Investigating the Effects of Dietary Supplementation and High-Intensity Motor Learning on Nutritional Status, Body Composition, and Muscle Strength in Children with Moderate Thinness in Southwest Ethiopia: A Cluster-Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Area and Period
2.2. Study Design
2.3. Population and Eligibility
2.4. Sample Size Determination and Sampling Technique
2.5. Recruitment of Study Participants and Randomization
2.6. Interventions
2.6.1. Ready-to-Use Supplementary Food (RUSF)
2.6.2. High-Intensity Motor Learning (HiML)
2.7. Data Collection Methods and Measurement
2.7.1. Anthropometric Measurements
2.7.2. Body Composition
2.8. Data Quality Control
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Parents/Caregivers at Baseline
3.2. Characteristics of the Children at Baseline
Sociodemographic Characteristics
3.3. Intervention
4. Discussion
4.1. Impact of RUSF Only
4.2. Impact of RUSF Combined with HiML Training
4.3. Implications for Society and Future Research
4.4. Strengths and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- ➢
- Three activities are performed every Monday, Tuesday, and Wednesday, and four activities are performed every Thursday and Friday.
- ➢
- The total time spent on activities each day is 1 h (60 min).
- ➢
- The recovery or rest time between each activity is 5 m, and the cooldown time is 5–10 min.
- ➢
- The children rest for 2–3 min between each intensive activity.
| Intervention | Dose/Day | Frequency | Duration | Systematic Treatment | Compliance Parameter | Considerations for Implementation | Responsible Person |
|---|---|---|---|---|---|---|---|
| RUSF | RUSF: 7 sachets/per child/week (1 per day). Follow national guidelines for SFP. 1 packet/day, 100 g/day. 500 kcal; 12.5 g Pro; 31 g Fat; 42.7 g CHO. | Daily follow-up | 12 weeks | Guidelines for OTP: If technical capacity and supplies (staff) are available/if health services are available. Deworming; amoxicillin vaccinations; malaria treatment. | Absence of intrahousehold sharing of RUSF rations. Counting of empty sachets. |
It can be consumed directly from the package with no dilution, mixing, or cooking necessary. | Research staff; school teachers; Health Extension Program workers; data collectors. |
| HiML | 5 times per week for 60 min. Training of fundamental gross motor skills: Ball skills; Locomotor skills; Cultural games. | Daily | 12 weeks | Number of HiML sessions attended by the child. | Research staff; school teachers; parents; Health Extension Program workers; data collectors. |
- Tester creates a fixed position for the Microfet device and the child tries to move against the Microfet2 device.
- Maximum force is measured 3 times. If a deviation of >20% exists within these three measurements, a fourth or fifth measurement will be performed until the range of 20% is reached.
- Strong verbal encouragement needs to be given during the repeated measurements so that the child produces maximum force.
- During each attempt, the child gradually builds up force against the HHD for about 5 s.
- Positions for placement are standardized. See Table A2.
- The lever arm is measured between the landmarks with a hard tape measure. See Table A2 below.
| Muscle Group | Position | Stabilization | HHD Placement | Direction of Resistance-Creating Block |
|---|---|---|---|---|
| Elbow flexors (m. biceps brachii) | Sitting/supine. Shoulder adducted, elbow 90° flexed, forearm supinated, and closed fist; or shoulder 30° abducted, elbow 90° flexed, and forearm supinated. | Pelvis stabilized using a belt or manual stabilization. | Pelvis stabilized using a belt or manual stabilization. | Block placed distally to the forearm. |
| Knee extensor (mm. quadriceps) | Sitting. Hip and knees at 90-degree angles. | Pelvis stabilized in the chair using hands or a belt. | Anterior tibia 5 cm proximal from bimalleolar line. | Block on the frontal side of the tibia. |
| Knee flexor (mm. hamstrings) | Sitting. Hip and knees at 90-degree angles. | Pelvis stabilized in the chair using hands or a belt. | Anterior tibia 5 cm proximal from bimalleolar line. | Block on the dorsal side of the lower leg. |
| Ankle plantar flexor (m. gastrocnemius) | Supine. Knees extended, ankle in a neutral position. Foot free from the table. | Pelvis stabilized using a belt or manual stabilization. | Metatarsal heads. | Block on the sole of the foot. |
| Grip strength | Upright. Elbow bent at a 90° angle. | Handle is adjusted so that the subject’s fingers can grasp and squeeze it. | With Jamar Dynamometer. | Subject squeezes as hard as possible and relaxes. |
References
- Larson-Nath, C.; Goday, P. Malnutrition in children with chronic disease. Nutr. Clin. Pract. 2019, 34, 349–358. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, A.W. Malnutrition: The specter at the feast. J. R. Coll. Physicians Edinb. 2008, 38, 317–321. [Google Scholar]
- World Health Organization. Fact Sheets—Malnutrition. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 30 March 2024).
- Cashin, K.; Oot, L. Guide to Anthropometry: A Practical Tool for Program Planners, Managers, and Implementers; Food and Nutrition Technical Assistance III Project (FANTA)/FHI 360; U.S. Agency for International Development: Washington, DC, USA, 2018; Available online: https://www.fantaproject.org/sites/default/files/resources/FANTA-Anthropometry-Guide-May2018.pdf (accessed on 29 July 2024).
- Suri, D.J.; Potani, I.; Singh, A.; Griswold, S.; Wong, W.W.; Langlois, B.; Shen, Y.; Chui, K.H.K.; Rosenberg, I.H.; Webb, P.; et al. Body Composition Changes in Children during Treatment for Moderate Acute Malnutrition: Findings from a 4-Arm Cluster-Randomized Trial in Sierra Leone. J. Nutr. 2021, 151, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Levels and Trends in Child Malnutrition UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates Key Findings of the 2023 Edition. Available online: https://data.unicef.org/resources/jme-report-2023/ (accessed on 23 December 2023).
- Saavedra, J.M.; Prentice, A.M. Nutrition in school-age children: A rationale for revisiting priorities. Nutr. Rev. 2023, 81, 823–843. [Google Scholar] [CrossRef] [PubMed]
- Rawe, K.; A Life Free from Hunger: Tackling Child Malnutrition. Save the Children. 2012. Available online: http://www.savethechildren.org.uk/sites/default/files/docs/A-Life-Free-From-Hunger-UK-low-res.pdf (accessed on 29 July 2024).
- EPHI. National Food and Nutrition Strategy Baseline Survey: Key Findings Preliminary Report. March 2023. Available online: https://ephi.gov.et/wpcontent/uploads/2023/03/FNS_baseline_survey_preliminary_findings.pdf (accessed on 23 December 2023).
- Assemie, M.A.; Alamneh, A.A.; Ketema, D.B.; Adem, A.M.; Desta, M.; Petrucka, P.; Ambaw, M.M. High burden of undernutrition among primary school-aged children and its determinant factors in Ethiopia; a systematic review and meta-analysis. Ital. J. Pediatr. 2020, 46, 118. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Galler, J.R.; Bringas-Vega, M.L.; Tang, Q.; Rabinowitz, A.G.; Musa, K.I.; Chai, W.J.; Omar, H.; Rahman, M.R.; Abd Hamid, A.I.; Abdullah, J.M.; et al. Neurodevelopmental effects of childhood malnutrition: A neuroimaging perspective. Neuroimage 2021, 231, 117828. [Google Scholar]
- World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO. Available online: https://cdn.who.int/media/docs/default-source/gho-documents/world-health-statistic-reports/2023/world-health-statistics-2023_20230519_.pdf (accessed on 29 July 2024).
- Akombi, B.J.; Agho, K.E.; Merom, D.; Renzaho, A.M.; Hall, J.J. Child malnutrition in sub-Saharan Africa: A meta-analysis of demographic and health surveys (2006–2016). PLoS ONE 2017, 12, e0177338. [Google Scholar] [CrossRef]
- Kalu, R.E.; Etim, K.D. Factors associated with malnutrition among under-five children in developing countries: A review. Glob. J. Pure Appl. Sci. 2018, 4, 69–74. [Google Scholar] [CrossRef]
- Belay, D.G.; Chilot, D.; Alem, A.Z.; Aragaw, F.M.; Asratie, M.H. Spatial distribution and associated factors of severe malnutrition among under-five children in Ethiopia: Further analysis of 2019 mini EDHS. BMC Public Health 2023, 23, 791. [Google Scholar] [CrossRef]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; De Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J.; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Faizan, U.; Rouster, A.S. Nutrition and Hydration Requirements in Children and Adults. [Updated 28 August 2023]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562207/ (accessed on 29 July 2024).
- Verbecque, E.; Coetzee, D.; Smits-Engelsman, B. Underweight children are agile but lack power. BMC Pediatr. 2022, 22, 490. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Metcalfe, J.S. The mountain of motor development: A metaphor. Mot. Dev. Res. Rev. 2002, 2, 183–202. [Google Scholar]
- Palmer, K.K.; Chinn, K.M.; Robinson, L.E. Using achievement goal theory in motor skill instruction: A systematic review. Sports Med. 2017, 47, 2569–2583. [Google Scholar] [CrossRef]
- Robinson, L.E.; Stodden, D.F.; Barnett, L.M.; Lopes, V.P.; Logan, S.W.; Rodrigues, L.P.; D’Hondt, E. Motor competence and its effect on positive developmental trajectories of health. Sports Med. 2015, 45, 1273–1284. [Google Scholar] [CrossRef]
- Stodden, D.F.; Goodway, J.D.; Langendorfer, S.J.; Roberton, M.A.; Rudisill, M.E.; Garcia, C.; Garcia, L.E. A developmental perspective on the role of motor skill competence in physical activity: An emergent relationship. Quest 2008, 60, 290–306. [Google Scholar] [CrossRef]
- Nikièma, L.; Huybregts, L.; Kolsteren, P.; Lanou, H.; Tiendrebeogo, S.; Bouckaert, K.; Kouanda, S.; Sondo, B.; Roberfroid, D. Treating moderate acute malnutrition in first-line health services: An effectiveness cluster-randomized trial in Burkina Faso. Am. J. Clin. Nutr. 2014, 100, 241–249. [Google Scholar] [CrossRef]
- Azimi, F.; Esmaillzadeh, A.; Alipoor, E.; Moslemi, M.; Yaseri, M.; Hosseinzadeh-Attar, M. Effect of a newly developed ready-to-use supplementary food on growth indicators in children with mild to moderate malnutrition. Public Health 2020, 185, 290–297. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.H.; Kerac, M.; Trehan, I.; Briend, A. Severe childhood malnutrition. Nat. Rev. Dis. Primers 2017, 3, 17067. [Google Scholar]
- Lagrone, L.; Cole, S.; Schondelmeyer, A.; Maleta, K.; Manary, M.J. Locally produced ready-to-use supplementary food is an effective treatment of moderate acute malnutrition in an operational setting. Ann. Trop. Paediatr. 2010, 30, 103–108. [Google Scholar] [CrossRef]
- Training Module on the National Guidelines on the Management of Moderate Acute Malnutrition for Children under Five Years. Revised on 2020 May. Available online: https://docs.wfp.org/api/documents/WFP-0000116054/download/ (accessed on 29 July 2024).
- Teshome, M.S.; Lema, T.B.; Abessa, T.G.; Mingels, S.; Granitzer, M.; Rameckers, E.; Verbecque, E. Current evidence on the effectiveness of Ready-to-Use Supplementary Foods in children with moderate acute malnutrition: A systematic review and meta-analysis. J. Nutr. Sci. 2023, 12, e130. [Google Scholar] [CrossRef] [PubMed]
- Fetriyuna, F.; Purwestri, R.C.; Jati, I.R.; Setiawan, B.; Huda, S.; Wirawan, N.N.; Andoyo, R. Ready-to-use therapeutic/supplementary foods from local food resources: Technology accessibility, program effectiveness, and sustainability, a review. Heliyon 2023, 9, e22478. [Google Scholar] [CrossRef] [PubMed]
- Merino-Andrés, J.; de Mateos-López, A.G.; Damiano, D.L.; Sánchez-Sierra, A. Effect of muscle strength training in children and adolescents with spastic cerebral palsy: A systematic review and meta-analysis. Clin. Rehabil. 2022, 36, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Bleyenheuft, Y.; Ebner-Karestinos, D.; Surana, B.; Paradis, J.; Sidiropoulos, A.; Renders, A.; Friel, K.M.; Brandao, M.; Rameckers, E.; Gordon, A.M. Intensive upper-and lower-extremity training for children with bilateral cerebral palsy: A quasi-randomized trial. Dev. Med. Child Neurol. 2017, 59, 625–633. [Google Scholar] [CrossRef]
- Rafique, N. Effects of task-oriented training on walking in children with cerebral palsy. J. Med. Sci. 2022, 30, 87–91. [Google Scholar] [CrossRef]
- Sanli, B.B.; Potten, Y.J.; Rameckers, E.A.; Meeuwsen, I.; Coenen, M.; Caponi, L.; Roijen, R.; Teeuwen, L.; Berge, G.v.D.; de Haan, C.; et al. Effect on Quality of Life in Children and Adolescents with Disabilities after a Functional Intensive Therapy Approach. Biomed. J. Sci. Tech. Res. 2020, 31, 24146–24151. [Google Scholar] [CrossRef]
- Jackman, M.; Lannin, N.; Galea, C.; Sakzewski, L.; Miller, L.; Novak, I. What is the threshold dose of upper limb training for children with cerebral palsy to improve function? A systematic review. Aust. Occup. Ther. J. 2020, 67, 269–280. [Google Scholar] [CrossRef]
- Brandão, M.B.; Mancini, M.C.; Ferre, C.L.; Figueiredo, P.R.; Oliveira, R.H.S.; Gonçalves, S.C.; Dias, M.C.; Gordon, A.M. Does dosage matter? A pilot study of hand-arm bimanual intensive training (HABIT) dose and dosing schedule in children with unilateral cerebral palsy. Phys. Occup. Ther. Pediatr. 2018, 38, 227–242. [Google Scholar] [CrossRef]
- Sakzewski, L.; Provan, K.; Ziviani, J.; Boyd, R.N. Comparison of dosage of intensive upper limb therapy for children with unilateral cerebral palsy: How big should the therapy pill be? Res. Dev. Disabil. 2015, 37, 9–16. [Google Scholar] [CrossRef]
- Sakzewski, L.; Ziviani, J.; Boyd, R.N. Efficacy of Upper Limb Therapies for Unilateral Cerebral Palsy: A Meta-analysis. Pediatrics 2014, 133, e175–e204. [Google Scholar] [CrossRef]
- Sutapa, P.; Pratama, K.W.; Rosly, M.M.; Ali, S.K.S.; Karakauki, M. Improving Motor Skills in Early Childhood through Goal-Oriented Play Activity. Children 2021, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Kirk, I.J.; Waldie, K.E. High-intensity training enhances executive function in children in a randomized, placebo-controlled trial. Elife 2017, 6, e25062. [Google Scholar] [PubMed]
- Smits-Engelsman, B.C.M.; Blank, R.; van der Kaay, A.; van der Meijs, R.M.; van den Brand, E.V.; Polatajko, H.J.; Wilson, P.H. Efficacy of interventions to improve motor performance in children with developmental coordination disorder: A combined systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 229–237. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Sadler, K.; Wondafrash, M.; Argaw, A.; Luo, H.; Geleta, B.; Kedir, K.; Getnet, Y.; Belachew, T.; Bahwere, P. Children with moderate acute malnutrition with no access to supplementary feeding programs experience high rates of deterioration and no improvement: Results from a prospective cohort study in rural Ethiopia. PLoS ONE 2016, 11, e0153530. [Google Scholar] [CrossRef]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Changes in grey matter induced by training. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef]
- Campbell, M.K.; Piaggio, G.; Elbourne, D.R.; Altman, D.G. Consort 2010 statement: Extension to cluster randomized trials. BMJ 2012, 345, e5661. [Google Scholar] [CrossRef]
- Polit, D.F.; Beck, C. Nursing Research: Principles and Methods, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Browne, R.H. On the use of a pilot sample for sample size determination. Stat. Med. 1995, 14, 1933–1940. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Van Belle, G. Statistical Rules of Thumb; John Wiley and Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Hertzog, M.A. Considerations in determining sample size for pilot studies. Res. Nurs. Health 2008, 31, 180–191. [Google Scholar] [CrossRef]
- NIHR, Biomedical Research Centre. Procedure for Measuring the Height of Children over 2. 2–5 June 2017. Available online: http://www.uhs.nhs.uk/Media/Southampton-Clinical-Research/Procedures/BRCProcedures/Procedure-for-height-of-children-over-2.pdf (accessed on 29 July 2024).
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height, and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 4 January 2020).
- NHANES, C.; Muscle Strength Procedures Manual. 40 April 2011. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/muscle_strength_proc_manual.pdf (accessed on 29 July 2024).
- Eek, M.N.; Kroksmark, A.K.; Beckung, E. Isometric muscle torque in children 5 to 15 years of age: Normative data. Arch. Phys. Med. Rehabil. 2006, 87, 1091–1099. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Body Composition Procedures Manual. 2018. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/Body_Composition_Procedures_Manual_2018.pdf (accessed on 29 July 2024).
- PERFORM Operating Document Use and Cleaning Procedures for Bodystat QuadScan 4000PC-POD-CP-012-v01. 18 June 2020. Available online: https://perform.concordia.ca/GettingStarted/pdf/compliance/PC-POD-CP-012-V01%20USE%20AND%20CLEANING%20PROCEDURES%20FOR%20BODYSTAT%20QUADSCAN%204000.pdf (accessed on 29 July 2024).
- Golden, M.H. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr. Bull. 2009, 30, S267–S342. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Camprubi-Robles, M.; Bear, D.; Cederholm, T.; Malafarina, V.; Welch, A.; Cruz-Jentoft, A. Muscle loss: The new malnutrition challenge in clinical practice. Clin. Nutr. 2019, 38, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Ackatia-Armah, R.S.; Doumbia, S.; Kupka, R.; Duggan, C.P.; Brown, K.H. Percent fat mass increases with recovery, but does not vary according to dietary therapy in young malian children treated for moderate acute malnutrition. J. Nutr. 2019, 149, 1089–1096. [Google Scholar] [CrossRef]
- Steenkamp, L.; Lategan, R.; Raubenheimer, J. The impact of Ready-to-Use Supplementary Food (RUSF) in targeted supplementation of children with moderate acute malnutrition (MAM) in South Africa. S. Afr. Fam. Pract. 2015, 57, 322–325. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Henretty, N.M.; Delnatus, J.R.; Previl, W.; Stehl, T.; Vorkoper, S.; Bodden, J.; Maust, A.; Smidt, R.; Nash, M.L.; et al. Ready-to-use supplementary food increases fat mass and BMI in Haitian school-aged children. J. Nutr. 2015, 145, 813–822. [Google Scholar] [CrossRef]
- Makori, N.; Masanja, H.; Masumo, R.; Rashid, S.; Jumbe, T.; Tegeye, M.; Esau, D.; Muiruri, J.; Mchau, G.; Mafung’a, S.H.; et al. Efficacy of ready-to-use food supplement for treatment of moderate acute malnutrition among children aged 6 to 59 months. Matern. Child Nutr. 2024, 20, e13602. [Google Scholar] [CrossRef] [PubMed]
- Fabiansen, C.; Yaméogo, C.W.; Iuel-Brockdorf, A.S.; Cichon, B.; Rytter, M.J.; Kurpad, A.; Wells, J.C.; Ritz, C.; Ashorn, P.; Filteau, S.; et al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomized 2 × 2 × 3 factorial trial in Burkina Faso. PLoS Med. 2017, 14, e1002387. [Google Scholar] [CrossRef]
- Ackatia-Armah, R.S.; McDonald, C.M.; Doumbia, S.; Erhardt, J.G.; Hamer, D.H.; Brown, K.H. Malian children with moderate acute malnutrition who are treated with lipid-based dietary supplements have greater weight gains and recovery rates than those treated with locally produced cereal-legume products: A community-based, cluster-randomized trial. Am. J. Clin. Nutr. 2015, 101, 632–645. [Google Scholar] [CrossRef]
- Garraza, M.; Forte, L.M.; Navone, G.T.; Oyhenart, E.E. Desnutrición, composición y proporción corporales en escolares de dos departamentos de Mendoza, Argentina. Intersecc. Antropol. 2014, 15, 167–175. [Google Scholar]
- Oyhenart, E.E.; Torres, M.F.; Luis, M.A.; Garraza, M.; Navazo, B.; Quintero, F.A.; Cesani, M.F. Body composition in relation to nutritional status and socio-environmental conditions in schoolchildren living in the urban periphery of La Plata, Argentina. Arch. Latinoam. Nutr. 2020, 70, 81–94. [Google Scholar] [CrossRef]
- Teshome, M.S.; Bekele, T.; Verbecque, E.; Mingels, S.; Granitzer, M.; Abessa, T.G.; Lema, T.B.; Rameckers, E. Body composition and associated factors among 5–7-year-old children with moderate acute malnutrition in Jimma town in southwest Ethiopia: A comparative cross-sectional study. Matern. Child Nutr. 2024, 20, e13655. [Google Scholar] [PubMed]
- Medoua, G.N.; Ntsama, P.M.; Ndzana, A.C.A.; Essa’a, V.J.; Tsafack, J.J.T.; Dimodi, H.T. Recovery rate of children with moderate acute malnutrition treated with ready-to-use supplementary food (RUSF) or improved corn–soya blend (CSB+): A randomized controlled trial. Public Health Nutr. 2016, 19, 363–370. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2021 Physical Activity Factsheets for the European Union Member States in the WHO European Region; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2021; Available online: https://iris.who.int/bitstream/handle/10665/345335/WHO-EURO-2021-3409-43168-60449-eng.pdf;sequence=2 (accessed on 29 July 2024).
- Ernandini, E.; Alvin Wiryaputra, J. Making Physical Activities a Part of a Child’s Life [Internet]; Updates on Physical Fitness in Children; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Bardid, F. Early Childhood Motor Development: Measuring, Understanding and Promoting Motor Competence. Ph.D. Thesis, Ghent University, Gent, Belgium, 2016. Available online: http://hdl.handle.net/1854/LU-8058200 (accessed on 29 July 2024).
- Logan, S.W.; Robinson, L.E.; Wilson, A.E.; Lucas, W.A. Getting the fundamentals of movement: A meta-analysis of the effectiveness of motor skill interventions in children. Child Care Health Dev. 2011, 38, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Riethmuller, A.M.; Jones, R.A.; Okely, A.D. Efficacy of interventions to improve motor development in young children: A systematic review. Pediatrics 2009, 124, e782–e792. [Google Scholar] [CrossRef]
- Bardid, F.; Deconinck, F.J.; Descamps, S.; Verhoeven, L.; De Pooter, G.; Lenoir, M.; D’hondt, E. The effectiveness of a fundamental motor skill intervention in pre-schoolers with motor problems depends on gender but not environmental context. Res. Dev. Disabil. 2013, 34, 4571–4581. [Google Scholar] [CrossRef]

| Variables | Category | Types of Intervention | Total | p | ||
|---|---|---|---|---|---|---|
| Control (n = 21) | RUSF (n = 23) | RUSF + HIML (n = 25) | ||||
| n (%) | n (%) | n (%) | n (%) | |||
| Marital status of the caregiver | Married | 18 (85.7) | 18 (78.3) | 20 (80.0) | 56 (81.2) | 0.270 |
| Divorced | 3 (14.3) | 1 (4.3) | 3 (12) | 7 (10.1) | ||
| Widowed | 0 (0.0) | 1 (4.3) | 1 (4.0) | 2 (2.9) | ||
| Separated | 0 (0.0) | 0 (0.0) | 1 (4.0) | 1 (1.4) | ||
| Single | 0 (0.0) | 3 (13.0) | 0 (0.0) | 3 (4.3) | ||
| Age (years) of the mother/caregiver | <30 | 13 (61.9) | 11 (47.8) | 16 (64.0) | 40 (58.0) | 0.478 |
| ≥30 | 8 (38.1) | 12 (52.2) | 9 (31.0) | 29 (42.0) | ||
| Family size | <5 | 19 (90.5) | 21 (91.3) | 13 (52.0) | 53 (76.8) | 0.001 |
| ≥5 | 2 (9.5) | 2 (8.7) | 12 (48.0) | 16 (23.2) | ||
| Salary per month (ETB) | <3500 | 10 (100.0) | 19 (100.0) | 15 (93.8) | 44 (97.8) | 0.396 |
| ≥3500 | 0 (0.0) | 0 (0.0) | 1 (6.3) | 1 (2.2) | ||
| Educational status of the mother | Could not read and write | 2 (9.5) | 5 (21.7) | 6 (24.0) | 13 (18.8) | 0.956 |
| Could read and write | 2 (9.5) | 3 (13.0) | 3 (12.0) | 8 (11.6) | ||
| Primary (0–8) | 10 (47.6) | 8 (34.8) | 9 (36.0) | 27 (39.1) | ||
| Secondary (9–12) | 5 (23.8) | 5 (21.7) | 4 (16.0) | 14 (20.3) | ||
| Above secondary (> 12) | 2 (9.5) | 2 (8.7) | 3 (12.0) | 7 (10.1) | ||
| Educational status of the husband | Could not read and write | 1 (4.8) | 2 (8.7) | 1 (4.0) | 4 (5.8) | 0.704 |
| Could read and write | 2 (9.5) | 0 (0.0) | 1 (4.0) | 3 (4.3) | ||
| Primary (0–8) | 7 (33.3) | 10 (43.5) | 14 (56.0) | 31 (44.9) | ||
| Secondary (9–12) | 9 (42.9) | 8 (34.8) | 6 (24.0) | 23 (33.3) | ||
| Above secondary (> 12) | 2 (9.5) | 3 (13.0) | 3 (12.0) | 8 (11.6) | ||
| Occupation of the mother | Housewife | 12 (57.1) | 7 (30.4) | 9 (36.0) | 28 (40.6) | 0.658 |
| Merchant | 0 (0.0) | 1 (4.3) | 2 (8.0) | 3 (4.3) | ||
| Gov’t employee | 5 (23.8) | 6 (26.1) | 6 (24.0) | 17 (24.6) | ||
| Self-employee | 3 (14.3) | 7 (30.4) | 5 (20.0) | 15 (21.7) | ||
| Other (e.g., daily laborer, wood seller) | 1 (4.8) | 2 (8.7) | 3 (12.0) | 6 (8.7) | ||
| Occupation of the husband | Farmer | 0 (0.0) | 1 (4.3) | 2 (8.3) | 3 (4.4) | 0.851 |
| Merchant | 1 (4.8) | 3 (13.0) | 2 (8.3) | 6 (8.8) | ||
| Gov’t employee | 8 (38.1) | 6 (26.1) | 5 (20.8) | 19 (27.9) | ||
| Private employee | 10 (47.6) | 11 (47.8) | 12 (50.0) | 33 (48.5) | ||
| Other (as specified) | 2 (9.5) | 2 (8.7) | 3 (12.5) | 7 (10.3) | ||
| Religion | Muslim | 9 (42.9) | 8 (34.8) | 17 (68.0) | 34 (49.3) | 0.110 |
| Orthodox | 11 (52.4) | 11 (47.8) | 6 (24.0) | 28 (40.6) | ||
| Protestant | 1 (4.8) | 4 (17.4) | 2 (8.0) | 7 (10.1) | ||
| Head of household | Father | 15 (71.4) | 17 (73.9) | 18 (72.0) | 50 (72.5) | 0.981 |
| Mother | 6 (28.6) | 6 (26.1) | 7 (28.0) | 19 (27.5) | ||
| Wealth index | Poor | 3 (14.3) | 10 (43.5) | 8 (32.0) | 21 (30.4) | 0.237 |
| Medium | 16 (76.2) | 11 (47.8) | 13 (52.0) | 40 (58.0) | ||
| Rich | 2 (9.5) | 2 (8.7) | 4 (16.0) | 8 (11.6) | ||
| HFIA category | Severe food insecurity | 21 (100.0) | 23 (100.0) | 25 (100.0) | 69 (100.0) | |
| Latrine facility in the compound | Yes | 20 (95.2) | 21 (91.3) | 23 (92.0) | 64 (92.8) | 0.867 |
| No | 1 (4.8) | 2 (8.7) | 2 (8.0) | 5 (7.2) | ||
| Type of latrine | Flush toilet | 0 (0.0) | 4 (18.2) | 2 (8.7) | 6 (9.2) | 0.126 |
| Pit latrine | 20 (100.0) | 18 (81.8) | 21 (91.3) | 59 (90.8) | ||
| Variables | Category | Types of Intervention | Total | p | ||
|---|---|---|---|---|---|---|
| Control | RUSF | RUSF + HIML | ||||
| n (%) | n (%) | n (%) | ||||
| Sex of child | Male | 10 (47.6) | 11 (47.8) | 10 (40.0) | 31 (44.9) | 0.825 |
| Female | 11 (52.4) | 12 (52.2) | 15 (60.0) | 38 (55.1) | ||
| Age of child (years) | 5 | 6 (28.6) | 7 (30.4) | 4 (16.0) | 17 (24.6) | 0.180 |
| 6 | 2 (9.5) | 2 (8.7) | 8 (32.0) | 12 (17.4) | ||
| 7 | 13 (61.9) | 14 (60.9) | 13 (52.0) | 40 (58.0) | ||
| Grade level | KG or Zero-Grade | 8 (38.1) | 8 (34.8) | 12 (48.0) | 28 (40.6) | 0.623 |
| Grade One | 13 (61.9) | 15 (65.2) | 13 (52.0) | 41 (59.4) | ||
| Place of delivery | At a public health institute | 16 (76.2) | 19 (82.6) | 19 (76.0) | 54 (78.3) | 0.150 |
| At a private health institute | 1 (4.8) | 3 (13.0) | 0 (0.0) | 4 (5.8) | ||
| At home | 4 (19.0) | 1 (4.3) | 6 (24.0) | 11 (15.9) | ||
| Means of transportation | Walking | 21 (100.0) | 23 (100.0) | 25 (100.0) | 69 (100.0) | |
| Child fully immunized | Yes | 19 (90.5) | 22 (95.7) | 24 (96.0) | 65 (94.2) | 0.680 |
| No | 2 (9.5) | 1 (4.3) | 1 (4.0) | 4 (5.8) | ||
| Deworming tablet in the last 6 months | Yes | 15 (71.4) | 18 (78.3) | 13 (52.0) | 46 (66.7) | 0.134 |
| No | 6 (28.6) | 5 (21.7) | 12 (48.0) | 23 (33.3) | ||
| Child receiving vitamin A supplementation | Yes | 15 (71.4) | 18 (78.3) | 6 (24.0) | 39 (56.5) | <0.001 |
| No | 6 (28.6) | 5 (21.7) | 19 (76.0) | 30 (43.5) | ||
| Any illness in the past 2 weeks | Yes | 11 (52.4) | 8 (34.8) | 11 (44.0) | 30 (43.5) | 0.500 |
| No | 10 (47.6) | 15 (65.2) | 14 (56.0) | 39 (56.5) | ||
| EBF for the first 6 months | yes | 14 (66.7) | 10 (43.5) | 15 (60.0) | 39 (56.5) | 0.273 |
| No | 7 (33.3) | 13 (56.5) | 10 (40.0) | 30 (43.5) | ||
| Complementary feeding | Before 6 months | 7 (33.3) | 13 (56.5) | 10 (40.0) | 30 (43.5) | 0.030 |
| At 6 months | 1 (4.8) | 4 (17.4) | 8 (32.0) | 13 (18.8) | ||
| After 6 months | 13 (61.9) | 6 (26.1) | 7 (28.0) | 26 (37.7) | ||
| Distance from the school (minutes) | <30 min | 20 (95.2) | 16 (69.6) | 19 (76.0) | 55 (79.7) | 0.090 |
| ≥30 min | 1 (4.8) | 7 (30.4) | 6 (24.0) | 14 (20.3) | ||
| School absenteeism in days | <4 days | 20 (95.2) | 20 (87.0) | 21 (84.0) | 61 (88.4) | 0.478 |
| ≥4 days | 1 (4.8) | 3 (13.0) | 4 (16.0) | 8 (11.6) | ||
| Variables | Intervention Type | n | Baseline | Endline | Difference | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Lower Bound | Upper Bound | ||||
| Height (cm) | No intervention | 21 | 114.97 ± 7.80 | 115.69 ± 7.96 | 0.72 ± 1.11 | 0.22 | 1.22 | <0.001 |
| RUSF | 23 | 117.53 ± 7.98 | 119.45 ± 7.62 | 1.92 ± 0.70 | 1.62 | 2.22 | ||
| RUSF + HiML | 25 | 117.03 ± 5.03 | 119.95 ± 5.14 | 2.92 ± 0.96 | 2.52 | 3.32 | ||
| Total | 69 | 116.57± 6.97 | 118.49 ± 7.09 | 1.92 ± 1.29 | 1.61 | 2.23 | ||
| Weight (kg) | No intervention | 21 | 17.54 ± 2.40 | 18.92 ± 2.54 | 1.38 ± 0.91 | 0.97 | 1.80 | <0.001 |
| RUSF | 23 | 17.13 ± 2.27 | 20.02 ± 2.41 | 2.89 ± 0.80 | 2.54 | 3.23 | ||
| RUSF + HiML | 25 | 17.03 ± 1.73 | 20.03 ± 1.72 | 3.00 ± 0.93 | 2.62 | 3.39 | ||
| Total | 69 | 17.22 ± 2.12 | 19.69 ± 2.26 | 2.47 ± 1.13 | 2.20 | 2.74 | ||
| Grip strength (kg) | No intervention | 21 | 6.77 ± 2.39 | 7.05 ± 2.14 | 0.28 ± 1.04 | −0.19 | 0.75 | <0.001 |
| RUSF | 23 | 7.42 ± 2.24 | 8.73 ± 2.00 | 1.32 ± 1.91 | 0.49 | 2.14 | ||
| RUSF + HiML | 25 | 5.99 ± 1.69 | 9.07 ± 1.05 | 3.08 ± 1.53 | 2.45 | 3.71 | ||
| Total | 69 | 6.71 ± 2.16 | 8.34 ± 1.95 | 1.64 ± 1.92 | 1.18 | 2.10 | ||
| Elbow flexor (N) | No intervention | 21 | 68.70 ± 11.88 | 74.17 ± 12.07 | 5.46 ± 6.79 | 2.37 | 8.55 | <0.001 |
| RUSF | 23 | 69.61 ± 15.03 | 95.71 ± 19.44 | 26.09 ± 14.02 | 20.03 | 32.15 | ||
| RUSF + HiML | 25 | 71.25 ± 10.14 | 92.64 ± 11.72 | 21.39 ± 9.55 | 17.45 | 25.33 | ||
| Total | 69 | 69.93 ± 12.33 | 88.04 ± 17.34 | 18.11 ± 13.57 | 14.85 | 21.37 | ||
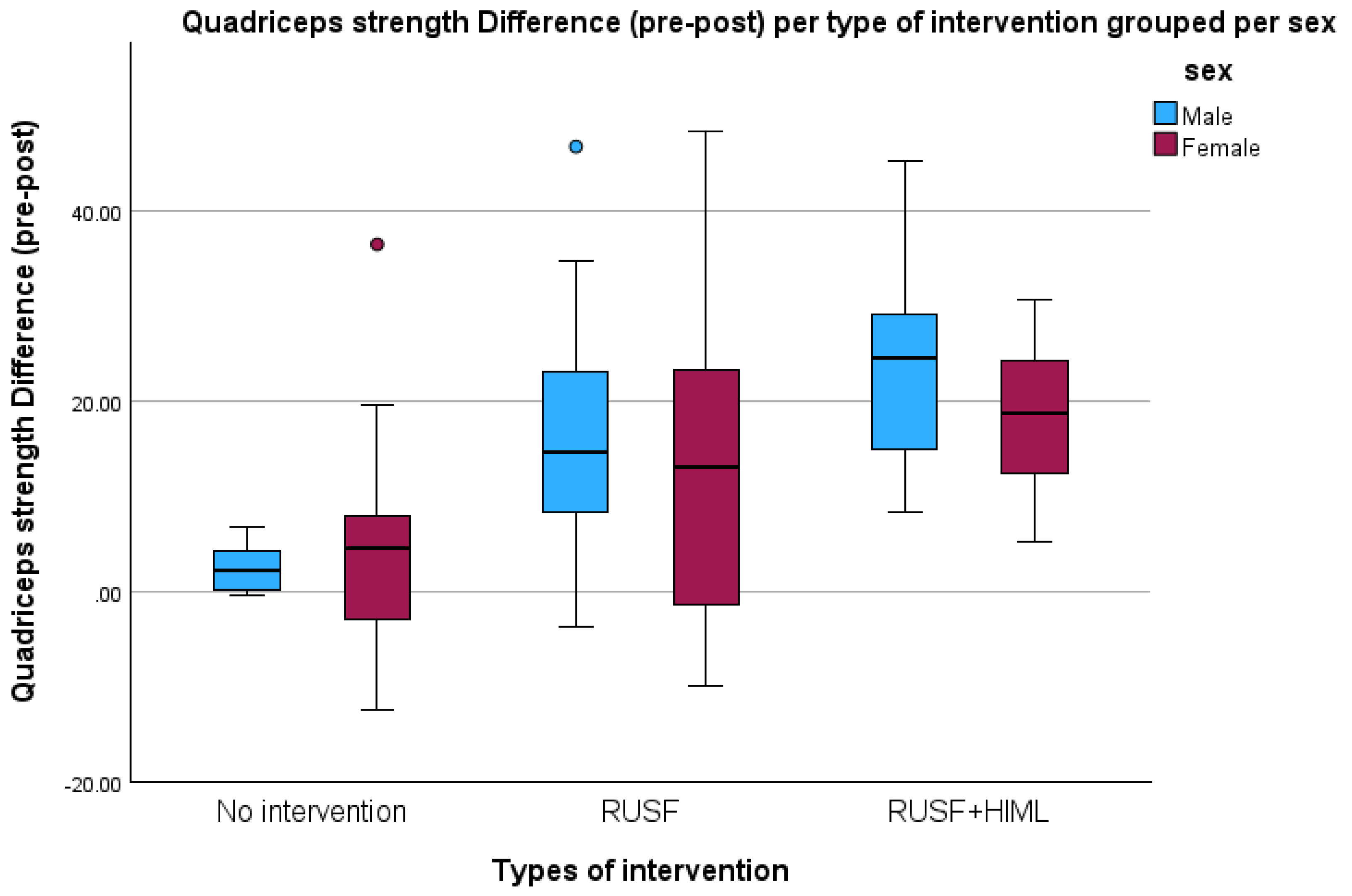
| Quadriceps (N) | No intervention | 21 | 79.37 ± 17.49 | 83.51 ± 15.74 | 4.14 ± 9.69 | −0.28 | 8.55 | <0.001 |
| RUSF | 23 | 79.57 ± 18.08 | 94.70 ± 19.05 | 15.13 ± 16.12 | 8.16 | 22.11 | ||
| RUSF + HiML | 25 | 76.00 ± 15.20 | 96.87 ± 13.37 | 20.87 ± 10.59 | 16.50 | 25.24 | ||
| Total | 69 | 78.21 ± 16.73 | 92.08 ± 16.94 | 13.86 ± 14.11 | 10.47 | 17.25 | ||
| Gastrocnemius sup flexor of the leg (N) | No intervention | 21 | 87.12 ± 13.15 | 93.88 ± 14.35 | 6.76 ± 8.90 | 2.71 | 10.82 | <0.001 |
| RUSF | 23 | 84.91 ± 13.88 | 110.62 ± 12.88 | 25.72 ± 14.58 | 19.41 | 32.02 | ||
| RUSF + HiML | 25 | 80.14 ± 11.22 | 96.65 ± 14.30 | 16.51 ± 9.65 | 12.53 | 20.50 | ||
| Total | 69 | 83.85 ± 12.90 | 100.47 ± 15.49 | 16.61 ± 13.53 | 13.36 | 19.86 | ||
| Fat mass (kg) | No intervention | 21 | 5.80 ± 1.22 | 5.60 ± 1.11 | −0.20 ± 0.80 | −0.56 | 0.16 | 0.032 |
| RUSF | 23 | 5.98 ± 1.49 | 6.60 ± 1.31 | 0.63 ± 1.59 | −0.06 | 1.31 | ||
| RUSF + HiML | 25 | 5.28 ± 1.18 | 5.88 ± 1.38 | 0.60 ± 0.92 | 0.22 | 0.98 | ||
| Total | 69 | 5.67 ± 1.32 | 6.03± 1.33 | 0.37 ± 1.20 | 0.08 | 0.65 | ||
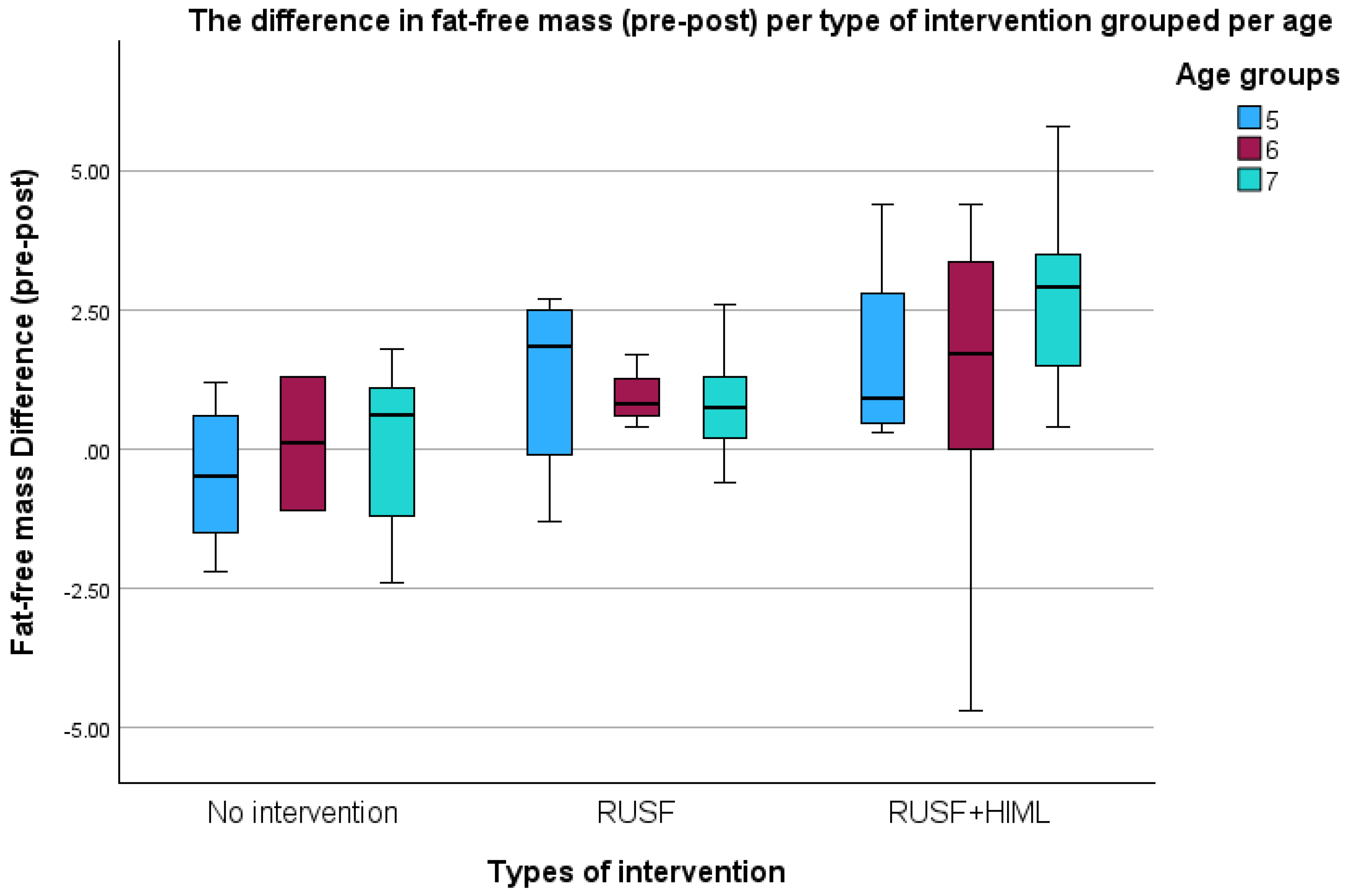
| Fat-free mass (kg) | No intervention | 21 | 13.40 ± 2.45 | 13.28± 2.10 | −0.12 ± 1.36 | −0.74 | 0.50 | <0.001 |
| RUSF | 23 | 12.57 ± 2.07 | 13.49 ± 1.78 | 0.91 ± 1.05 | 0.46 | 1.37 | ||
| RUSF + HiML | 25 | 11.97 ± 2.28 | 14.04 ± 1.50 | 2.08 ± 2.20 | 1.17 | 2.98 | ||
| Total | 69 | 12.60 ± 2.31 | 13.62 ± 1.79 | 1.02 ± 1.85 | 0.58 | 1.46 | ||
| Dependent Variable | Types of Intervention | Mean Difference | Std. Err | 95% CI | p | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Height (cm) difference | RUSF vs. No intervention | 1.20 | 0.28 | 0.64 | 1.76 | <0.001 |
| RUSF + HiML vs. No intervention | 2.20 | 0.28 | 1.65 | 2.75 | <0.001 | |
| RUSF + HiML vs. RUSF | 1.00 | 0.27 | 0.46 | 1.54 | <0.001 | |
| Weight (kg) difference | RUSF vs. No intervention | 1.50 | 0.27 | 0.97 | 2.03 | <0.001 |
| RUSF + HiML vs. No intervention | 1.62 | 0.26 | 1.10 | 2.14 | <0.001 | |
| RUSF + HiML vs. RUSF | 0.11 | 0.25 | −0.39 | 0.62 | 0.653 | |
| Grip strength (kg) difference | RUSF vs. No intervention | 1.04 | 0.46 | −0.08 | 2.16 | 0.074 |
| RUSF + HiML vs. No intervention | 2.80 | 0.38 | 1.88 | 3.72 | <0.001 | |
| RUSF + HiML vs. RUSF | 1.76 | 0.50 | 0.54 | 2.98 | 0.003 | |
| Elbow flexor (N) difference | RUSF vs. No intervention | 20.63 | 3.28 | 12.58 | 28.68 | <0.001 |
| RUSF + HiML vs. No intervention | 15.93 | 2.42 | 10.06 | 21.80 | <0.001 | |
| RUSF + HiML vs. RUSF | −4.70 | 3.49 | −13.21 | 3.81 | 0.379 | |
| Quadricep strength (N) difference | RUSF vs. No intervention | 11.00 | 3.97 | 1.29 | 20.70 | 0.023 |
| RUSF + HiML vs. No intervention | 16.73 | 2.99 | 9.47 | 23.99 | <0.001 | |
| RUSF + HiML vs. RUSF | 5.74 | 3.97 | −3.96 | 15.43 | 0.329 | |
| Gastrocnemius sup flexor of the leg (N) difference | RUSF vs. No intervention | 18.95 | 3.42 | 12.11 | 25.79 | <0.001 |
| RUSF + HiML vs. No intervention | 9.75 | 3.36 | 3.05 | 16.46 | 0.005 | |
| RUSF + HiML vs. RUSF | −9.20 | 3.28 | −15.75 | −2.65 | 0.007 | |
| Fat mass (kg) difference | RUSF vs. No intervention | 0.83 | 0.35 | 0.13 | 1.52 | 0.021 |
| RUSF + HiML vs. No intervention | 0.80 | 0.34 | 0.12 | 1.48 | 0.022 | |
| RUSF+ HiML vs. RUSF | −0.03 | 0.33 | −0.69 | 0.64 | 0.938 | |
| Fat-free mass (kg) difference | RUSF vs. No intervention | 1.03 | 0.37 | 0.13 | 1.93 | 0.022 |
| RUSF + HiML vs. No intervention | 2.20 | 0.53 | 0.90 | 3.49 | <0.001 | |
| RUSF + HiML vs. RUSF | 1.16 | 0.49 | −0.04 | 2.37 | 0.059 | |
| Outcome Variable | Predictors | β | 95% CI | p | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Height (cm) | RUSF + HiML | 2.286 | 1.743 | 2.830 | <0.001 | |
| RUSF | 1.206 | 0.673 | 1.739 | <0.001 | ||
| No intervention | Ref. | . | . | . | ||
| Sex (F) | −0.232 | −0.648 | 0.184 | 0.275 | ||
| Age (yrs) | 7 | −0.057 | −0.647 | 0.533 | 0.850 | |
| 6 | 0.041 | −0.669 | 0.751 | 0.910 | ||
| 5 | Ref. | |||||
| Weight (kg) | RUSF + HiML | 1.730 | 1.199 | 2.261 | <0.001 | |
| RUSF | 1.505 | 1.030 | 1.979 | <0.001 | ||
| No intervention | Ref. | |||||
| Sex (F) | 0.417 | 0.012 | 0.822 | 0.044 | ||
| Age (yrs) | 7 | 0.244 | −0.233 | 0.722 | 0.316 | |
| 6 | 0.086 | −0.407 | 0.579 | 0.733 | ||
| 5 | Ref. | |||||
| Grip strength (kg) | RUSF + HiML | 2.783 | 2.017 | 3.549 | <0.001 | |
| RUSF | 1.046 | 0.070 | 2.022 | 0.036 | ||
| No intervention | Ref. | |||||
| Sex (F) | −0.090 | −0.810 | 0.631 | 0.807 | ||
| Age (yrs) | 7 | −0.493 | −1.318 | 0.332 | 0.241 | |
| 6 | −0.269 | −1.667 | 1.129 | 0.706 | ||
| 5 | Ref. | |||||
| Elbow flexor (N) | RUSF + HiML | 17.171 | 12.359 | 21.982 | <0.001 | |
| RUSF | 20.620 | 14.996 | 26.244 | <0.001 | ||
| No intervention | Ref. | |||||
| Sex (F) | −2.400 | −7.173 | 2.373 | 0.324 | ||
| Age (yrs) | 7 | 2.902 | −3.049 | 8.852 | 0.339 | |
| 6 | −2.816 | −10.057 | 4.426 | 0.446 | ||
| 5 | Ref. | |||||
| Quadricep strength (N) | RUSF + HiML | 15.890 | 9.303 | 22.478 | <0.001 | |
| RUSF | 10.298 | 2.269 | 18.328 | 0.012 | ||
| No intervention | Ref. | |||||
| Sex (F) | 2.067 | −3.964 | 8.099 | 0.502 | ||
| Age (yrs) | 7 | −3.619 | −10.339 | 3.100 | 0.291 | |
| 6 | −2.975 | −11.078 | 5.128 | 0.472 | ||
| 5 | Ref. | |||||
| Gastrocnemius sup flexor of the leg (N) | RUSF + HiML | 9.626 | 3.613 | 15.640 | 0.002 | |
| RUSF | 19.055 | 12.741 | 25.370 | <0.001 | ||
| No intervention | Ref. | |||||
| Sex (F) | 1.804 | −3.265 | 6.873 | 0.486 | ||
| Age (yrs) | 7 | 4.869 | −0.195 | 9.934 | 0.060 | |
| 6 | −5.131 | −14.086 | 3.824 | 0.261 | ||
| 5 | Ref. | |||||
| Fat mass (kg) | RUSF + HiML | 0.892 | 0.275 | 1.508 | 0.005 | |
| RUSF | 1.020 | 0.419 | 1.620 | 0.001 | ||
| No intervention | Ref. | |||||
| Sex (F) | 0.047 | −0.472 | 0.565 | 0.860 | ||
| Age (yrs) | 7 | −0.131 | −0.683 | 0.421 | 0.642 | |
| 6 | −0.314 | −0.883 | 0.256 | 0.280 | ||
| 5 | Ref. | |||||
| Fat-free mass (kg) | RUSF + HiML | 2.025 | 0.951 | 3.100 | <0.001 | |
| RUSF | 0.932 | 0.195 | 1.670 | 0.013 | ||
| No intervention | Ref. | |||||
| Sex (F) | 0.609 | −0.091 | 1.309 | 0.088 | ||
| Age (yrs) | 7 | 0.443 | −0.373 | 1.258 | 0.287 | |
| 6 | −0.515 | −1.895 | 0.865 | 0.464 | ||
| 5 | Ref. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teshome, M.S.; Verbecque, E.; Mingels, S.; Granitzer, M.; Abessa, T.G.; Bruckers, L.; Belachew, T.; Rameckers, E. Investigating the Effects of Dietary Supplementation and High-Intensity Motor Learning on Nutritional Status, Body Composition, and Muscle Strength in Children with Moderate Thinness in Southwest Ethiopia: A Cluster-Randomized Controlled Trial. Nutrients 2024, 16, 3118. https://doi.org/10.3390/nu16183118
Teshome MS, Verbecque E, Mingels S, Granitzer M, Abessa TG, Bruckers L, Belachew T, Rameckers E. Investigating the Effects of Dietary Supplementation and High-Intensity Motor Learning on Nutritional Status, Body Composition, and Muscle Strength in Children with Moderate Thinness in Southwest Ethiopia: A Cluster-Randomized Controlled Trial. Nutrients. 2024; 16(18):3118. https://doi.org/10.3390/nu16183118
Chicago/Turabian StyleTeshome, Melese Sinaga, Evi Verbecque, Sarah Mingels, Marita Granitzer, Teklu Gemechu Abessa, Liesbeth Bruckers, Tefera Belachew, and Eugene Rameckers. 2024. "Investigating the Effects of Dietary Supplementation and High-Intensity Motor Learning on Nutritional Status, Body Composition, and Muscle Strength in Children with Moderate Thinness in Southwest Ethiopia: A Cluster-Randomized Controlled Trial" Nutrients 16, no. 18: 3118. https://doi.org/10.3390/nu16183118
APA StyleTeshome, M. S., Verbecque, E., Mingels, S., Granitzer, M., Abessa, T. G., Bruckers, L., Belachew, T., & Rameckers, E. (2024). Investigating the Effects of Dietary Supplementation and High-Intensity Motor Learning on Nutritional Status, Body Composition, and Muscle Strength in Children with Moderate Thinness in Southwest Ethiopia: A Cluster-Randomized Controlled Trial. Nutrients, 16(18), 3118. https://doi.org/10.3390/nu16183118






