Abstract
Phenylketonuria (PKU) is an inherited metabolic disorder that requires lifelong adherence to a low-phenylalanine (Phe) diet to prevent severe neurological complications. However, maintaining dietary adherence can be challenging for patients and their families. This systematic review aimed to comprehensively evaluate the factors affecting adherence to a low-Phe diet in patients with PKU. A systematic search of multiple databases was conducted, and 49 studies were included in the final analysis. The quality of evidence was assessed using the Joanna Briggs Institute levels of evidence and the Quality Assessment with Diverse Studies tool. The review identified four main categories of factors influencing dietary adherence: family-related factors (social, psychological, behavioral, and educational), patient-specific factors (psychological, behavioral, educational, and demographic), environmental factors (healthcare professional support, educational and camp-based interventions, and the COVID-19 pandemic), and therapy-related factors (protein substitute formulation, clinic visits, blood tests, and telemedicine). The findings highlight the complex interplay between elements contributing to dietary adherence in PKU patients and underscore the importance of a multifaceted approach to support patients and their families. Future research should prioritize high-quality longitudinal and experimental studies to provide stronger evidence for the PKU community.
Keywords:
phenylketonuria; PKU; dietary adherence; low-Phe diet; compliance; barriers; facilitators; systematic review 1. Introduction
Phenylketonuria (PKU) is an inborn error of metabolism caused by a deficiency in the enzyme phenylalanine hydroxylase (PAH), which is responsible for converting the amino acid phenylalanine (Phe) into tyrosine [1]. The resulting accumulation of Phe in the blood can lead to severe intellectual disability, seizures, and other neurological problems if left untreated [2]. The primary treatment for PKU is a lifelong low-Phe diet, which involves restricting the intake of high-protein foods and supplementing patients with Phe-free or low-Phe amino acid mixtures [3]. Adherence to this strict dietary regimen is crucial for optimal health outcomes, but it can be challenging for patients and their families [4]. Dietary adherence in PKU is a complex behavior influenced by a multitude of factors, including individual patient characteristics, family dynamics, social support, and healthcare system factors [5]. Understanding these factors is essential for developing targeted interventions to improve adherence and, ultimately, health outcomes for patients with PKU. Previous studies have identified several barriers to dietary adherence, such as the restrictive nature of the diet, a lack of social support, and limited access to specialized care [6,7]. However, a comprehensive understanding of the factors influencing adherence across different age groups and settings is lacking.
Systematic reviews are a valuable tool for synthesizing evidence from multiple studies and identifying gaps in the literature [8]. By systematically searching, appraising, and synthesizing the available evidence, systematic reviews can provide a more reliable and comprehensive understanding of a topic than individual studies [9]. In the context of dietary adherence in PKU, a systematic review can help us to identify the most important factors influencing adherence and inform the development of evidence-based interventions to improve adherence and health outcomes.
Previous research has identified the influence of demographic and psychosocial factors on treatment adherence in children and adolescents with PKU [10]. While this review provided valuable insights into the factors affecting dietary adherence in pediatric population, there is a need for a comprehensive and up-to-date systematic review on the factors affecting dietary adherence in PKU across all age groups and settings to synthesize the available evidence and identify consistent patterns across studies.
2. Materials and Methods
2.1. Search Strategy
The search process used in this systematic review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11]. The review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO registration ID number CRD42023412538) [12]. A systematic search of MEDLINE/PubMed, The Cochrane Library, lens.org, and dimensions.ai databases was performed in November 2023. Abstracts of articles containing data on the prevalence of and/or factors associated with dietary adherence in patients with PKU were identified using keywords with search terms including “phenylketonuria”, “hyperPhemia”, “Phe hydroxylase deficiency”, “adherence”, and “low-Phe diet”, as well as their synonyms and combinations. Details of the search strategy are available in Table S1.
2.2. Eligibility Criteria
Eligibility criteria were developed in accordance with the Population, Intervention, Comparison, Outcomes and Study (PICOS) statement [13].
Population: Patients with PKU diagnosed according to well-defined criteria. Intervention: Low-Phe diet in patients with PKU.
Comparator: Comparisons were made between adherent and non-adherent patients with PKU to identify factors that influence dietary adherence.
Outcomes: Studies included a statistical examination of the correlation between therapy-related, socio-demographic, disease-related, and/or psychosocial factors and adherence to the low-Phe diet. For the prevalence of adherence to the low-Phe diet in patients with PKU, adherence was measured by self-report (or parent report), dietitian assessment, physician assessment, or serological markers. Additional outcomes: The documentation of other factors associated with adherence to the low-Phe diet (e.g., environmental factors, family-related factors, patient-specific factors).
Types of studies included: We included qualitative, cross-sectional, case–control, correlational, cohort, randomized controlled trial, and mixed-methods studies available in full-text form and published in English. Letters to the editor, commentaries, abstracts, Editorials, studies centered on animals, systematic reviews, and case reports were excluded.
2.3. Study Selection and Data Extraction
Two researchers (KK, VS) independently performed title and abstract screening to identify potentially relevant studies. Full-text articles were then assessed for eligibility based on the predetermined inclusion and exclusion criteria. Any disagreements were resolved through discussion and consensus.
Data extraction was conducted using a standardized form, capturing key information from each included study. The extracted data included the authors, year of publication, article title, study design, participant characteristics (age and sex), sample size, methods for assessing adherence to the low-Phe diet, factors associated with dietary adherence, key findings, and relevant statistical information. Summary tables were created to present the main features and findings of the included studies.
2.4. Quality Assessment
To ensure the validity and integrity of the findings of this systematic review, a rigorous quality assessment process was undertaken. Quality assessment was completed independently by two researchers (KK, VS), and conflicts were resolved through consensus. The Joanna Briggs Institute (JBI) levels of evidence (LOEs) were employed to rate the quality of evidence of the included studies [14]. The JBI LOE categorizes studies based on their methodological rigor and susceptibility to bias, with Level 1 representing the highest quality of evidence and Level 5 the lowest.
The JBI levels of evidence hierarchy encompasses the following:
Level 1: Experimental designs, including systematic reviews of randomized controlled trials (RCTs) and other experimental studies;
Level 2: Quasi-experimental designs, such as prospectively controlled studies without randomization;
Level 3: Observational analytic designs, which includes cohort and case–control studies;
Level 4: Observational descriptive studies, such as cross-sectional and case series studies;
Level 5: Expert opinion and bench research, including consensus and single expert opinions.
For the purpose of this review, each included study was classified according to the JBI LOE, and its methodological quality and risk of bias were further evaluated using the Quality Assessment with Diverse Studies (QuADS) developed by Harrison et al. (2021) [15]. The QuADS tool is well validated for assessing research of various designs, and it demonstrates good reliability in differentiating between high- and low-quality studies.
The assessment with QuADS covered 13 key items relevant to the qualitative and quantitative aspects of the studies. Each item was evaluated on a 4-point Likert scale, ranging from ‘not at all’ (0) to ‘complete’ (3). The items addressed critical aspects of study design, including the clarity of aims, appropriateness of methodology, use of robust data collection methods, and relevance of analyses. The scores for each item were summed to provide a total quality score for each study, with a maximum possible score of 39. This quantitative scoring enabled a standardized assessment of study quality, allowing for a clear comparison across studies with diverse methodologies.
2.5. Data Synthesis
A narrative synthesis approach was employed to summarize the results reported by each study. This involved identifying commonalities and differences among the studies, evaluating the methodologies used, synthesizing the main findings, and assessing the quality of evidence. Due to the heterogeneity in study populations, methods, and reported outcomes, a meta-analysis was not deemed appropriate. The narrative synthesis allowed for a comprehensive overview of the current evidence on factors affecting adherence to a low-Phe diet in patients with PKU.
3. Results
3.1. Study Selection
Our comprehensive search across multiple databases initially identified a total of 1637 articles. The screening of titles and abstracts led to the exclusion of 776 duplicates. After removing duplicates, the remaining 861 articles were subjected to a thorough review of titles and abstracts, which resulted in the further exclusion of 750 articles deemed irrelevant for the current review. Consequently, 111 full-text articles were assessed for their eligibility based on the predetermined inclusion and exclusion criteria. Of these, 62 articles were excluded for not meeting the criteria, leaving 49 studies that were included in the final analysis. These studies were evaluated for their exploration of various factors affecting adherence to a low-Phe diet in patients with PKU. The selection process is summarized in a flow diagram provided in Figure 1.
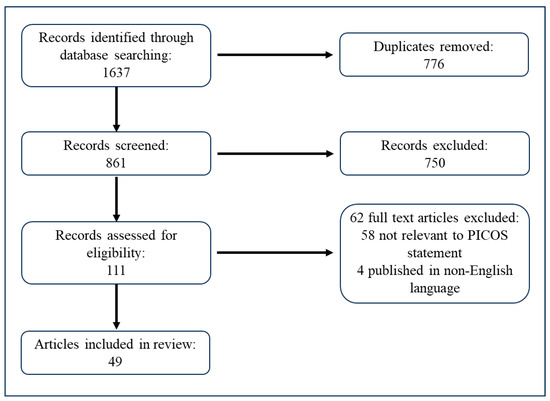
Figure 1.
A flow diagram illustrating the search and selection process.
3.2. Quality and Risk of Bias Assessment
The systematic review included a diverse array of study designs, as evidenced by the JBI levels of evidence score attributed to each study. Based on the JBI levels, the quality of evidence was varied, ranging from randomized controlled trials (RCTs) to observational studies without control groups.
Out of the 49 studies included in the review, 3 studies were categorized as Level 1.c, 2 studies were classified as Level 2.c, and studies were classified 2 as Level 2.d. Level 3 studies, which included observational analytic designs, represented the largest proportion of the included studies. This category encompassed 17 studies classified as Level 3.e (observational study without a control group) and 5 studies classified as Level 3.c (cohort study with control group). These studies contribute to the body of evidence but are subject to more bias compared to experimental studies due to the lack of randomization and control. The review also encompassed a significant number of descriptive studies, with 17 studies falling into Level 4.b (cross-sectional study) and 3 studies classified as Level 4.c (case series). These studies offer insights into associations between variables but do not provide a strong basis for causality due to their observational nature and potential confounding factors.
The quality of studies and risk of bias was further evaluated using the QuADS tool, which includes 13 criteria weighted toward methodological quality. The QuADS tool demonstrated good reliability and validity in assessing the risk of bias and quality across diverse study designs. The QuADS assessment revealed a range of methodological quality among the included studies, with total scores ranging from 18 to 34 out of a maximum of 39 (Table S2) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. On average, studies had a quality score of 27.76 out of 39 across the 13 QuADS items. The quality appraisal found that all of the studies scored high (2–3) in several key areas, indicating a lower risk of bias due to the clear description of the research setting and target population, the appropriateness of the study design to address the stated research aims, the format and content of the data collection tool being appropriate to address the stated research aims, and the method of analysis being appropriate to achieve the research aims. However, studies consistently scored lower (0–1), suggesting a higher risk of bias in other important methodological areas, such as the consideration and involvement of research stakeholders in study design or conduct (82%), evidence of consideration of the sampling approach (55%), the justification for the choice of analytic method (33%), the provision of recruitment data (29%), and critical discussion of study strengths and limitations (24%).
3.3. Description of Included Studies
Table 1 shows the overview of the studies included in the systematic review, encompassing details such as the study year, the number of participants, participants’ sex, the ages(s) of participants, the level of evidence, and the study design.

Table 1.
An overview of the included studies.
The studies were categorized based on the age groups of participants and the primary focus of the research as follows:
- Children and adolescents (<18 years): Studies that primarily involve children or adolescent participants or focus on issues specific to this age group;
- Adults (>20 years): These studies exclusively involve adult participants or focus on issues specific to adults with PKU;
- Mixed age groups: Studies that include participants from various age groups or do not specify a particular age range, covering a broad spectrum of PKU patients;
- Caregivers/Parents: These studies focus on the roles of caregivers or parents in managing PKU, their knowledge, and its impact on patient outcomes.
This categorization allows for a distinction between studies focusing on specific age groups while also recognizing the importance of caregiver/parent involvement and studies that span multiple age groups.
Our comprehensive review, encompassing a diverse range of studies spanning several decades and involving patients of all ages, caregivers, and healthcare providers, identified four main categories of factors influencing adherence to a low-Phe diet in patients with PKU: family-related factors, patient-specific factors, environmental factors, and therapy-related factors. It is important to note that the dietary treatment of PKU has evolved significantly over the years, particularly since 2000, with improvements in protein substitutes and the increased availability of low-protein foods. While we included pre-2000 studies, we carefully considered their context and relevance. These earlier studies primarily focused on psychosocial, family-related, and patient-specific factors rather than therapy-related aspects. As these factors remain relevant to adherence regardless of changes in dietary treatment, we deemed their inclusion valuable. This approach allowed us to leverage historical insights on enduring psychosocial factors while maintaining awareness of the evolving nature of PKU dietary management.
3.4. Family-Related Factors
We recognize that the family constitutes the primary support system for individuals managing PKU, and the dynamics within this unit can either facilitate or hinder dietary compliance. Family-related factors encompass a range of social, psychological, and behavioral components within the family unit that collectively influence dietary compliance. An overview of the research discussed in this section is presented in Table 2.

Table 2.
An overview of studies investigating the influence of family-related factors on dietary compliance in PKU.
3.4.1. Social Factors
Several social factors related to family structure and dynamics have been associated with adherence to a low-Phe diet in patients with PKU.
Family structure plays a role in dietary adherence, with compliant families of both high (M = 55.63, SD = 13.50) and low (M = 57.87, SD = 9.01) socioeconomic statuses having a more structured family environment and more firmly fixed rules than noncompliant families of high (M = 44.25, SD = 8.06) and low (M = 52.00, SD = 10.42) socioeconomic statuses [16].
Family size has been investigated in relation to dietary adherence, although the findings are mixed. One study found no significant relationship between family size and dietary adherence, with blood Phe concentrations not increasing significantly with an increase in family members [41]. However, another study found that among patients who lived with only one parent (n = 17/55, 30.9%), 15 (27.3%) were non-adherent (p = 0.027), and adjusted analysis revealed that living with both parents is a protective factor for treatment adherence (RRs 0.59, 95% CI 0.39–0.80, p = 0.001) [44].
Parental employment status has also been associated with dietary adherence. One study found a significant association between unemployed parents and higher levels of blood Phe concentration (p = 0.03) [41]. Although specific “Phe free” formulas are given at no cost to families in some referral hospitals, unemployed families may be under pressure to pay for other requirements for their children. A significant positive correlation was found between the number of affected children in the family and blood Phe concentrations (r = 0.43, p < 0.001), suggesting that an increase in affected children may lead to more financial pressure on families, as special foods or supplements are not always available free of charge [41].
Patients with separated or divorced parents were more likely to have higher Phe levels, and this association was robust and not diminished by adjustment for potential confounding factors [34]. Among patients with parents who were still together, the statement “I drink amino acids 3–4 times/day” was statistically significantly associated with the recommended Phe level (adjusted odds ratio of 0.07, 95% CI 0.006 to 0.783, p = 0.031). Another study found a significant association between divorced parents and higher levels of blood Phe concentration (p = 0.02), with the mean plasma Phe concentration in patients with divorced and not divorced parents being 14.56 mg/dL and 7.86 mg/dL, respectively [41].
The mother’s country of birth impacts children’s Phe levels in PKU management, with foreign-born mothers’ children typically showing higher Phe concentrations. This is likely due to language barriers, cultural dietary differences, and varying adherence to medical instructions [30]. Healthcare workers often struggle to provide effective dietary advice across cultures due to limited knowledge of diverse eating habits and ingredients [30]. To improve PKU management in diverse populations, culturally sensitive approaches, including translated materials, cultural mediators, and education on various dietary practices, are essential.
3.4.2. Psychological and Behavioral Factors
Parental attitudes and behaviors have been shown to significantly impact adherence to a low-Phe diet in patients with PKU. A study found strong correlations between Phe levels and the external locus of control for both behavioral dysregulation (r = 0.69, p < 0.001) and academic difficulties (r = 0.52, p < 0.001) [29]. Children exhibited a similar relationship between Phe levels and the external locus of control for behavioral dysregulation (r = 9.61, p < 0.001) [29]. In the context of PKU management, individuals with a higher external locus of control tend to believe that factors outside their personal influence predominantly shape their circumstances. Consequently, these individuals are more likely to attribute elevated Phe levels and the resulting behavioral or academic challenges to external factors beyond their control. Such external factors may include chance occurrences, predetermined fate, or the actions of others, rather than recognizing the direct impact of their own dietary choices and adherence to the prescribed low-Phe diet. This perspective can potentially hinder effective self-management of PKU, as it may lead to a reduced sense of personal responsibility for maintaining optimal Phe levels through dietary compliance. Notably, mothers were found to be more disturbed by behavioral dysregulation than fathers [29], which may influence family dynamics in managing PKU and adhering to dietary restrictions.
Parents’ problem-solving skills were related to their children’s level of disease control [16]. On verbal measures of problem-solving, which involved parents verbally responding to hypothetical disease management scenarios, the quality of response varied with the level of dietary control (p < 0.001), with parents of children with good dietary control producing a higher quality verbal response (M = 1.99, SD = 0.24) than noncompliant parents [16]. The number of verbal alternatives suggested also varied with the level of dietary control (p < 0.01), with parents of children with good dietary control giving responses that specified a greater number of behaviors in response to each situation (M = 1.38, SD = 0.20) compared to mothers of children with poor dietary control (M = 1.24, SD = 0.11) [16].
Parental wellbeing and family support play crucial roles in a child’s adherence to a low-phenylalanine diet. Medford et al. [45] found that including factors such as perceived family support, parental wellbeing, and the child’s level of dependency in their regression model more than doubled the variance in adherence from 14.7% to 34.1%. This substantial increase highlights the critical importance of family dynamics in successful dietary management. Interestingly, Borghi et al. [56] reported that optimal diet adherence was associated with some unexpected parental characteristics: lower social functioning, greater control of anger expression, and more somatic depressive symptoms. Conversely, parents who tended to express anger outwardly and experience higher mental stress were more likely to have children with elevated Phe levels, indicating poorer adherence. These findings suggest a complex relationship between parents’ psychological factors and their children’s dietary compliance in PKU management.
A study revealed a significant association between caregiver experiences with dietary management and children’s Phe levels in PKU [31]. Children exhibited notably lower Phe levels when their caregivers reported less frequent, less challenging, and less emotionally distressing dietary problems. Moreover, these caregivers perceived their strategies for addressing dietary issues as more effective. All of these associations were statistically significant (p values < 0.05) [31]. Caregivers who reported using strategies coded as representing an authoritarian parenting style to solve dietary problems were significantly more likely to have older children with higher Phe levels (all p values < 0.05) [31]. The number of formula-related and total problems reported by caregivers was significantly related to children’s Phe levels (r = 0.51, p = 0.03 and r = 0.47, p = 0.05, respectively), with more problems being related to worse adherence [31]. Caregivers’ perceptions of the effectiveness of dietary solutions were significantly related to their children’s Phe levels (r = 0.64, p < 0.01), with greater effectiveness ratings associated with better adherence levels [31]. Children who reported trying strategies coded as being maladaptive for adherence (i.e., eating inappropriate foods or ignoring/avoiding problems) had significantly higher Phe levels, i.e., F(1, 8) = 8.38, p < 0.05 [31].
3.4.3. Educational Factors
Higher parent education levels were associated with lower blood Phe levels, with significant correlations found between blood Phe and maternal education (r = −0.27, p < 0.05) and paternal education (r = -0.28, p < 0.05) [17]. However, a multiple logistic regression analysis did not find a statistically significant association between parental educational level and Phe levels [34]. When considering the quantity or excess of Phe intake compared to the prescribed diet, children with mothers with a lower educational level showed a significant excess (median 158 mg vs. −36 mg, p = 0.045), while no difference was found based on fathers’ education levels (median −35.5 vs. −2.00, p = 0.9) [40]. Additionally, patients whose mothers had 4 years of formal education or less were at a greater risk of non-adherence (RR 1.59, 95% CI 1.01–2.51, p = 0.044) [44].
Parental disease-related knowledge levels also play a role in adherence to a low-Phe diet. A study evaluating the success of a PKU education program found that subjects who successfully completed the program had a lower baseline blood Phe concentration (13.4 mg/dL) compared to nonsuccessful subjects (17.9 mg/dL, p < 0.05) [18]. A significant relationship (r = −0.485) was found between baseline blood Phe concentration and success with the program [18].
Linear regression analysis showed that greater knowledge was associated with lower Phe concentrations, but this association was statistically significant only in the parent group (parents: b = −28, 95% CI −39/−17; patients: b = −20, 95% CI −42/2) [27]. However, after adjusting for confounders (pre-treatment Phe concentrations, dietary Phe tolerance, patient age, parental educational level, and ethnicity), this association disappeared (parents: b = 0.4, 95% CI -8/7; patients: b = 1.5, 95% CI -10/7) [27]. A negative correlation was found between maternal knowledge about Phe exchange and median blood Phe concentration in the child (p < 0.05) [36]. “Phe exchange”, or “PKU exchange”, is a dietary management tool used for individuals with phenylketonuria (PKU). It refers to a measured amount of Phe in food. One standard exchange is defined as 15 mg of Phe. This system allows patients and caregivers to categorize and measure foods in terms of these exchanges, facilitating precise tracking and control of Phe intake. Maternal knowledge about a standard 15 mg Phe exchange system was correlated with dietary compliance, as measured by blood Phe concentrations [36].
3.5. Patient-Specific Factors
We identified three main categories of patient-specific factors that may influence adherence to a low-Phe diet in patients with PKU: psychological and behavioral factors, educational factors, and demographic factors. The key findings from the studies explored in this section are summarized in Table 3.

Table 3.
An overview of studies investigating the influence of patient-specific factors on dietary compliance in PKU.
3.5.1. Psychological and Behavioral Factors
In a pre-2000 study, Waisbren et al. (1997) found that adult women (>18 years) who felt that they did not receive support from health professionals needed extensive help from their parents and believed that life’s circumstances were determined through chance (an external locus of control) were at greater risk of poor metabolic control [20].
Improved attitudes and reduced negative health beliefs among children and adolescents were not accompanied by sustained reductions in blood Phe levels [24]. Believing that the costs of treatment add to the complications of the diet was associated with late metabolic control (RR, 4.2; 95% CI, 1.4, 23.3) [25]. Other factors associated with increased risk were age (<25 years) (RR, 8.4; 95% CI, 1.9, 147.8), having a high school education or less, and dependence on state assistance programs [25].
Significant correlations were found between Phe levels and the external locus of control for academic difficulties (p < 0.001) and between Phe levels and parents’ emotional responses to their children’s behavior/academic difficulties (p < 0.001) [29]. Children’s perceptions of the effectiveness of strategies were significantly associated with their Phe levels for dietary problems (p < 0.05) [31]. Children who reported using maladaptive strategies (e.g., eating inappropriate foods or ignoring/avoiding problems) had significantly higher Phe levels, p < 0.05 [31].
Among adult PKU patients, full-time work was associated with poorer metabolic compliance (mean Phe blood levels > 281.11 μMol/L) compared to part-time work. Shift work was linked to even worse compliance, with mean Phe plasma levels exceeding 356.73 μMol/L [47]. Perceived barriers related to PKU treatment also affected adherence. Adolescents reported fewer barriers compared to adults (U = 8.000, p = 0.008), and patients with better recent metabolic control reported fewer perceived barriers than patients with poor adherence (U = 20.000, p = 0.009) [53]. The number of perceived barriers was positively associated with recent blood Phe concentration (Kendall’s tau(b) = 0.41; p = 0.001) [53].
3.5.2. Educational Factors
Patients’ education level has been shown to influence adherence to a low-Phe diet in patients with PKU. Brown et al. (2002) found that among adults (>18 years), having a high school education or less was associated with an increased risk of late metabolic control (RR, 8.4; 95% CI, 1.9, 147.8) [25]. Additionally, patients who achieved a university degree were found to adhere to the PKU special diet throughout their lives, as they understood the reasons for being on a special diet and thus avoided any damage to their psychomotor functions [50]. Patients’ disease-related knowledge also plays a crucial role in adherence to a low-Phe diet. Children and adolescents (<18 years) who successfully completed an educational program had a significantly lower baseline blood Phe concentration (13.4 mg/dL) compared to nonsuccessful subjects (17.9 mg/dL) (p < 0.05) [18]. However, increased knowledge, improved attitudes, and reduced negative health beliefs were not always accompanied by sustained reductions in blood Phe levels [24,35]. A significant difference in the extent of change in knowledge score between baseline and 1 month was found in favor of the intervention group (p < 0.05), but this improvement in knowledge was not accompanied by a significant improvement in measures of compliance [35]. Another study found significant associations between knowledge scores (total knowledge, PKU knowledge, PKU diet knowledge) and whether an adult respondent always followed a PKU diet, had returned to diet, or was currently off diet [64]. Post hoc analysis showed significantly lower correct scores among those who were currently off diet (mean ± SD total knowledge score 69.1% ± 15.4%) compared to those who had always followed their recommended PKU diet (78.0% ± 12.0%, p = 0.005) [64].
3.5.3. Demographic Factors
Patient age was found to be a significant factor affecting adherence to a low-Phe diet in patients with PKU across multiple studies involving various age groups [19,21,23,25,30,33,34,39,40,43,45,46,47,48,49,51,54]. Blood Phe concentrations were significantly higher in older age groups compared to younger groups [19,23,39,43,48]. Pre-2000 studies by McMurry et al. (1992) and Schulz and Bremer (1996), both involving mixed age groups, reported that blood Phe concentrations were significantly higher in older age groups compared to younger groups, with age positively correlated with mean Phe concentrations [19,21]. Non-adherence to clinic-recommended target Phe concentrations increased with age [46]. A linear relationship was found between age and mean Phe blood levels, with an average annual increase of 30.56 μMol/L (95% CI: 7.53; 53.60) [47]. Age was the single most robust and statistically significant predictor of Phe concentrations, with a baseline concentration of 140 μmol/L and an increase of 22 μmol/L with every additional year of age (p < 0.0001) [49]. Dietary compliance, as measured by questionnaire, was lower in older subjects compared to younger groups and negatively correlated with age (r = −0.689, p < 0.0001) [19]. Factors associated with late metabolic control included age < 25 years (RR, 8.4; 95% CI, 1.9, 147.8) [25]. Child age accounted for significant variance in the proportion of blood Phe concentrations in the target range (b = 1.879, p = 0.009, r2 = 0.147) [45]. Logistic regression analyses identified higher Phe levels in the age ranges of 12–23 months and 6–8 years as predictors of treatment discontinuation before 13 years of age [48].
The impact of gender on adherence to a low-Phe diet in PKU patients was less consistent across studies involving various age groups [23,34,40,42,43,54]. Some studies found no significant difference in blood Phe levels between boys and girls [23,40,43]. However, one study reported that girls had lower Phe levels than boys, with sex differences achieving borderline statistical significance (adjusted odds ratio of 0.004, 95% CI 0.000 to 1.011, p = 0.05) [34]. Another study found that the mean proportion of females (all ages, 57.5%) with 70% or more of their Phe concentrations within the target range was higher than that for males (all ages, 49.8%) [42]. In contrast, one study reported that those who had a blood Phe concentration higher than the recommended threshold were more likely to be female (39.5% of females vs. 21.5% of males, p = 0.0202) [54]. However, in this study population, females (median = 16.6 years) were older than males (median = 9.6 years) (p = 0.0088), which may have skewed the differences in blood Phe concentration [54].
3.6. Environmental Factors
Environmental factors encompass a wide range of external influences that can affect a patient’s ability to adhere to a low-Phe diet. An overview of the research discussed in this section is presented in Table 4, which summarizes key studies, their participants, and their main findings related to environmental factors affecting dietary compliance in PKU.

Table 4.
An overview of studies investigating the influence of environmental factors on dietary compliance in PKU.
Multiple logistic regression analyses revealed that adult women who felt that they did not receive support from health professionals were at greater risk of poor metabolic control [20]. On the other hand, all adult participants who were either mildly or moderately depressed experienced a beneficial effect on their depression when they were psychologically supported, which was further enforced by the reduction in Phe blood levels after their psychological support [50]. These findings highlight the importance of support from health professionals in promoting adherence to a low-Phe diet and improving metabolic control in patients with PKU.
Educational and camp-based interventions have been shown to improve adherence to a low-Phe diet in patients with PKU [22,24,38,57,62]. A week-long camping experience for girls and young women resulted in enhanced ongoing social support networks and improved metabolic control, with blood Phe concentrations reduced in 96% (24/25) of the young women during camp and remaining down in 75% (18/24) of these women at follow-up (p < 0.05) [22]. However, increased knowledge gained from educational interventions was not always accompanied by sustained reductions in blood Phe levels [24]. An educational intervention was found to be more effective at lowering children’s Phe levels compared to a control group (F = 4.68, p = 0.03), with the percentage of children in the educational group with a normal range of Phe levels increasing from 26% and 38% at baseline to 73.9% at 24-month follow-up [57]. Additionally, a camp-based intervention for mixed age groups led to decreased plasma Phe concentrations [median change: −173 µmol/L (IQR: −325, −28 µmol/L)], with 70% of PKU participants demonstrating improved dietary adherence by decreasing Phe intake and/or increasing medical food consumption [62].
A study comparing concurrent biochemical markers (Phe, tyrosine, and Phe to tyrosine ratio) at holiday and non-holiday time points found no significant difference in concurrent Phe levels across time using a within-subjects t-test (t = 1.029, p = 0.331) [37]. Furthermore, no significant differences were observed between Phe levels in 2005 and the two testing occasions, even when adjusted for age and different dietary restrictions. No significant differences were found between lifetime Phe levels and concurrent Phe levels on holiday [t(7) = 1.886, p = 0.101] or non-holiday [t(7) = 0.819, p = 0.440] occasions [37]. These findings suggest that adherence to a low-Phe diet may not differ significantly between holiday and non-holiday periods.
Several studies involving mixed age groups have investigated the influence of the relationship between the distance to the PKU clinic and clinic staffing resources on adherence to a low-Phe diet in patients with PKU. In one study, no significant correlation was found between the distance in miles and the Phe target (p = 0.62) [43]. Similarly, another study reported that the distance between the patient’s town of origin and the PKU clinic did not correlate with median Phe concentration in the 12 months prior to study inclusion, both for adherent patients (p = 0.629) and non-adherent patients (p = 0.72) [44]. However, clinic staffing resources, defined as the number of specialists per 100 actively managed PKU patients, were found to be correlated with the proportion of non-adherent patients (those not following clinic recommendations for blood Phe concentrations) in different age groups [46]. This suggests that while the physical distance to the clinic may not directly impact adherence, the availability of specialized staff at the clinic could play a role in helping patients to maintain a low-Phe diet.
The COVID-19 pandemic has had varying effects on adherence to a low-Phe diet in PKU patients. One study found that among the child population (GROUP A), metabolic control did not differ significantly during March–April–May (MAM) 2019 compared to MAM 2020. However, adolescent and adult patients (GROUP B) demonstrated a significant decrease in blood Phe concentrations, with significant improvements in metabolic control (556.4 ± 301 μmol/L in MAM 2019 vs. 454 ± 252 μmol/L in MAM 2020) [58]. The authors suggest that this improvement in adherence among adolescent and adult patients may be related to a more favorable social environment with fewer external influences during the pandemic, allowing them to concentrate more on their diet [58]. Another study found that self-reported high stress intensity was associated with higher odds ratios for an increase in Phe concentrations (p = 0.0023) and non-PKU-related health problems [59]. Better compliance was associated with higher odds of acceptance of remote contact, reporting fewer problems with contacting the doctor, and lower odds of missing Phe testing. However, a third study reported that the median Phe level significantly increased in both age groups during the COVID-19 era (CE) compared to the non-COVID-19 era (NCE). There was a decreasing tendency in the number of patients within the target Phe range in both age groups during CE [63]. Significant negative correlations were found between the dried blood spot (DBS) testing frequencies and Phe levels in both age groups during NCE (children r = −0.43, p = 0.002; adolescents r = −0.37, p = 0.012) and in the adolescent group during CE (r = −0.6, p = 0.006) [63]. These findings suggest that the COVID-19 pandemic has had mixed effects on adherence to a low-Phe diet in PKU patients, with some studies reporting improvements in adherence, particularly among adolescent and adult patients, while others have reported worsening adherence and increased Phe levels. The impact of the pandemic on adherence may depend on various factors, such as stress levels, access to remote healthcare, and changes in social environments and routines.
3.7. Therapy-Related Factors
The management of phenylketonuria (PKU) involves various therapeutic approaches, each with its own set of challenges and potential impacts on dietary compliance. Therapy-related factors include a diverse array of elements, such as the type and formulation of protein substitutes, treatment protocols, the frequency of clinical visits and blood tests, and the use of innovative technologies in patient care. Table 5 synthesizes the key findings from various studies examining the relationship between therapy-related factors and dietary compliance among individuals with PKU.

Table 5.
An overview of studies investigating the influence of therapy-related factors on dietary compliance in PKU.
The formulation of protein substitutes has been shown to impact compliance across different age groups. In a study involving mixed age groups, MacDonald et al. (2003) found that 90% (18 out of 20) of patients took Phe-free amino acid tablets as prescribed compared to only 65% (13 out of 20) who were fully compliant with their usual protein substitute. Additionally, plasma Phe levels were lower when taking tablets, with a median difference of 46 µmol/L (95% CI 14.8 to 89.0, p = 0.02), indicating better metabolic control. Similarly, a liquid protein substitute was found to be more popular and efficacious, reducing self-consciousness and improving overall compliance in teenagers and adults with PKU (p < 0.0001) [32]. The use of large neutral amino acid (LNAA) supplements was associated with improved adherence, with all patients self-reporting high adherence to medication after 12 months of LNAA treatment, compared to only 3 out of 12 patients reporting ‘medium’ adherence before treatment. Tyrosine levels also increased significantly in 92% of patients (mean 75 ± 16 µmol/L; p = 0.0195) [52]. It is important to note that recent research suggests that GMP-based formulas may be more acceptable to patients struggling with dietary adherence when compared to amino acid-based formulas, potentially enhancing compliance and improving health outcomes, especially in adults. [65].
The formulation of the protein substitute appears to impact compliance, with a study showing that 90% of patients took Phe-free amino acid tablets as prescribed, compared to only 65% who were fully compliant with their usual protein substitute. Moreover, plasma Phe levels were significantly lower in patients on the amino acid tablets (p = 0.02) [26].
The frequency of visits to a specialist and blood Phe tests was found to be higher in the first year of life compared to subsequent years (visits: OR = 6.8267, 95% CI = 2.827–16.5163, p < 0.0001; blood tests: OR = 2.7875, 95% CI = 1.0467–7.4234, p < 0.0402) [51]. Phe levels were correlated with the number of visits to a specialist (ρ = 0.39), and the number of Phe blood tests was correlated with the index of dietary control (ρ = −0.33) [51].
The clinic’s Phe target also influenced adherence, with clinics using a higher upper target (600 μM vs. 360 μM) for adult patients having more non-adherent patients when evaluated against a single adherence criterion of 360 μM (p < 0.05) [46]. However, adherence to clinic-recommended blood Phe concentrations was not significantly correlated with adherence to blood frequency testing recommendations or actual blood frequency testing, except for patients who were pregnant or planning to become pregnant.
Patients with classical PKU showed higher current blood Phe levels than those with mild PKU (U = 37.000, p = 0.003) [55]. Lifetime and childhood Phe levels were associated with recent metabolic control, and the perception of barriers to treatment was associated with higher blood Phe levels.
The implementation of a transition program from pediatric to adult services was found to be successful, with a positive impact on metabolic control and high attendance rates [60]. The mean ± SD of median blood Phe remained stable (525 ± 248 µmol/L vs. 552 ± 225 µmol/L; p = 0.100), while the median percentage of blood Phe < 480 µmol/L decreased (p = 0.041) [60]. The median number of clinic appointments increased from the first to the second study period (p < 0.001) [60].
The treatment approach also played a role in adherence, with children aged 2 to 12 years who received care from an expert, coordinated team in Nova Scotia having better Phe control and more medical visits than those in New Brunswick (p < 0.01) [28].
Lastly, the use of telemedicine was associated with a statistically higher ratio of samples with Phe levels in the recommended ranges across all treatment modalities (p < 0.05) [61]. In the low-Phe diet group, the decrease in Phe washout frequency and the increase in Phe tolerance were statistically significant during the pandemic (p < 0.05) [61].
4. Discussion
Numerous factors influence dietary adherence in PKU patients, and they can be classified as facilitators, barriers, or mixed evidence based on their impact (Figure 2).
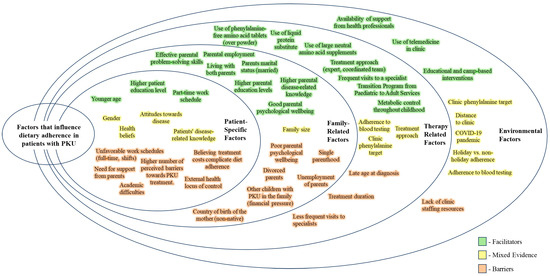
Figure 2.
A summary of facilitators and barriers to dietary adherence.
When considering patient-specific factors, younger age, higher education level, and part-time work schedules are linked to better adherence. Conversely, reliance on parental support, an external health locus of control, the belief that treatment costs complicate diet adherence, academic difficulties, older age, unfavorable work schedules, and a higher number of perceived barriers to PKU treatment are obstacles to adherence.
Family-related factors play a crucial role in promoting adherence, especially for children and adolescents. Effective parental problem-solving skills, parental employment, living with both parents, higher parental education levels, good parental psychological well-being, and better parental disease-related knowledge all contribute positively to adherence. On the other hand, adherence is hindered by poor parental mental health, single parenthood, parental unemployment, having siblings with PKU, parental divorce, and having a mother born outside the country.
Therapy-related factors such as the formulation of the protein substitute (with tablets or liquid form being preferable), more frequent visits to specialists and blood Phe tests, lower clinic Phe targets, and the implementation of transition programs from pediatric to adult services are associated with better adherence. Classical PKU, higher perceived barriers to treatment, and lower adherence to blood testing frequency remain challenges across all age groups.
Lastly, the availability of support from health professionals, educational and camp-based interventions, and the use of telemedicine in clinics are environmental factors that facilitate adherence, while a lack of clinic staffing resources poses a challenge.
Several limitations should be acknowledged. While the dietary management of PKU has evolved significantly over time, with substantial improvements in protein substitutes and increased availability of low-protein foods, we determined that the inclusion of pre-2000 studies was scientifically justified for several reasons. Pre-2000 studies predominantly explored psychosocial, family-related, and patient-specific factors influencing dietary adherence. These factors, such as family dynamics, patient attitudes, and psychological and social mechanisms underlying adherence, are less likely to be affected by changes in dietary treatment modalities over time. Therefore, insights from earlier studies on these aspects remain relevant to contemporary PKU management. Including earlier studies provides a valuable historical context, allowing for the examination of trends and persistent challenges in PKU management over time. We noted that pre-2000 studies contained minimal data on therapy-related factors that would be outdated by current standards. To address this, we were careful to interpret pre-2000 data within their historical context, particularly when discussing therapy-related aspects.
PKU is classified as an orphan disease, meaning that it affects a relatively small number of individuals. This inherently limits the potential recruitment pool for studies, often resulting in small sample sizes. Small cohorts can lead to a lack of statistical power to detect meaningful differences or associations.
A considerable number of included studies were categorized as observational or cross-sectional studies. These studies were prone to selection bias and could not control for confounding variables as effectively as randomized controlled trials. The absence of longitudinal or follow-up data in cross-sectional and most observational studies limits the understanding of the long-term effects and sustainability of the outcomes reported. In observational studies without control groups, the inability to control for all potential confounding variables may lead to biased estimates of effect sizes or associations.
The included studies demonstrated strengths in clearly stating their research aims, describing the study context, and selecting appropriate designs; there is significant room for improvement in involving stakeholders, justifying analytic approaches, and transparently discussing study limitations. While this systematic review contributed valuable insights into PKU management, there is a clear need for more high-quality research, particularly longitudinal and experimental studies, that can provide stronger evidence for the PKU community. Future research should prioritize high-quality RCTs and longitudinal studies that can provide more definitive evidence regarding the efficacy of interventions.
5. Conclusions
In this systematic review, we have identified a nuanced landscape of influences that span social, psychological, behavioral, educational, and environmental factors influencing dietary adherence in PKU patients across their lifespans. Family-related factors, including parental education, family dynamics, and socioeconomic status, play a significant role in dietary adherence, particularly for children and adolescents. Higher levels of parental education correlate with better dietary control, indicating the need for targeted educational programs for caregivers. Stable family structures and problem-solving abilities further enhance adherence, pointing toward the benefit of psychological and social support services for PKU families. As patients age, the challenges to adherence evolve. Patient-specific factors, such as age and the need for support, also impact adherence, with adolescents and adults facing more significant challenges. This suggests a potential gap in transitional care from pediatric to adult services, highlighting the importance of age-appropriate interventions and support mechanisms to ensure continued adherence throughout a patient’s life. Environmental factors, such as the availability of healthcare support and educational interventions, are essential in facilitating adherence for all age groups. Camp-based programs and telemedicine have demonstrated effectiveness in improving metabolic control, emphasizing the need for accessible and engaging support infrastructures. Therapeutic factors, including treatment modalities and formulations, influence adherence across all age groups. Preferences for Phe-free amino acid tablets and liquid protein substitutes suggest that patient-centered treatment choices can improve adherence.
In conclusion, adherence to a low-Phe diet in PKU is a multifaceted behavior influenced by the interplay of family-related, patient-specific, environmental, and therapy-related factors. The impacts of these factors vary across different age groups, emphasizing the need for a personalized, age-appropriate approach to PKU management. By recognizing and addressing these diverse influences, healthcare providers can develop more effective strategies to improve dietary adherence and, consequently, health outcomes for PKU patients throughout their lives.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16183119/s1, Table S1: Search terms per database, Table S2: QuADS item scores per study.
Author Contributions
Conceptualization, R.Y., A.K. and K.K.; formal analysis, V.S., M.P. and K.K.; investigation, V.S. and K.K.; data curation V.S. and K.K.; writing—original draft preparation, K.K.; writing—review and editing, R.Y., A.K., V.S., M.P. and K.K.; supervision, R.Y.; project administration, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A.; et al. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef]
- Giovannini, M.; Verduci, E.; Salvatici, E.; Paci, S.; Riva, E. Phenylketonuria: Nutritional advances and challenges. Nutr. Metab. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Gokmen-Ozel, H.; van Rijn, M.; Burgard, P. The reality of dietary compliance in the management of phenylketonuria. J. Inherit. Metab. Dis. 2010, 33, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Bilginsoy, C.; Waitzman, N.; Leonard, C.O.; Ernst, S.L. Living with phenylketonuria: Perspectives of patients and their families. J. Inherit. Metab. Dis. 2005, 28, 639–649. [Google Scholar] [CrossRef]
- Ford, S.; O’Driscoll, M.; MacDonald, A. Living with Phenylketonuria: Lessons from the PKU community. Mol. Genet. Metab. Rep. 2018, 17, 57–63. [Google Scholar] [CrossRef]
- Gokmen-Ozel, H.; Ferguson, C.; Evans, S.; Daly, A.; MacDonald, A. Does a lower carbohydrate protein substitute impact on blood phenylalanine control, growth and appetite in children with PKU? Mol. Genet. Metab. 2011, 104, S64–S67. [Google Scholar] [CrossRef]
- Uman, L.S. Systematic Reviews and Meta-Analyses. J. Can. Acad. Child Adolesc. Psychiatry 2011, 20, 57–59. [Google Scholar]
- Petticrew, M.; Roberts, H. Systematic Reviews in the Social Sciences: A Practical Guide, 1st ed.; Blackwell Pub: Malden, MA, USA; Oxford, UK, 2006; 336p. [Google Scholar]
- Medford, E.; Hare, D.J.; Wittkowski, A. Demographic and Psychosocial Influences on Treatment Adherence for Children and Adolescents with PKU: A Systematic Review. JIMD Rep. 2018, 39, 107–116. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- National Institute of Health and Care Research (NIHR). Factors Affecting Adherence to a Low Phenylalanine Diet in Children with Phenylketonuria: A Systematic Review [Internet]. PROSPERO International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=412538 (accessed on 23 March 2024).
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Wolters Kluwer Health. JBI Levels of Evidence. Levels of Evidence—Effectiveness [Internet]. JBI EBP Database. Available online: https://ospguides.ovid.com/OSPguides/jbidb.htm (accessed on 23 March 2024).
- Harrison, R.; Jones, B.; Gardner, P.; Lawton, R. Quality assessment with diverse studies (QuADS): An appraisal tool for methodological and reporting quality in systematic reviews of mixed- or multi-method studies. BMC Health Serv. Res. 2021, 21, 144. [Google Scholar]
- Fehrenbach, A.M.; Peterson, L. Parental problem-solving skills, stress, and dietary compliance in phenylketonuria. J. Consult. Clin. Psychol. 1989, 57, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Shulman, S.; Fisch, R.O.; Zempel, C.E.; Gadish, O.; Chang, P.N. Children with phenylketonuria: The interface of family and child functioning. J. Dev. Behav. Pediatr. 1991, 12, 315–321. [Google Scholar] [CrossRef]
- Gleason, L.A.; Michals, K.; Matalon, R.; Langenberg, P.; Kamath, S. A treatment program for adolescents with phenylketonuria. Clin. Pediatr. 1992, 31, 331–335. [Google Scholar] [CrossRef]
- McMurry, M.P.; Chan, G.M.; Leonard, C.O.; Ernst, S.L. Bone mineral status in children with phenylketonuria—Relationship to nutritional intake and phenylalanine control. Am. J. Clin. Nutr. 1992, 55, 997–1004. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Hamilton, B.D.; St James, P.J.; Shiloh, S.; Levy, H.L. Psychosocial factors in maternal phenylketonuria: Women’s adherence to medical recommendations. Am. J. Public. Health 1995, 85, 1636–1641. [Google Scholar] [CrossRef]
- Schulz, B.; Bremer, H.J. Nutrient intake and food consumption of adolescents and young adults with phenylketonuria. Acta Paediatr. 1995, 84, 743–748. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Rokni, H.; Bailey, I.; Rohr, F.; Brown, T.; Warner-Rogers, J. Social factors and the meaning of food in adherence to medical diets: Results of a maternal phenylketonuria summer camp. J. Inherit. Metab. Dis. 1997, 20, 21–27. [Google Scholar] [CrossRef]
- Al-Qadreh, A.; Schulpis, K.H.; Athanasopoulou, H.; Mengreli, C.; Skarpalezou, A.; Voskaki, I. Bone mineral status in children with phenylketonuria under treatment. Acta Paediatr. 1998, 87, 1162–1166. [Google Scholar] [CrossRef]
- Singh, R.H.; Kable, J.A.; Guerrero, N.V.; Sullivan, K.M.; Elsas, L.J. Impact of a camp experience on phenylalanine levels, knowledge, attitudes, and health beliefs relevant to nutrition management of phenylketonuria in adolescent girls. J. Am. Diet. Assoc. 2000, 100, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Fernhoff, P.M.; Waisbren, S.E.; Frazier, D.M.; Singh, R.; Rohr, F.; Morris, J.M.; Kenneson, A.; MacDonald, P.; Gwinn, M.; et al. Barriers to successful dietary control among pregnant women with phenylketonuria. Genet. Med. 2002, 4, 84–89. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Ferguson, C.; Rylance, G.; Morris, A.; Asplin, D.; Hall, S.; Booth, I. Are tablets a practical source of protein substitute in phenylketonuria? Arch. Dis. Child. 2003, 88, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Bekhof, J.; van Spronsen, F.J.; Crone, M.R.; van Rijn, M.; Oudshoorn, C.G.M.; Verkerk, P.H. Influence of knowledge of the disease on metabolic control in phenylketonuria. Eur. J. Pediatr. 2003, 162, 440–442. [Google Scholar] [CrossRef]
- Camfield, C.S.; Joseph, M.; Hurley, T.; Campbell, K.; Sanderson, S.; Camfield, P.R. Optimal management of phenylketonuria: A centralized expert team is more successful than a decentralized model of care. J. Pediatr. 2004, 145, 53–57. [Google Scholar] [CrossRef]
- Antshel, K.M.; Brewster, S.; Waisbren, S.E. Child and parent attributions in chronic pediatric conditions: Phenylketonuria (PKU) as an exemplar. J. Child. Psychol. Psychiatry 2004, 45, 622–630. [Google Scholar] [CrossRef]
- Crone, M.R.; van Spronsen, F.J.; Oudshoorn, K.; Bekhof, J.; van Rijn, G.; Verkerk, P.H. Behavioural factors related to metabolic control in patients with phenylketonuria. J. Inherit. Metab. Dis. 2005, 28, 627–637. [Google Scholar] [CrossRef]
- Ievers-Landis, C.E.; Hoff, A.L.; Brez, C.; Cancilliere, M.K.; McConnell, J.; Kerr, D. Situational analysis of dietary challenges of the treatment regimen for children and adolescents with phenylketonuria and their primary caregivers. J. Dev. Behav. Pediatr. 2005, 26, 186–193. [Google Scholar] [CrossRef]
- MacDonald, A.; Lilburn, M.; Davies, P.; Evans, S.; Daly, A.; Hall, S.K.; Hendriksz, C.; Chakrapani, A.; Lee, P. “Ready to drink” protein substitute is easier is for people with phenylketonuria. J. Inherit. Metab. Dis. 2006, 29, 526–531. [Google Scholar] [CrossRef]
- VanZutphen, K.H.; Packman, W.; Sporri, L.; Needham, M.C.; Morgan, C.; Weisiger, K.; Packman, S. Executive functioning in children and adolescents with phenylketonuria. Clin. Genet. 2007, 72, 13–18. [Google Scholar] [CrossRef]
- Olsson, G.M.; Montgomery, S.M.; Alm, J. Family conditions and dietary control in phenylketonuria. J. Inherit. Metab. Dis. 2007, 30, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Durham-Shearer, S.J.; Judd, P.A.; Whelan, K.; Thomas, J.E. Knowledge, compliance and serum phenylalanine concentrations in adolescents and adults with phenylketonuria and the effect of a patient-focused educational resource. J. Hum. Nutr. Diet. 2008, 21, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Ozel, H.G.; Kucukkasap, T.; Koksal, G.; Sivri, H.S.K.; Dursun, A.; Tokatli, A.; Coskun, T. Does maternal knowledge impact blood phenylalanine concentration in Turkish children with phenylketonuria? J. Inherit. Metab. Dis. 2008, 31 (Suppl. 2), S213–S217. [Google Scholar] [CrossRef] [PubMed]
- Sharman, R.; Sullivan, K.; Young, R.; McGill, J. Biochemical markers associated with executive function in adolescents with early and continuously treated phenylketonuria. Clin. Genet. 2009, 75, 169–174. [Google Scholar] [CrossRef][Green Version]
- Peipert, J.; Rohr, F.; Phornphutkul, C.; Waisbren, S. Changes in Metabolic Control of Phenylketonuria in Children Attending a Summer Camp: Pre- and Postassessment of a Nutritional Intervention. ICAN Infant Child Adolesc. Nutr. 2010, 2, 117–119. [Google Scholar] [CrossRef][Green Version]
- Viau, K.S.; Wengreen, H.J.; Ernst, S.L.; Cantor, N.L.; Furtado, L.V.; Longo, N. Correlation of age-specific phenylalanine levels with intellectual outcome in patients with phenylketonuria. J. Inherit. Metab. Dis. 2011, 34, 963–971. [Google Scholar] [CrossRef]
- Cotugno, G.; Nicolò, R.; Cappelletti, S.; Goffredo, B.M.; Dionisi Vici, C.; Di Ciommo, V. Adherence to diet and quality of life in patients with phenylketonuria. Acta Paediatr. 2011, 100, 1144–1149. [Google Scholar] [CrossRef]
- Alaei, M.; Asadzadeh-Totonchi, G.; Gachkar, L.; Farivar, S. Family Social Status and Dietary Adherence of Patients with Phenylketonuria. Iran. J. Pediatr. 2011, 21, 379–384. [Google Scholar]
- Macdonald, A.; Nanuwa, K.; Parkes, L.; Nathan, M.; Chauhan, D. Retrospective, observational data collection of the treatment of phenylketonuria in the UK, and associated clinical and health outcomes. Curr. Med. Res. Opin. 2011, 27, 1211–1222. [Google Scholar] [CrossRef]
- Freehauf, C.; Van Hove, J.L.K.; Gao, D.; Bernstein, L.; Thomas, J.A. Impact of geographic access to care on compliance and metabolic control in phenylketonuria. Mol. Genet. Metab. 2013, 108, 13–17. [Google Scholar] [CrossRef]
- Vieira, T.A.; Nalin, T.; Krug, B.C.; Bittar, C.M.; Netto, C.B.O.; Schwartz, I.V.D. Adherence to Treatment of Phenylketonuria: A Study in Southern Brazilian Patients. J. Inborn Errors Metab. Screen. 2015, 3, 2326409815579861. [Google Scholar] [CrossRef]
- Medford, E.; Hare, D.J.; Carpenter, K.; Rust, S.; Jones, S.; Wittkowski, A. Treatment Adherence and Psychological Wellbeing in Maternal Carers of Children with Phenylketonuria (PKU). JIMD Rep. 2017, 37, 107–114. [Google Scholar] [PubMed]
- Jurecki, E.R.; Cederbaum, S.; Kopesky, J.; Perry, K.; Rohr, F.; Sanchez-Valle, A.; Viau, K.S.; Sheinin, M.Y.; Cohen-Pfeffer, J.L. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol. Genet. Metab. 2017, 120, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Riva, M.A.; Madotto, F.; Turato, M.; Salvatici, E.; Indovina, S.; Giovannini, M.; Riva, E.; Cesana, G. Work activity and phenylalanine levels in a population of young adults with classic PKU. Med. Lav. 2017, 108, 118–122. [Google Scholar] [PubMed]
- García, M.I.; Araya, G.; Coo, S.; Waisbren, S.E.; de la Parra, A. Treatment adherence during childhood in individuals with phenylketonuria: Early signs of treatment discontinuation. Mol. Genet. Metab. Rep. 2017, 11, 54–58. [Google Scholar] [CrossRef]
- Mlčoch, T.; Puda, R.; Ješina, P.; Lhotáková, M.; Štěrbová, Š.; Doležal, T. Dietary patterns, cost and compliance with low-protein diet of phenylketonuria and other inherited metabolic diseases. Eur. J. Clin. Nutr. 2018, 72, 87–92. [Google Scholar] [CrossRef]
- Iakovou, K.; Schulpis, K. The significant role of educational status in PKU patients: The beneficial effect of psychological support in depression. Int. J. Adolesc. Med. Health 2019, 33, 20180233. [Google Scholar] [CrossRef]
- Walkowiak, D.; Bukowska-Posadzy, A.; Kałużny, Ł.; Ołtarzewski, M.; Staszewski, R.; Musielak, M.; Walkowiak, J. Therapy compliance in children with phenylketonuria younger than 5 years: A cohort study. Adv. Clin. Exp. Med. 2019, 28, 1385–1391. [Google Scholar] [CrossRef]
- Burlina, A.P.; Cazzorla, C.; Massa, P.; Loro, C.; Gueraldi, D.; Burlina, A.B. The Impact of a Slow-Release Large Neutral Amino Acids Supplement on Treatment Adherence in Adult Patients with Phenylketonuria. Nutrients 2020, 12, 2078. [Google Scholar] [CrossRef]
- Teruya, K.I.; Remor, E.; Schwartz, I.V.D. Development of an inventory to assess perceived barriers related to PKU treatment. J. Patient Rep. Outcomes 2020, 4, 29. [Google Scholar] [CrossRef]
- Kenneson, A.; Singh, R.H. Natural history of children and adults with phenylketonuria in the NBS-PKU Connect registry. Mol. Genet. Metab. 2021, 134, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Teruya, K.I.; Remor, E.; Schwartz, I.V.D. Factors that increase risk for poor adherence to phenylketonuria treatment in Brazilian patients. Am. J. Med. Genet. A 2021, 185, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Salvatici, E.; Banderali, G.; Riva, E.; Giovannini, M.; Vegni, E. Psychological wellbeing in parents of children with phenylketonuria and association with treatment adherence. Minerva Pediatr. 2021, 73, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Zamani, R.; Karimi-Shahanjarini, A.; Tapak, L.; Moeini, B. Improving phenylalanine and micronutrients status of children with phenylketonuria: A pilot randomized study. Orphanet J. Rare Dis. 2021, 16, 475. [Google Scholar] [CrossRef]
- Rovelli, V.; Zuvadelli, J.; Ercoli, V.; Montanari, C.; Paci, S.; Dionigi, A.R.; Scopari, A.; Salvatici, E.; Cefalo, G.; Banderali, G. PKU and COVID19: How the pandemic changed metabolic control. Mol. Genet. Metab. Rep. 2021, 27, 100759. [Google Scholar] [CrossRef]
- Walkowiak, D.; Mikołuć, B.; Mozrzymas, R.; Kałużny, Ł.; Didycz, B.; Korycińska-Chaaban, D.; Patalan, M.; Jagłowska, J.; Chrobot, A.; Starostecka, E.; et al. The Impact of the COVID-19 Pandemic on the Perception of Health and Treatment-Related Issues among Patients with Phenylketonuria in Poland-The Results of a National Online Survey. Int. J. Environ. Res. Public Health 2021, 18, 6399. [Google Scholar] [CrossRef]
- Peres, M.; Almeida, M.F.; Pinto, É.J.; Carmona, C.; Rocha, S.; Guimas, A.; Ribeiro, R.; Martins, E.; Bandeira, A.; MacDonald, A.; et al. Implementing a Transition Program from Paediatric to Adult Services in Phenylketonuria: Results after Two Years of Follow-Up with an Adult Team. Nutrients 2021, 13, 799. [Google Scholar] [CrossRef]
- Zubarioglu, T.; Hopurcuoglu, D.; Uygur, E.; Ahmadzada, S.; Oge-Enver, E.; Isat, E.; Cansever, M.S.; Kiykim, E.; Aktuglu-Zeybek, C. The Impact of Telemedicine for Monitoring and Treatment of Phenylketonuria Patients on Metabolic Outcome During Coronavirus Disease-19 Outbreak. Telemed. J. E Health 2022, 28, 258–265. [Google Scholar] [CrossRef]
- Schoen, M.S.; Singh, R.H. Plasma metabolomic profile changes in females with phenylketonuria following a camp intervention. Am. J. Clin. Nutr. 2022, 115, 811–821. [Google Scholar] [CrossRef]
- Becsei, D.; Kiss, E.; Szatmári, I.; Arató, A.; Reusz, G.; Szabó, A.J.; Bókay, J.; Zsidegh, P. A retrospective analysis of metabolic control in children with PKU in the COVID-19 era. Mol. Genet. Metab. Rep. 2022, 32, 100897. [Google Scholar]
- Firman, S.J.; Ramachandran, R.; Whelan, K. Knowledge, perceptions and behaviours regarding dietary management of adults living with phenylketonuria. J. Hum. Nutr. Diet. 2022, 35, 1016–1029. [Google Scholar] [CrossRef]
- Proserpio, C.; Pagliarini, E.; Zuvadelli, J.; Paci, S.; Re Dionigi, A.; Banderali, G.; Cattaneo, C.; Verduci, E. Exploring Drivers of Liking of Low-Phenylalanine Products in Subjects with Phenyilketonuria Using Check-All-That-Apply Method. Nutrients 2018, 10, 1179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).