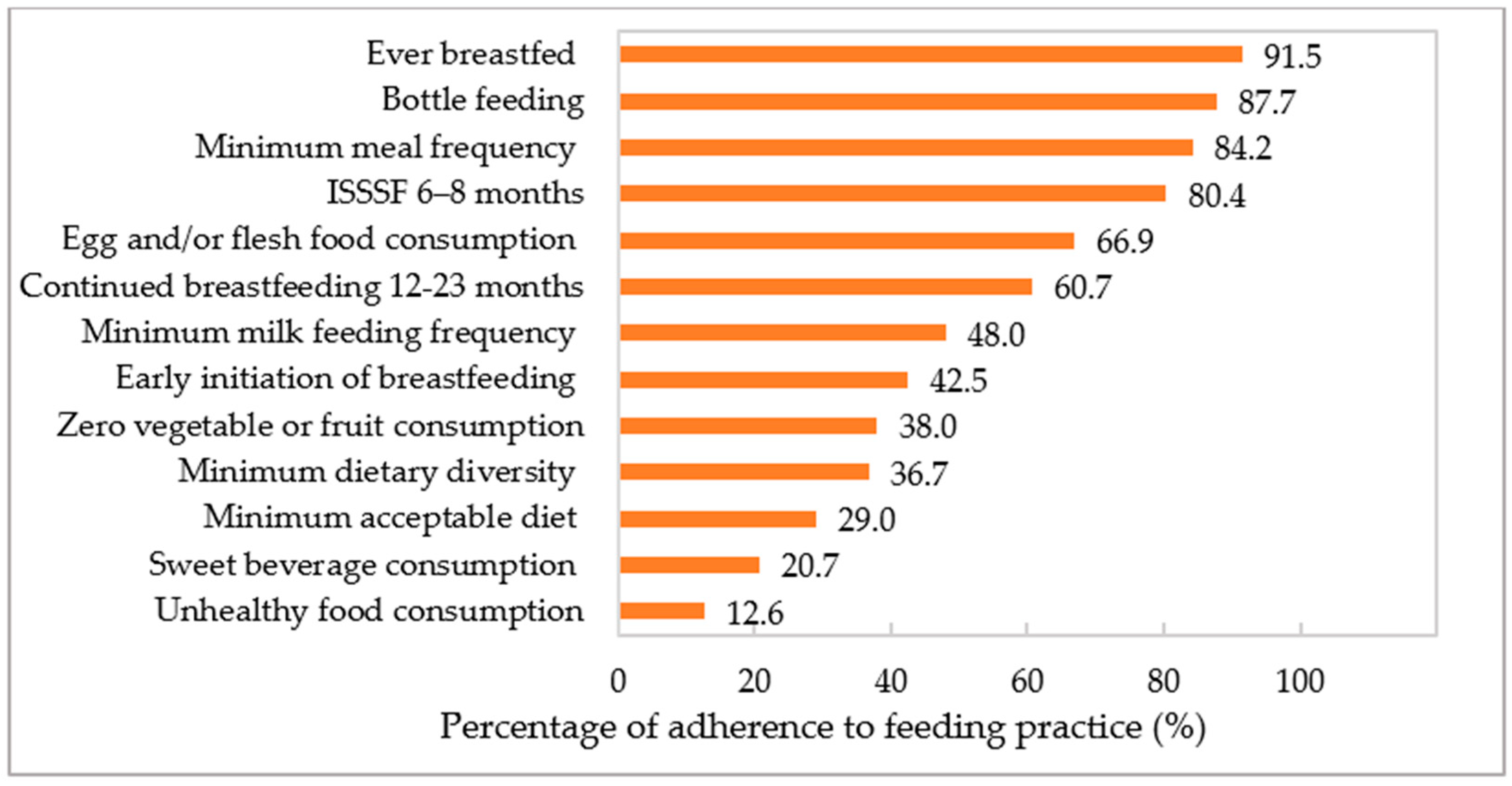
| Indicators | Definition |
|---|---|
| Ever breastfed (EvBF) | Proportion of children aged 6–23 months who were ever breastfed. |
| Early initiation of breastfeeding (EIBF) | Proportion of newborns who were put to the breast within one hour of birth. |
| Continued breastfeeding 12–23 months (CBF) | Proportion of children aged 6–23 months who continued breastfeeding. |
| Bottle feeding | Proportion of children aged 6–23 months who were bottle-fed. |
| Introduction of solid, semi-solid, or soft foods after 6–8 months (ISSSF) | Proportion of children aged 6–8 months who received solid, semi-solid, or soft foods. |
| Minimum dietary diversity (MDD) | Proportion of children aged 6–23 months who consumed at least five out of eight food groups which included (1) breast milk, (2) grains, roots, and tubers, (3) legumes, nuts, and seeds, (4) dairy products, (5) flesh foods, (6) eggs, (7) vitamin A-rich fruits and vegetables, and (8) other fruits and vegetables. |
| Minimum meal frequency (MMF) | Proportion of breastfed and non-breastfed children aged 6–23 months who consumed solid, semi-solid, or soft foods the minimum number of times (i.e., ≥2 times for children aged 6–8 months, ≥3 times for breastfed children aged 9–23 months, and ≥4 times for non-breastfed children). |
| Minimum milk feeding frequency (MMFF) | Proportion of non-breastfed children aged 6–23 months who consumed ≥ 2 milk feeds. |
| Minimum acceptable diet (MAD) | Proportion of children aged 6–23 months who had adequate minimum dietary diversity and minimum meal frequency. |
| Egg and/or flesh food consumption (EFF) | Proportion of children aged 6–23 months who consumed meats, poultry, fish, eggs, or organ meats. |
| Sweet beverage consumption (SWB) | Proportion of children aged 6–23 months who consumed sugar-sweetened beverages. |
| Unhealthy food consumption (UFC) | Proportion of children aged 6–23 months who consumed fried foods (i.e., fried dough), salty snacks (i.e., chips, crisps), and sweet snacks (i.e., sweet, ice cream, chocolate, and cakes). |
| Zero vegetable and fruit consumption (ZVF) | Proportion of children aged 6–23 months who did not consume vegetables or fruits. |
| Variables | Total (n = 2039) | Males (n = 1037) | Females (n = 1002) | t-Test/Chi Square p-Value |
|---|---|---|---|---|
| Demographic and socioeconomic indicators | ||||
| Age (months) | 14 ± 5 | 14 ± 5 | 14 ± 5 | t = 3.75, p = 0.068 |
| Age groups | χ2 = 8.07, p = 0.117 | |||
| 6–11 months | 730 (37.3) | 347 (34.3) | 383 (40.4) | |
| 12–23 months | 1309 (62.7) | 690 (65.7) | 619 (59.6) | |
| Geographical area | χ2 = 9.02, p = 0.075 | |||
| Northern mountains | 299 (15.0) | 167 (16.8) | 132 (13.1) | |
| Red River Delta | 340 (27.6) | 165 (25.7) | 175 (29.4) | |
| North central and central coastal | 365 (23.1) | 174 (22.4) | 191 (23.9) | |
| Central highlands | 334 (6.1) | 172 (6.4) | 162 (5.9) | |
| Southeast | 316 (14.8) | 165 (15.6) | 151 (13.9) | |
| Mekong River Delta | 385 (13.4) | 194 (13.0) | 191 (13.8) | |
| Area of residence | χ2 = 0.02, p = 0.899 | |||
| Urban | 634 (27.5) | 323 (27.7) | 311 (27.4) | |
| Rural | 1405 (72.5) | 714 (72.3) | 691 (72.6) | |
| Ethnicity | χ2 = 2.57, p = 0.134 | |||
| Kinh | 1619 (84.6) | 814 (83.3) | 805 (85.9) | |
| Others | 420 (15.4) | 223 (16.7) | 197 (14.1) | |
| Wealth quintiles | χ2 = 3.10, p = 0.521 | |||
| Poorest | 308 (12.5) | 169 (13.5) | 139 (11.4) | |
| Poorer | 489 (17.6) | 214 (17.0) | 248 (18.2) | |
| Middle | 509 (21.9) | 255 (21.1) | 254 (22.8) | |
| Richer | 490 (30.8) | 250 (30.7) | 240 (30.8) | |
| Richest | 243 (17.2) | 122 (17.7) | 121 (16.7) | |
| Anthropometric parameters | ||||
| Height (cm) | 76.9 ± 12.0 | 76.3 ± 5.9 | 77.5 ± 15.9 | t = 0.22, p = 0.646 |
| Weight (kg) | 10.1 ± 5.4 | 9.7 ± 1.7 | 10.4 ± 7.4 | t = 0.28, p = 0.602 |
| Height-for-age z score (HAZ) | −0.45 ± 1.37 | −0.57 ± 1.39 | −0.33 ± 1.34 | t = 8.25, p = 0.010 |
| Stunting (HAZ < −2 SD) | 213 (10.9) | 136 (14.0) | 77 (7.7) | χ2 = 17.67, p = 0.001 |
| Weight-for-age z score (WAZ) | −0.35 ± 1.15 | −0.40 ± 1.20 | −0.30 ± 1.09 | t = 2.29, p = 0.146 |
| Underweight (WAZ < −2 SD) | 117 (5.6) | 76 (7.2) | 41 (3.9) | χ2 = 8.75, p = 0.003 |
| Weight-for-height z score (WHZ) | −0.16 ± 1.06 | −0.14 ± 1.10 | −0.19 ± 1.01 | t = 1.61, p = 0.219 |
| Normal-weight (−2 SD ≤WHZ ≤ + 2 SD) | 1617 (93.6) | 816 (93.5) | 801 (93.7) | χ2 = 0.56, p = 0.836 |
| Wasting (WHZ < −2 SD) | 64 (3.4) | 37 (3.6) | 27 (3.2) | |
| Overweight (+2 SD < WHZ ≤ +3 SD) | 42 (2.5) | 21 (2.3) | 21 (2.6) | |
| Obesity (WHZ > +3 SD) | 10 (0.6) | 7 (0.7) | 3 (0.5) | |
| Biomarkers of micronutrients | ||||
| Hemoglobin (g/dL) | 11.4 ± 1.1 | 11.3 ± 1.2 | 11.4 ± 1.1 | t = 1.24, p = 0.279 |
| Serum ferritin (µg/L) a | 19.3 ± 0.6 | 17.2 ± 0.7 | 21.8 ± 1.0 | t = 21.32, p < 0.001 |
| Serum transferrin receptor (mg/L) | 8.1 ± 3.8 | 8.6 ± 4.1 | 7.5 ± 3.2 | t = 30.45, p < 0.001 |
| Body iron store | 1.9 ± 4.4 | 1.3 ± 4.4 | 2.6 ± 4.3 | t = 20.42, p < 0.001 |
| Serum zinc (µmol/L) | 9.7 ± 6.1 | 9.7 ± 6.1 | 9.7 ± 6.1 | t = 0.001, p = 0.979 |
| Serum retinol (µmol/L) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | t = 1.57, p = 0.226 |
| Retinol-binding protein (µmol/L) | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.0 ± 0.4 | t = 1.83, p = 0.192 |
| C-reactive protein (mg/L) | 1.9 ± 5.7 | 2.1 ± 6.3 | 1.6 ± 4.9 | t = 2.17, p = 0.158 |
| Alpha-1 acid glycoprotein (mg/L) | 0.67 ± 0.3 | 0.69 ± 0.4 | 0.65 ± 0.3 | t = 5.19, p = 0.035 |
| Micronutrient deficiencies (MNDs) | ||||
| Anemia | 583 (31.2) | 310 (32.4) | 273 (29.9) | χ2 = 1.33, p = 0.202 |
| Iron deficiency | 626 (34.6) | 379 (40.0) | 247 (28.8) | χ2 = 23.93, p = 0.009 |
| Iron deficiency anemia | 347 (17.8) | 212 (21.0) | 135 (14.3) | χ2 = 13.80, p = 0.001 |
| Low serum zinc | 1066 (56.7) | 549 (56.1) | 517 (57.5) | χ2 = 0.36, p = 0.499 |
| Low serum retinol | 254 (14.3) | 24 (14.3) | 123 (14.3) | χ2 = 0.0003, p = 0.989 |
| Total number of MNDs * | ||||
| 0–1 | 981 (48.9) | 467 (46.0) | 514 (52.0) | χ2 = 7.29, p = 0.018 |
| 2–4 | 1058 (51.1) | 570 (54.0) | 488 (48.0) | |
| Inflammation | 335 (18.5) | 182 (19.4) | 153 (17.4) | χ2 = 1.17, p = 0.331 |
| Variables | Early Initiation of Breastfeeding | Continued Breastfeeding (12–23 Months) | Minimum Dietary Diversity | Minimum Meal Frequency | Minimum Milk Feeding Frequency | Minimum Acceptable Diet | Egg and/or Flesh Foods Consumption | Sweet Beverage Consumption | Unhealthy Food Consumption | Zero Vegetable or Fruit Consumption | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Age (Ref: 6–11 months) | 12–23 months | 1.91 (1.49, 2.46) *** | 4.26 (3.00, 6.06) *** | 2.15 (1.61, 2.86) *** | 1.69 (1.26, 2.25) *** | 2.52 (1.60, 3.95) *** | 3.37 (2.53, 4.49) *** | ||||
| Area of residence (Ref: urban) | Rural | 0.98 (0.68, 1.40) | 0.65 (0.49, 0.86) ** | 0.77 (0.60, 0.98) * | 1.02 (0.76, 1.38) | ||||||
| Mountainous | 0.40 (0.22, 0.71) ** | 0.44 (0.26, 0.74) ** | 0.48 (0.23, 1.00) | 0.52 (0.32, 0.85) * | |||||||
| Ethnicity (Ref: Kinh major) | Minorities | 0.39 (0.21, 0.71) ** | 0.65 (0.54, 0.79) *** | 0.56 (0.34, 0.91) * | 0.36 (0.20, 0.64) ** | 0.41 (0.29, 0.57) *** | 1.86 (1.32, 2.63) *** | ||||
| Wealth quintiles (Ref: poorest) | Poorer | 1.84 (0.92, 3.68) | 1.19 (0.66, 2.15) | 1.50 (0.94, 2.40) | 1.55 (0.88, 2.70) | 0.85 (0.50, 1.43) | |||||
| Middle | 2.19 (0.95, 5.03) | 1.65 (0.94, 2.90) | 1.67 (0.98, 2.86) | 2.76 (1.72, 4.41) *** | 0.56 (0.35, 0.89) * | ||||||
| Richer | 2.79 (1.22, 6.35) * | 1.80 (0.97, 3.32) | 2.43 (1.29, 4.57) ** | 2.82 (1.51, 5.28) ** | 0.49 (0.30, 0.78) ** | ||||||
| Richest | 2.32 (1.04, 5.19) * | 2.69 (1.28, 5.67) * | 2.23 (1.41, 3.54) ** | 2.89 (1.72, 4.83) *** | 0.42 (0.25, 0.70) ** | ||||||
| Geographical area (Ref: Northern mountains) | Red River Delta | 1.93 (0.30, 12.34) | 2.54 (1.14, 5.65) * | 1.81 (1.06, 3.10) * | 1.64 (0.79, 3.40) | 0.67 (0.29, 1.53) | |||||
| North central and central coastal | 5.20 (1.22, 22.15) * | 1.81 (0.87, 3.75) | 1.04 (0.49, 2.22) | 2.19 (1.02, 3.96) * | 0.45 (0.20, 0.99) * | ||||||
| Central highlands | 4.08 (0.70, 23.77) | 1.33 (0.34, 5.24) | 0.89 (0.26, 3.05) | 0.77 (0.32, 1.82) | 0.41 (0.13, 1.38) | ||||||
| Southeast | 2.17 (0.61, 7.77) | 2.63 (1.42, 4.89) *** | 2.39 (1.36, 4.21) ** | 1.69 (0.77, 3.72) | 0.80 (0.33, 1.99) | ||||||
| Mekong River Delta | 5.88 (1.61, 21.40) ** | 2.63 (1.42, 4.89) ** | 1.02 (0.54, 1.94) | 0.98 (0.37, 2.60) | 0.91 (0.37, 2.23) | ||||||
| Variables | Stunting (HAZ < −2 SD) | Underweight (WAZ < −2 SD) | Wasting (WHZ < −2 SD) | Overweight (WHZ > +2 SD) |
|---|---|---|---|---|
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | |
| Continued breastfeeding (12–23 months) | 0.50 (0.27, 0.92) * | |||
| Introduction of solid, semi-solid, or soft foods at 6–8 months | 0.13 (0.04, 0.40) *** | |||
| Adequate minimum dietary diversity | 0.61 (0.41, 0.92) * | |||
| Adequate minimum milk feeding frequency | 3.33 (1.01, 11.09) * | |||
| Egg and/or flesh foods consumption | 0.68 (0.48, 0.97) * | |||
| Sweet beverage consumption | 0.64 (0.42, 0.99) * | |||
| Zero vegetable or fruit consumption | 1.55 (1.18, 2.04) ** |
| Variables | Anemia | Iron Deficiency | Iron Deficiency Anemia | Low Serum Zinc | Low Serum Retinol |
|---|---|---|---|---|---|
| AOR (95% CI) b | AOR (95% CI) a | AOR (95% CI) a | AOR (95% CI) b | AOR (95% CI) b | |
| Continued breastfeeding (12–23 months) | 0.75 (0.58, 0.98) * | 0.70 (0.52, 0.96) * | |||
| Introduction of solid, semi-solid, or soft foods at 6–8 months | 0.37 (0.16, 0.89) * | ||||
| Adequate minimum dietary diversity | 0.69 (0.54, 0.88) ** | 0.63 (0.46, 0.88) ** | |||
| Adequate minimum milk feeding frequency | 0.56 (0.38, 0.82) ** | ||||
| Adequate minimum acceptable diet | 0.72 (0.57, 0.91) ** | 0.66 (0.52, 0.84) ** | 0.56 (0.42, 0.75) *** | 0.63 (0.41, 0.99) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, P.Y.; Som, S.V.; Nguyen, S.D.; Tran, D.T.; Tran, N.T.; Tran, V.K.; Dye, L.; Moore, J.B.; Caton, S.; Ensaff, H.; et al. The Role of Complementary Feeding Practices in Addressing the Double Burden of Malnutrition among Children Aged 6–23 Months: Insight from the Vietnamese General Nutrition Survey 2020. Nutrients 2024, 16, 3240. https://doi.org/10.3390/nu16193240
Tan PY, Som SV, Nguyen SD, Tran DT, Tran NT, Tran VK, Dye L, Moore JB, Caton S, Ensaff H, et al. The Role of Complementary Feeding Practices in Addressing the Double Burden of Malnutrition among Children Aged 6–23 Months: Insight from the Vietnamese General Nutrition Survey 2020. Nutrients. 2024; 16(19):3240. https://doi.org/10.3390/nu16193240
Chicago/Turabian StyleTan, Pui Yee, Somphos Vicheth Som, Son Duy Nguyen, Do Tranh Tran, Nga Thuy Tran, Van Khanh Tran, Louise Dye, J. Bernadette Moore, Samantha Caton, Hannah Ensaff, and et al. 2024. "The Role of Complementary Feeding Practices in Addressing the Double Burden of Malnutrition among Children Aged 6–23 Months: Insight from the Vietnamese General Nutrition Survey 2020" Nutrients 16, no. 19: 3240. https://doi.org/10.3390/nu16193240
APA StyleTan, P. Y., Som, S. V., Nguyen, S. D., Tran, D. T., Tran, N. T., Tran, V. K., Dye, L., Moore, J. B., Caton, S., Ensaff, H., Lin, X., Smith, G., Chan, P., & Gong, Y. Y. (2024). The Role of Complementary Feeding Practices in Addressing the Double Burden of Malnutrition among Children Aged 6–23 Months: Insight from the Vietnamese General Nutrition Survey 2020. Nutrients, 16(19), 3240. https://doi.org/10.3390/nu16193240





